Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives
Abstract
:1. Introduction
2. Oligosaccharides, Bacteria, and Microbial Metabolites in Human Milk
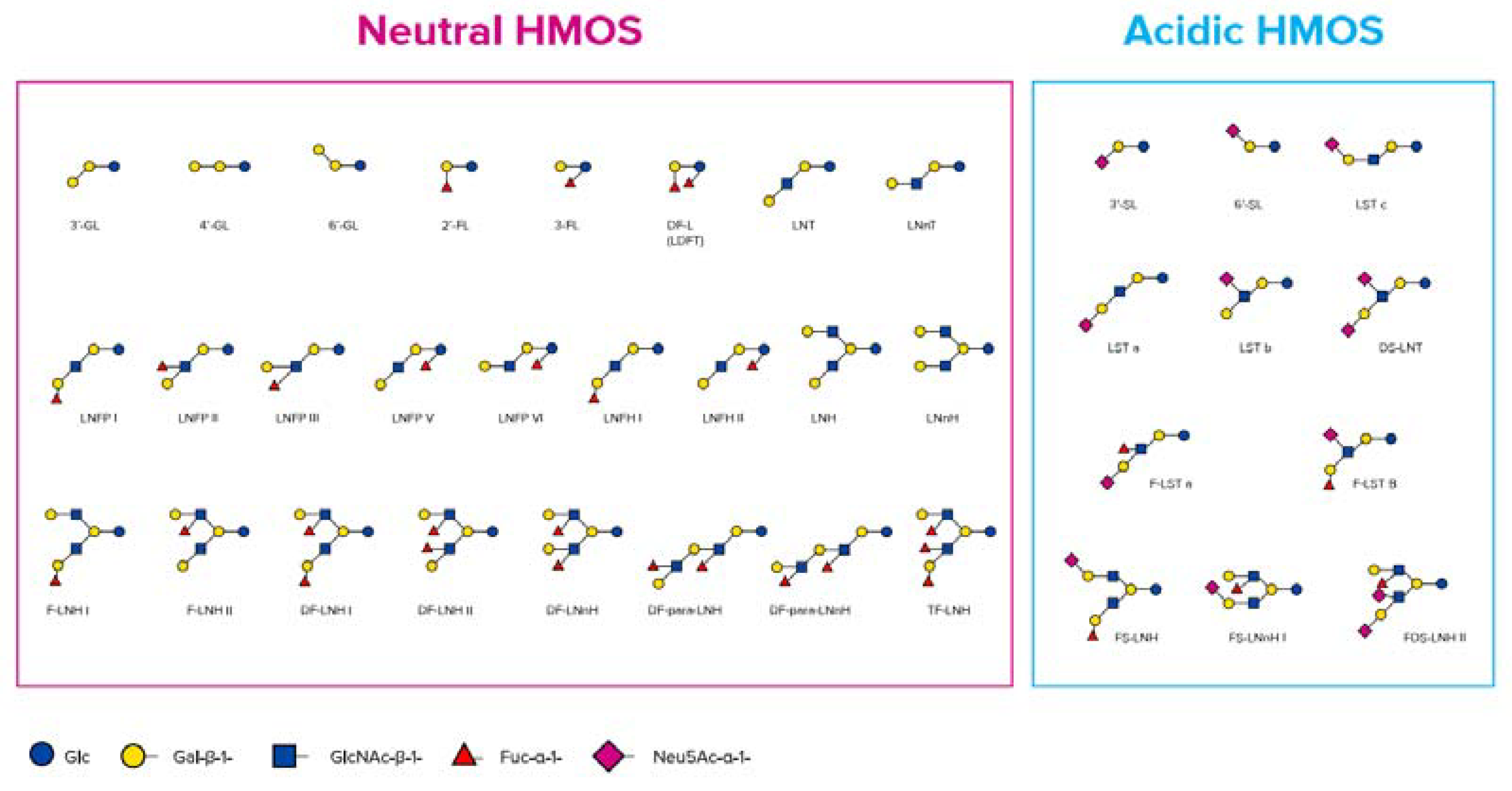
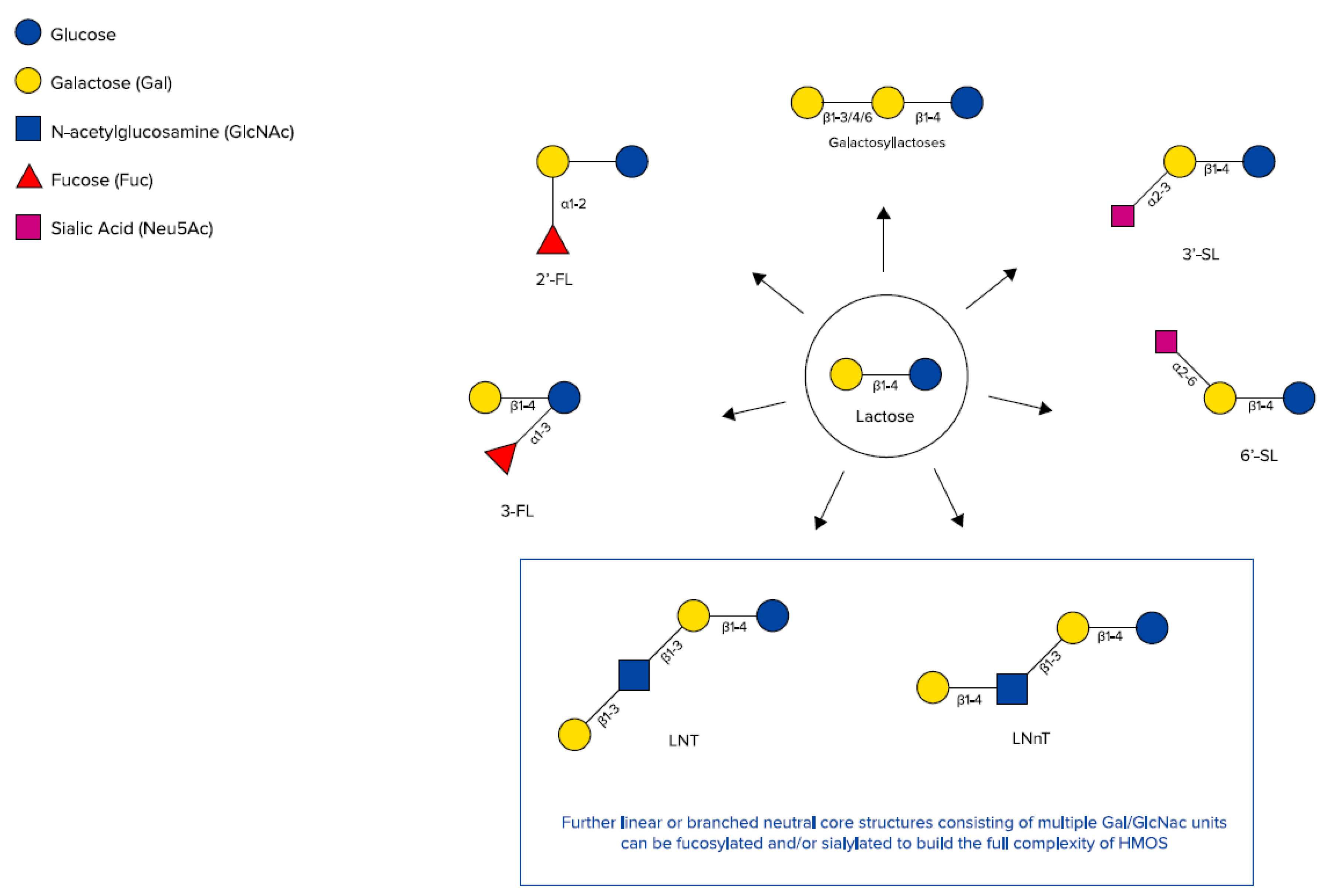
2.1. Human Milk Oligosaccharides (HMOs)
2.2. Bacteria in Human Milk
2.3. Microbial Metabolites in Human Milk
