Heart Rate Variability Behavior during Exercise and Short-Term Recovery Following Energy Drink Consumption in Men and Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects
2.3. HRV Parameters Analysis
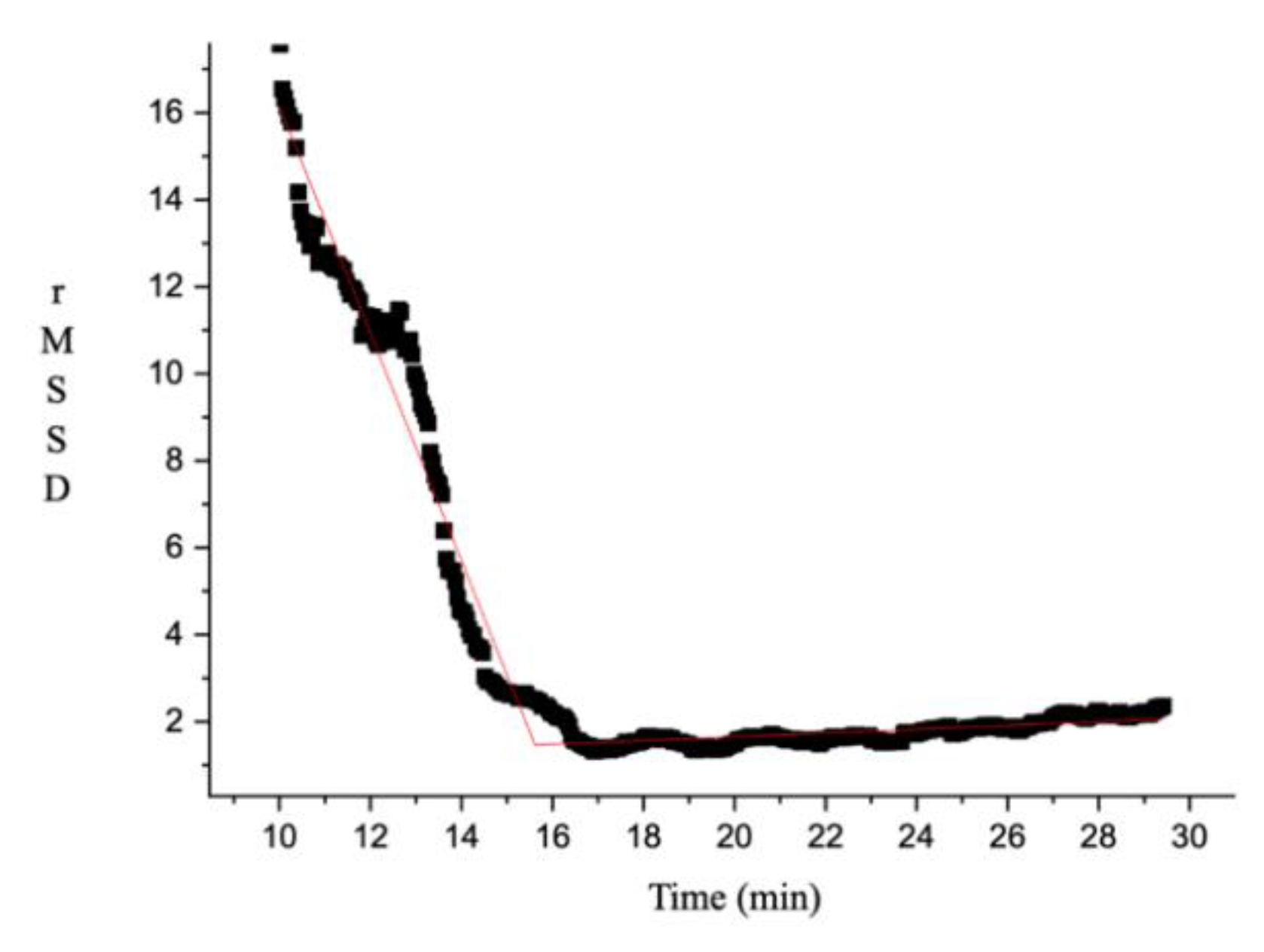
2.4. HRVT Determination
2.5. Statistical Analysis
3. Results
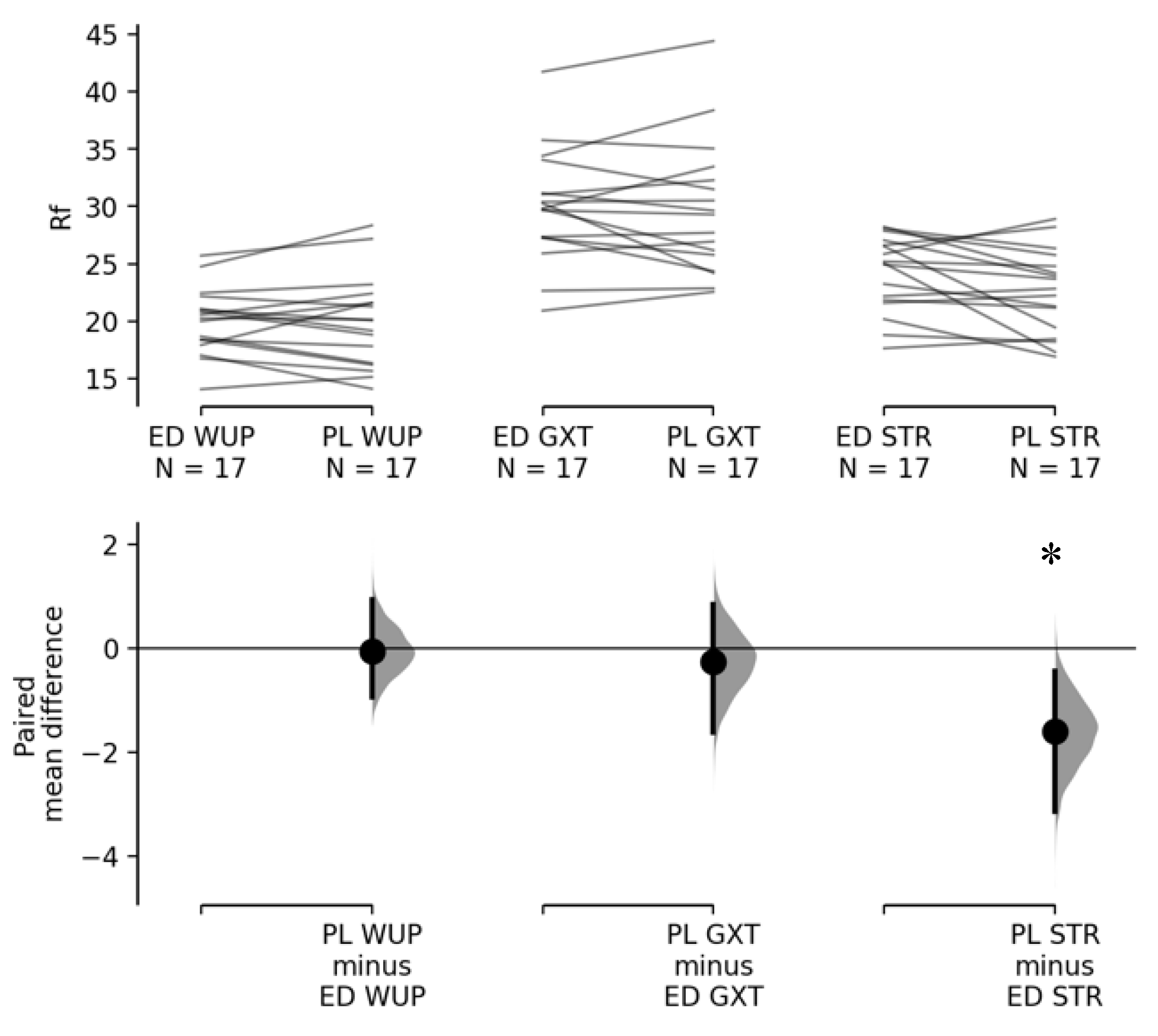
3.1. Effects of Energy Drink on HRV Parameters during Warm-Up, Graded Exercise Test, and Short-Term Rest
3.2. Effect of Energy Drink on HRVT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraak, V.I.; Davy, B.M.; Rockwell, M.S.; Kostelnik, S.; Hedrick, V.E. Policy Recommendations to Address Energy Drink Marketing and Consumption by Vulnerable Populations in the United States. J. Acad. Nutr. Diet. 2020, 120, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. Correction to: The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2425–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Taylor, L.; Willoughby, D.; Stout, J.; Graves, B.S.; et al. International society of sports nutrition position stand: Caffeine and performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.; Jarczok, M.N.; Kuhn, W.; Morsch, K.; Schäfer, A.; Hillecke, T.K.; Thayer, J.F. Impact of Caffeine on Heart Rate Variability: A Systematic Review. J. Caffeine Res. 2013, 3, 22–37. [Google Scholar] [CrossRef]
- Breda, J.J.; Whiting, S.H.; Encarnação, R.; Norberg, S.; Jones, R.; Reinap, M.; Jewell, J. Energy Drink Consumption in Europe: A Review of the Risks, Adverse Health Effects, and Policy Options to Respond. Front. Public Health 2014, 2, 134. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Maäkikallio, T.H.; Airaksinen, K.J.; Seppaänen, T.; Puukka, P.; Räihä, I.J.; Sourander, L.B. Power-Law Relationship of Heart Rate Variability as a Predictor of Mortality in the Elderly. Circulation 1998, 97, 2031–2036. [Google Scholar] [CrossRef] [Green Version]
- Tulppo, M.P.; Mäkikallio, T.H.; Seppänen, T.; Laukkanen, R.T.; Huikuri, H.V. Vagal modulation of heart rate during exercise: Effects of age and physical fitness. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H424–H429. [Google Scholar] [CrossRef]
- Albert, C.M. Triggering of Sudden Death from Cardiac Causes by Vigorous Exertion. N. Engl. J. Med. 2000, 343, 1355–1361. [Google Scholar] [CrossRef]
- Yeragani, V.K.; Krishnan, S.; Engels, H.J.; Gretebeck, R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress. Anxiety 2005, 21, 130–134. [Google Scholar] [CrossRef]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Balady, G.J.; Beasley, J.W.; Bricker, J.T.; Duvernoy, W.F.C.; Froelicher, V.F.; Mark, D.B.; Marwick, T.H.; McCallister, B.D. ACC/AHA guide-lines for exercise testing: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J. Am. Coll. Cardiol. 1997, 30, 260–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sondermeijer, H.P.; van Marle, A.G.J.; Kamen, P.; Krum, H. Acute effects of caffeine on heart rate variability. Am. J. Cardiol. 2002, 90, 906–907. [Google Scholar] [CrossRef]
- Rauh, R.; Burkert, M.; Siepmann, M.; Mueck-Weymann, M. Acute effects of caffeine on heart rate variability in habitual caffeine consumers. Clin. Physiol. Funct. Imaging 2006, 26, 163–166. [Google Scholar] [CrossRef] [PubMed]
- La Monica, M.B.; Fukuda, D.H.; Wang, R.; Gonzalez, A.M.; Wells, A.J.; Hoffman, J.R.; Stout, J.R. Maintenance of Vagal Tone with Time-Release Caffeine, But Vagal Withdrawal During Placebo in Caffeine-Habituated Men. J. Caffeine Adenosine Res. 2018, 8, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, Y.; Ikeda, T.; Takamatsu, M.; Kiso, Y.; Shibata, H.; Fushiki, T.; Moritani, T. Influence of caffeine ingestion on autonomic nervous activity during endurance exercise in humans. Eur. J. Appl. Physiol. 2002, 87, 475–480. [Google Scholar] [CrossRef]
- Benson, S.M.; Unice, K.M.; Glynn, M.E. Hourly and daily intake patterns among U.S. caffeinated beverage consumers based on the National Health and Nutrition Examination Survey (NHANES, 2013–2016). Food Chem. Toxicol. 2019, 125, 271–278. [Google Scholar] [CrossRef]