3. The ‘Biotic’ Family: Resembling Human Milk Benefits in Infant Formula
3.1. Probiotics
3.2. Prebiotics
3.3. Specific HMOs Added to and/or Present in Infant Formula
3.3.1. HMOs 2′-FL and LNnT
3.3.2. HMO 3′-GL
3.4. Synbiotics
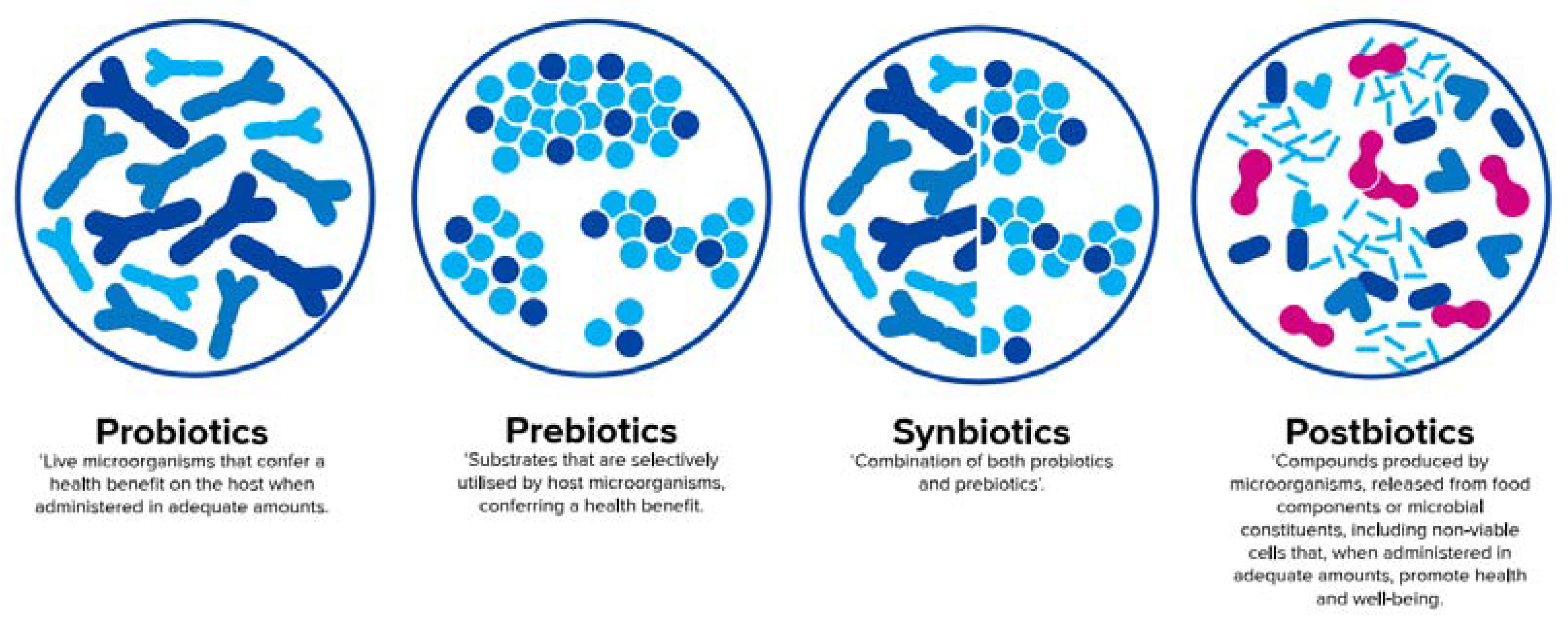
3.5. Postbiotics
3.5.1. Definition of Postbiotics
- Compounds deriving from bacterial metabolism, such as exopolysaccharides, vitamins, lactic acid, bacteriocins, enzymes, surfactants, antioxidants, and SCFAs.
- Complex molecules released from food compounds (enzymatically produced during food fermentation), such as peptides and galacto-oligosaccharides, e.g., 3′-GL and 6‘-GL.
- Components released from lysed cells including DNA, RNA, cell walls and, perhaps, other cytoplasmic components, and surface layer proteins.