- Farag, N.H.; Vincent, A.S.; McKey, B.S.; Whitsett, T.L.; Lovallo, W.R. Hemodynamic Mechanisms Underlying the Incomplete Tolerance to Caffeine’s Pressor Effects. Am. J. Cardiol. 2005, 95, 1389–1392. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Karapetian, G.K.; Engels, H.J.; Gretebeck, R.J. Use of Heart Rate Variability to Estimate LT and VT. Int. J. Sports Med. 2008, 29, 652–657. [Google Scholar] [CrossRef]
- Michele, R.D.; Gatta, G.; Leo, A.D.; Cortesi, M.; Andina, F.; Tam, E.; Boit, M.D.; Merni, F. Estimation of the Anaerobic Threshold from Heart Rate Variability in an Incremental Swimming Test. J. Strength Cond. Res. 2012, 26, 3059–3066. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Michael, S.; Rozenberg, R.; Stokla, S.; Stam, H.J.; Praet, S.F. Heart-rate Variability Threshold as an Alternative for Spiro-ergometry Testing: A Validation Study. J. Strength Cond. Res. 2017, 31, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Smith, P.M. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis. Scand. J. Med. Sci. Sports 2005, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Engels, H.J.; Haymes, E.M. Effects of caffeine ingestion on metabolic responses to prolonged walking in sedentary males. Int. J. Sport Nutr. 1992, 2, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mcnaughton, L. Two Levels of Caffeine Ingestion on Blood Lactate and Free Fatty Acid Responses during Incremental Exercise. Res. Quart. Exerc. Sport 1987, 58, 255–259. [Google Scholar] [CrossRef]
- Karapetian, G.K.; Engels, H.J.; Gretebeck, K.A.; Gretebeck, R.J. Effect of caffeine on LT, VT and HRVT. Int. J. Sports Med. 2012, 33, 507–513. [Google Scholar] [CrossRef]
- D’Agosto, T.; Peçanha, T.; Bartels, R.; Moreira, D.N.; Silva, L.P.; Nóbrega, A.C.L.; Lima, J.R.P. Cardiac Autonomic Responses at Onset of Exercise: Effects of Aerobic Fitness. Int. J. Sports Med. 2014, 35, 879–885. [Google Scholar] [CrossRef]