3.5.2. Postbiotics through Fermentation
3.5.3. Benefits of Postbiotics in Infant Formula
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-FL | 2′-fucosyllactose |
| 3′-GL | 3′-galactosyllactose |
| 4’-GL | 4’-galactosyllactose |
| AD | atopic dermatitis |
| AE | adverse event |
| ATCC | American Type Culture Collection |
| DFL | difucosyllactose |
| DP | degree of polymerisation |
| Caco | adenocarcinoma of the colon |
| CBA | chocolate blood agar |
| CFU | colony forming units |
| CMA | cow’s milk allergy |
| DNA | deoxyribonucleic acid |
| DSM | Dutch State Mines |
| EFSA | European Food Safety Authority |
| ESPGHAN | European Society for Paediatric Gastroenterology, Hepatology and Nutrition |
| FDA | U.S. Food & Drug Administration |
| FL | fucosyllactoses |
| FOS | fructo-oligosaccharides |
| FUT2 | fucosyltransferase 2 |
| GOS | galacto-oligosaccharides |
| GL | galactosyllactose |
| GRAS | generally recognized as safe |
| HM | human milk |
| HMO | human milk oligosaccharide |
| IF | infant formula |
| IgE | immunoglobulin E |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| kDa | kilodalton |
| LA | Lactobacillus acidophilus |
| LB | lysogeny broth |
| LNnT | lacto-N-neotetraose |
| LNT | lacto-N-tetraose |
| NCDO | National Collection of Dairy Organism |
| NCIMB | National Collection of Industrial, Food and Marine Bacteria |
| NEC | necrotizing enterocolitis |
| QPS | qualitative presumption of safety |
| RNA | ribonucleic acid |
| SCORAD | scoring atopic dermatitis |
| scGOS/lcFOS (9:1) | short-chain GOS/long-chain FOS (in a ratio of 9:1) |
| SCFA | short-chain fatty acid |
| SEM | standard error of the mean |
| SL | sialyllactose |
| spp | subspecies |
References
- Thurow, R. The First 1,000 Days: A Crucial Time for Mothers and Children—And the World. Breastfeed. Med. 2016, 11, 416–418. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; A França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C. ‘Gut health’: A new objective in medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Nauta, A.J.; Ben Amor, K.; Knippels, L.M.; Knol, J.; Garssen, J. Early life: Gut microbiota and immune development in infancy. Benef. Microbes 2010, 1, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Boix-Amorós, A.; Selma-Royo, M.; Mira, A.; Collado, M.C. Gut Microbiota and Mucosal Immunity in the Neonate. Med. Sci. 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Oozeer, R.; Rescigno, M.; Ross, R.P.; Knol, J.; Blaut, M.; Khlebnikov, A.; Dore, J. Gut health: Predictive biomarkers for preventive medicine and development of functional foods. Br. J. Nutr. 2010, 103, 1539–1544. [Google Scholar] [CrossRef] [Green Version]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; A Wilder, J.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula With 2′FL Show Growth and 2′FL Uptake Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz-Hoyos, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, V.; Fenet, B.; Praly, J.-P.; Lecroix, F.; Ta, C.D. Identification and synthesis of a trisaccharide produced from lactose by transgalactosylation. Carbohydr. Res. 2000, 325, 202–210. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; Van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; Land, B.V. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, H.; Rodríguez, J.M.; Salminen, S.; Szajewska, H. Probiotics in human milk and probiotic supplementation in infant nutrition: A workshop report. Br. J. Nutr. 2014, 112, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Engfer, M.B.; Stahl, B.; Finke, B.; Sawatzki, G.; Daniel, H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr. 2000, 71, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Langa, S.; Martín, V.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The microbiota of human milk in healthy women. Cell. Mol. Biol. 2013, 59, 31–42. [Google Scholar]
- Andreas, N.J.; Kampmann, B.; Le Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef]