- Clark, N.W.; Wells, A.J.; Coker, N.A.; Goldstein, E.R.; Herring, C.H.; Starling-Smith, T.M.; Varanoske, A.N.; Panissa, V.L.G.; Stout, J.R.; Fukuda, D.H. The acute effects of thermogenic fitness drink formulas containing 140 mg and 100 mg of caffeine on energy expenditure and fat metabolism at rest and during exercise. J. Int. Soc. Sports Nutr. 2020, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine Position Stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Landrum, R. College Students’ Use of Caffeine and Its Relationship to Personality. Coll. Stud. J. 1992, 26, 151–155. [Google Scholar]
- Quinart, S.; Mourot, L.; Nègre, V.; Simon-Rigaud, M.L.; Nicolet-Guénat, M.; Bertrand, A.M.; Meneveau, N.; Mougin, F. Ventilatory thresholds determined from HRV: Comparison of 2 methods in obese adolescents. Int. J. Sports Med. 2014, 35, 203–208. [Google Scholar] [CrossRef]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 3, 764–766. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Graham, K.S.; Davis, G.M.O. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, J.; Tuck, S.; Hughson, R.L.; Yamamoto, Y. Heart Rate Variability at Rest and Exercise: Influence of Age, Gender, and Physical Training. Can. J. Appl. Physiol. 1996, 21, 455–470. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Kliszczewicz, B.; Garner, D.M.; Cavalcante, T.C.F.; da Silva, A.A.M.; Santana, M.D.R.; Valenti, V.E. Is Caffeine Recommended Before Exercise? A Systematic Review to Investigate Its Impact on Cardiac Autonomic Control Via Heart Rate and Its Variability. J. Am. Coll. Nutr. 2019, 1–11. [Google Scholar] [CrossRef]
- Magkos, F.; Kavouras, S.A. Caffeine and Ephedrine: Physiological, Metabolic and Performance-Enhancing Effects. Sports Med. 2004, 34, 871–889. [Google Scholar] [CrossRef]
- Porto, A.A.; Valenti, V.E.; Tonon do Amaral, J.A.; Benjamim, C.J.R.; Garner, D.M.; Ferreira, C. Energy Drink before Exercise Did not Affect Autonomic Recovery Following Moderate Aerobic Exercise: A Crossover, Randomized and Controlled Trial. J. Am. Coll. Nutr. 2020, 1–7. [Google Scholar] [CrossRef]
- Nelson, M.T.; Biltz, G.R.; Dengel, D.R. Cardiovascular and ride time-to-exhaustion effects of an energy drink. J. Int. Soc. Sports Nutr. 2014, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Hill, L.K.; Siebenbrock, A. Are all measures created equal? Heart rate variability and respiration-biomed 2009. Biomed. Sci. Instrum. 2009, 45, 71–76. [Google Scholar]
- Bai, X.; Li, J.; Zhou, L.; Li, X. Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 765–774. [Google Scholar] [CrossRef]
| df | Energy Drink (ED) M ± SE | Placebo (PL) M ± SE | Within Drink ED vs. PL p-Value (Cohens-d) | Between Sex Male vs. Female p-Value (Cohens-d) | Interaction Drink x Sex p-Value | ||
|---|---|---|---|---|---|---|---|
| Warm up | LnHF | 14 | 5.65 ± 0.17 | 4.95 ± 0.17 | <0.001 * (1.731) | 0.799 | 0.781 |
| HF% | 14 | 41 ± 3 | 39 ± 3 | 0.592 | 0.111 | 0.655 | |
| LnLF | 15 | 5.91 ± 0.18 | 5.42 ± 0.18 | <0.001 * (1.098) | 0.630 | 0.688 | |
| LF% | 15 | 52 ± 33 | 54 ± 3 | 0.584 | 0.049 * (0.519) | 0.832 | |
| LF/HF | 13 | 0.24 ± 0.19 | 0.37 ± 0.19 | 0.342 | 0.116 | 0.950 | |
| LnTP | 14 | 6.60 ± 0.17 | 6.08 ± 0.17 | <0.001 * (1.500) | 0.238 | 0.229 | |
| Graded Exercise Test | LnHF | 15 | 2.55 ± 0.23 | 2.56 ± 0.23 | 0.937 | 0.149 | 0.038 * |
| HF% | 15 | 37 ± 3 | 35 ± 3 | 0.261 | 0.271 | 0.022 * | |
| LnLF | 15 | 2.97 ± 0.19 | 3.05 ± 0.19 | 0.691 | 0.010 * (0.716) | 0.258 | |
| LF% | 13 | 52 ± 3 | 52 ± 3 | 0.904 | 0.154 | 0.364 | |
| LF/HF | 15 | 0.42 ± 0.14 | 0.48 ± 0.14 | 0.532 | 0.252 | 0.049 * | |
| LnTP | 14 | 3.55 ± 0.18 | 3.55 ± 0.18 | 0.998 | 0.069 | 0.208 | |
| Short Term Rest | LnHF | 15 | 3.06 ± 0.34 | 3 ± 0.34 | 0.058 | 0.834 | 0.885 |
| HF% | 12 | 14 ± 3 | 19 ± 3 | 0.059 | 0.086 | 0.180 | |
| LnLF | 15 | 4.59 ± 0.26 | 5.07 ± 0.26 | 0.078 | 0.442 | 0.819 | |
| LF% | 15 | 70 ± 3 | 68 ± 3 | 0.534 | 0.133 | 0.659 | |
| LF/HF | 14 | 1.51 ± 0.22 | 1.34 ± 0.22 | 0.151 | 0.218 | 0.741 | |
| LnTP | 14 | 4.94 ± 0.26 | 5.42 ± 0.26 | 0.096 | 0.725 | 0.973 |
| df | Energy Drink (ED) M ± SE | Placebo (PL) M ± SE | Within Drink ED vs. PL p-Value (Cohens-d) | Between Sex Male vs. Female p-Value (Cohens-d) | Interaction Drink x Sex p-Value | ||
|---|---|---|---|---|---|---|---|