- Wise, A.; Robertson, B.; Choudhury, B.; Rautava, S.; Isolauri, E.; Salminen, S.; Bode, L. Infants Are Exposed to Human Milk Oligosaccharides Already in utero. Front. Pediatr. 2018, 6, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; Van Poppel, M.N.M.; Köfeler, H.C.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of Human Milk Oligosaccharides in Cord Blood and Maternal-to-Fetal Transport across the Placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Köfeler, H.C.; Csapo, B.; Desoye, G.; Bode, L.; Van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human Milk Oligosaccharides as Essential Tools for Basic and Application Studies on Galectins. Trends Glycosci. Glycotechnol. 2018, 30, SJ11–SJ24. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2014, 77, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef]
- Eiwegger, T.; Stahl, B.; Haidl, P.; Schmitt, J.; Boehm, G.; Dehlink, E.; Urbanek, R.; Szépfalusi, Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 2010, 21, 1179–1188. [Google Scholar] [CrossRef]
- Eiwegger, T.; Stahl, B.; Schmitt, J.; Boehm, G.; Gerstmayr, M.; Pichler, J.; Dehlink, E.; Loibichler, C.; Urbanek, R.; Szepfalusi, Z.; et al. Human Milk–Derived Oligosaccharides and Plant-Derived Oligosaccharides Stimulate Cytokine Production of Cord Blood T-Cells In Vitro. Pediatr. Res. 2004, 56, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Engen, P.; Leusink-Muis, T.; Van Ark, I.; Stahl, B.; A Overbeek, S.; Garssen, J.; Naqib, A.; Green, S.J.; Keshavarzian, A.; et al. The Combination of 2′-Fucosyllactose with Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides that Enhance Influenza Vaccine Responses Is Associated with Mucosal Immune Regulation in Mice. J. Nutr. 2019, 149, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Altaye, M.; Chaturvedi, P.; Meinzen-Derr, J.; Guerrero, M.D.L.; Morrow, A.L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2003, 14, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Harvey, L.; Martin, R.; Van Der Beek, E.M.; Knol, J.; Cryan, J.F.; Renes, I. Targeting the gut microbiota to influence brain development and function in early life. Neurosci. Biobehav. Rev. 2018, 95, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Akkerman, R.; Kong, C.; Walvoort, M.T.C.; De Vos, P. More than sugar in the milk: Human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk123. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobata, A. Structures and functions of the sugar chains of glycoproteins. JBIC J. Boil. Inorg. Chem. 1992, 209, 483–501. [Google Scholar] [CrossRef]
- Newburg, D.; Ko, J.S.; Leone, S.; Nanthakumar, N.N. Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6’-Galactosyllactose and Attenuate Inflammation in Human T84, NCM-460, and H4 Cells and Intestinal Tissue Ex Vivo. J. Nutr. 2015, 146, 358–367. [Google Scholar] [CrossRef]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Nagasawa, T.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; et al. Galactosyllactoses in the Milk of Japanese Women: Changes in Concentration during the Course of Lactation. J. Appl. Glycosci. 2004, 51, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Donald, A.S.; Feeney, J. Separation of human milk oligosaccharides by recycling chromatography. First isolation of lacto-N-neo-difucohexaose II and 3′-galactosyllactose from this source. Carbohydr. Res. 1988, 178, 79–91. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Haase, G.; Jelen, P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]
- Sugawara, M.; Idota, T. A new oligosaccharide: 4’-galactosyllactose in human milk. In Proceedings of the Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry, Tokyo, Japan, 3 August 1995; p. 132. [Google Scholar]
- Yamashita, K.; Kobata, A. Oligosaccharides of human milk. Isolation and characterization of a new trisaccharide, 6’-galactosyllactose. Arch. Biochem. Biophys. 1974, 174, 582–591. [Google Scholar] [CrossRef]
- Mank, M.; Blijenberg, B.; Stahl, B. Label free targeted LC-ESI-MS2 analysis of 3′- and 6’-galactosyllactose in human milk with enhanced structural selectivity. J. Pediatr. Gastroenterol. Nutr. 2019, 68, N-P-126. [Google Scholar]
- Coppa, G.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999, 88, 89–94. [Google Scholar] [CrossRef]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Affolter, M.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.-F.; Fumeaux, C.J.F. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, T.; Kitaoka, M.; Terabayashi, T.; Fukuda, K.; Ohnishi, M.; Kobata, A. Milk oligosaccharides. In Oligosaccharides: Sources, Properties and Applications; Gordon, N.S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Stahl, B.; Thurl, S.; Zeng, J.; Karas, M.; Hillenkamp, F.; Steup, M.; Sawatzki, G. Oligosaccharides from Human Milk as Revealed by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Biochem. 1994, 223, 218–226. [Google Scholar] [CrossRef]
- Finke, B.; Stahl, B.; Pfenninger, A.; Karas, M.; Daniel, H.; Sawatzki, G. Analysis of high-molecular-weight oligosaccharides from human milk by liquid chromatography and MALDI-MS. Anal. Chem. 1999, 71, 3755–3762. [Google Scholar] [CrossRef]
- Van De Wiele, T.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J. Appl. Microbiol. 2007, 102, 452–460. [Google Scholar] [CrossRef]
- Jeurink, P.; Van Bergenhenegouwen, J.; Jiménez, E.; Knippels, L.; Fernández, L.; Garssen, J.; Knol, J.; Rodríguez, J.M.; Martin, R. Human milk: A source of more life than we imagine. Benef. Microbes 2013, 4, 17–30. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Boil. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Reyman, M.; Van Houten, M.A.; Van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2014, 77, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; et al. What’s Normal? Microbiomes in Human Milk and Infant Feces Are Related to Each Other but Vary Geographically: The INSPIRE Study. Front. Nutr. 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Pärnänen, K.M.M.; Karkman, A.; Hultman, J.; Lyra, C.; Bengtsson-Palme, J.; Larsson, D.G.J.; Rautava, S.; Isolauri, E.; Salminen, S.; Kumar, H.; et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 2018, 9, 3891. [Google Scholar] [CrossRef] [Green Version]
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between Milk Microbiota, Bacterial Load, Macronutrients, and Human Cells during Lactation. Front. Microbiol. 2016, 7, 3389. [Google Scholar] [CrossRef] [Green Version]
- Heikkila, M.; Saris, P.E.J. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003, 95, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Gueimonde, M.; Laitinen, K.; Salminen, S.; Isolauri, E. Breast Milk: A Source of Bifidobacteria for Infant Gut Development and Maturation? Neonatology 2007, 92, 64–66. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Fanos, V.; Strigini, F.A.L.; Artini, P.G.; Peroni, D.G. Human Breast Milk: Exploring the Linking Ring Among Emerging Components. Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Gallego, C.; Morales, J.M.; Monleon, D.; Du Toit, E.; Kumar, H.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Isolauri, E.; Salminen, S.; et al. Human Breast Milk NMR Metabolomic Profile across Specific Geographical Locations and Its Association with the Milk Microbiota. Nutrients 2018, 10, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collado, M.; Vinderola, G.; Salminen, S. Postbiotics: Facts and open questions. A position paper on the need for a consensus definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; De Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Seppo, A.E.; Kukkonen, A.K.; Kuitunen, M.; Savilahti, E.; Yonemitsu, C.; Bode, L.; Järvinen, K.M. Association of Maternal Probiotic Supplementation With Human Milk Oligosaccharide Composition. JAMA Pediatr. 2019, 173, 286. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- De Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics, and Synbiotics. Plant Cells 2008, 111, 1–66. [Google Scholar] [CrossRef]
- Salminen, S.; Szajewska, H.; Knol, J. The Biotics Family in Early Life; John Wiley and Sons Ltd.: Chichester, UK, 2019. [Google Scholar]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Braegger, C.; Chmielewska, A.; Decsi, T.; Kolaček, S.; Mihatsch, W.; Moreno, L.; Pieścik, M.; Puntis, J.; Shamir, R.; Szajewska, H.; et al. Supplementation of Infant Formula With Probiotics and/or Prebiotics: A Systematic Review and Comment by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Akker, C.H.V.D.; Van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Canani, R.B.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the ESPGHAN Committee on Nutrition and the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef]