| Warm up | Mean RR (ms) | 15 | 598 ± 17 | 610 ± 17 | 0.207 | 0.254 | 0.894 |
| HRmean (bpm) | 15 | 99 ± 3 | 99 ± 3 | 0.910 | 0.526 | 0.189 | |
| HRmax (bpm) | 14 | 115 ± 3 | 109 ± 3 | 0.008 * (0.770) | 0.630 | 0.688 | |
| HRmin (bpm) | 13 | 75 ± 3 | 71 ± 3 | 0.217 | 0.798 | 0.859 | |
| LnRMSSD | 13 | 2.9 ± 0.1 | 2.7 ± 0.1 | 0.006 * (0.792) | 0.671 | 0.999 | |
| Graded Exercise Test | Mean RR (ms) | 15 | 397 ± 6 | 413 ± 6 | 0.005 * (-0.805) | 0.065 | 0.515 |
| HRmean (bpm) | 15 | 155 ± 3 | 149 ± 3 | 0.016 * (0.685) | 0.111 | 0.635 | |
| HRmax (bpm) | 14 | 190 ± 2 | 184 ± 2 | 0.014 * (0.702) | 0.235 | 0.708 | |
| HRmin (bpm) | 14 | 106 ± 3 | 100 ± 3 | 0.028 * (0.613) | 0.645 | 0.898 | |
| LnRMSSD | 14 | 1.44 ± 0.09 | 1.39 ± 0.09 | 0.451 | 0.773 | 0.079 | |
| Short Term Rest | Mean RR (ms) | 13 | 509 ± 10 | 533 ± 10 | 0.023 * (-0.666) | 0.400 | 0.915 |
| HRmean (bpm) | 15 | 118 ± 3 | 112 ± 3 | 0.021 * (0.628) | 0.566 | 0.731 | |
| HRmax (bpm) | 15 | 187 ± 3 | 180 ± 3 | 0.041 * (0.541) | 0.131 | 0.377 | |
| HRmin (bpm) | 15 | 94 ± 3 | 87 ± 3 | 0.009 * (0.728) | 0.854 | 0.523 | |
| LnRMSSD | 11 | 1.63 ± 0.13 | 1.91 ± 0.13 | 0.104 | 0.546 | 0.673 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, N.W.; Herring, C.H.; Goldstein, E.R.; Stout, J.R.; Wells, A.J.; Fukuda, D.H. Heart Rate Variability Behavior during Exercise and Short-Term Recovery Following Energy Drink Consumption in Men and Women. Nutrients 2020, 12, 2372. https://doi.org/10.3390/nu12082372
Clark NW, Herring CH, Goldstein ER, Stout JR, Wells AJ, Fukuda DH. Heart Rate Variability Behavior during Exercise and Short-Term Recovery Following Energy Drink Consumption in Men and Women. Nutrients. 2020; 12(8):2372. https://doi.org/10.3390/nu12082372
Chicago/Turabian StyleClark, Nicolas W., Chad H. Herring, Erica R. Goldstein, Jeffrey R. Stout, Adam J. Wells, and David H. Fukuda. 2020. "Heart Rate Variability Behavior during Exercise and Short-Term Recovery Following Energy Drink Consumption in Men and Women" Nutrients 12, no. 8: 2372. https://doi.org/10.3390/nu12082372