- Miqdady, M.; Al Mistarihi, J.; Azaz, A.; Rawat, D. Prebiotics in the Infant Microbiome: The Past, Present, and Future. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Fanaro, S.; Jelinek, J.; Stahl, B.; Marini, A. Prebiotic concept for infant nutrition. Acta Paediatr. 2003, 92, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Moro, G. Structural and Functional Aspects of Prebiotics Used in Infant Nutrition. J. Nutr. 2008, 138, 1818S–1828S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, G.; Boehm, G. Clinical Outcomes of Prebiotic Intervention Trials during Infancy: A Review. Funct. Food Rev. 2012, 4, 101–113. [Google Scholar] [CrossRef]
- European Union. Ec. Commission Delegated Regulation (EU) 2016/127. Off. J. Eur. Union 2016, 127, L25. [Google Scholar]
- Bych, K.; Mikš-Krajnik, M.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs using microbial hosts—From cell engineering to large scale production. Curr. Opin. Biotechnol. 2019, 56, 130–137. [Google Scholar] [CrossRef]
- Thurl, S.; Offermanns, J.; Müller-Werner, B.; Sawatzki, G. Determination of neutral oligosaccharide fractions from human milk by gel permeation chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1991, 568, 291–300. [Google Scholar] [CrossRef]
- Obermeier, S.; Rudloff, S.; Pohlentz, G.; Lentze, M.J.; Kunz, C. Secretion of 13 C-Labelled Oligosaccharides into Human Milk and Infant’s Urine after an Oral 13 C-Galactose Load. Isot. Environ. Health Stud. 1999, 35, 119–125. [Google Scholar] [CrossRef]
- Thurl, S.; Henker, J.; Taut, H.; Tovar, K.; Sawatzki, G. Variations of neutral oligosaccharides and lactose in human milk during the feeding. Eur. J. Nutr. 1993, 32, 262–269. [Google Scholar] [CrossRef]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [Green Version]
- Craft, K.M.; Townsend, S.D. Synthesis of lacto-N-tetraose. Carbohydr. Res. 2017, 43–50. [Google Scholar] [CrossRef]
- Fair, R.J.; Hahm, H.S.; Seeberger, P.H. Combination of automated solid-phase and enzymatic oligosaccharide synthesis provides access to α(2,3)-sialylated glycans. Chem. Commun. 2015, 51, 6183–6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-H.; Hahm, H.S.; Liang, C.-F.; Seeberger, P.H. Automated solid-phase synthesis of oligosaccharides containing sialic acids. Beilstein J. Org. Chem. 2015, 11, 617–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.; Lane, J.A.; Van Sinderen, D.; Hickey, R.M. From lab bench to formulated ingredient: Characterization, production, and commercialization of human milk oligosaccharides. J. Funct. Foods 2020, 72, 104052. [Google Scholar] [CrossRef]
- Baumgärtner, F.; Seitz, L.; Sprenger, G.A.; Albermann, C. Construction of Escherichia coli strains with chromosomally integrated expression cassettes for the synthesis of 2′-fucosyllactose. Microb. Cell Factories 2013, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, F.; Conrad, J.; Sprenger, G.A.; Albermann, C. Synthesis of the Human Milk Oligosaccharide Lacto-N-Tetraose in Metabolically Engineered, Plasmid-Free E. coli. ChemBioChem 2014, 15, 1896–1900. [Google Scholar] [CrossRef]
- Sprenger, G.A.; Baumgärtner, F.; Albermann, C. Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations. J. Biotechnol. 2017, 258, 79–91. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Książyk, J.; Lagström, H.; Sánchez-Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Safety of lacto-N-neotetraose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA J. 2015, 13, 4183. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens. Safety of lacto-N-tetraose (LNT) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05907. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens. Safety of 2′-fucosyllactose/difucosyllactose mixture as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05717. [Google Scholar] [CrossRef] [Green Version]
- FDA. U.S. Food & Drug Administration GRAS Notices. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices (accessed on 18 February 2020).
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of Consumption of Human Milk Oligosaccharides by Infant Gut-associated Bifidobacteria*. J. Boil. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.-T.; Nanthakumar, N.N.; Newburg, D. The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuniBinds Intestinal H(O) Antigen (Fucα1, 2Galβ1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Boil. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichert, S.; Jennewein, S.; Hüfner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mezoff, E.A.; Hawkins, J.A.; Ollberding, N.J.; Karns, R.; Morrow, A.L.; Helmrath, M.A. The human milk oligosaccharide 2′-fucosyllactose augments the adaptive response to extensive intestinal. Am. J. Physiol. Liver Physiol. 2015, 310, G427–G438. [Google Scholar] [CrossRef] [Green Version]
- Bienenstock, J.; Buck, R.H.; Linke, H.; Forsythe, P.; Stanisz, A.M.; Kunze, W.A. Fucosylated but Not Sialylated Milk Oligosaccharides Diminish Colon Motor Contractions. PLoS ONE 2013, 8, e76236. [Google Scholar] [CrossRef] [Green Version]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Land, B.V.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation With 2′-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Elsen, L.V.D.; Tims, S.; Jones, A.; Stewart, A.; Stahl, B.; Garssen, J.; Knol, J.; Forbes-Blom, E.; Land, B.V. Prebiotic oligosaccharides in early life alter gut microbiome development in male mice while supporting influenza vaccination responses. Benef. Microbes 2019, 10, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A. In Vitro and Clinical Experiences with a Human Milk Oligosaccharide, Lacto-NneoTetraose, and Fructooligosaccharides. Foods Food Ingred. J. Jpn. 2005, 210, 1018–1030. [Google Scholar]
- Huang, X.; Zhu, B.; Jiang, T.; Yang, C.; Qiao, W.; Hou, J.; Han, Y.; Xiao, H.; Chen, L. Improved Simple Sample Pretreatment Method for Quantitation of Major Human Milk Oligosaccharides Using Ultrahigh Pressure Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2019, 67, 12237–12244. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Leone, S.; Newburg, D. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef]
- Varasteh, S.; Van’t Land, B.; Giziakis, I.; Mank, M.; Stahl, B.; Wiertsema, S.; Folkerts, G.; Garssen, J.; Braber, S. Human Milk Oligosaccharide 3′-galactosyllactose Can Protect the Intestinal Barrier to Challenges. J. Pediatr. Gastroenterol. Nutr. 2019, 68, N-P-016. [Google Scholar]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Van De Heijning, B.J.M.; Berton, A.; Bouritius, H.; Goulet, O. GI Symptoms in Infants Are a Potential Target for Fermented Infant Milk Formulae: A Review. Nutrients 2014, 6, 3942–3967. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, M.; Luron, I.; Blat, S. Effects of infant formula composition on long-term metabolic health. J. Dev. Orig. Health Dis. 2018, 9, 573–589. [Google Scholar] [CrossRef]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J. Clin. Investig. 2000, 106, 935–937. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B.B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Goh, A.E.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J.; et al. Effect of Synbiotic on the Gut Microbiota of Cesarean Delivered Infants: A Randomized, Double-blind, Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse-Berkeveld, M.; Alles, M.; Franke-Beckmann, E.; Helm, K.; Knecht, R.; Köllges, R.; Sandner, B.; Knol, J.; Ben Amor, K.; Bufe, A. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Van Der Aa, L.B.; Heymans, H.S.; Van Aalderen, W.M.; Smitt, J.H.S.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef]
- Van Der Aa, L.B.; Van Aalderen, W.M.C.; Heymans, H.S.A.; Smitt, J.H.S.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K.; Sprikkelman, A.B.; The Synbad Study Group. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; García-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernandez-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Sanders, M.E. Defining Emerging ‘Biotics’. Available online: https://isappscience.org/defining-emerging-biotics/ (accessed on 23 March 2020).
- Agostoni, C.; Goulet, O.; Kolacek, S.; Koletzko, B.; Moreno, L.; Puntis, J.; Rigo, J.; Shamir, R.; Szajewska, H.; Turck, D. Fermented Infant Formulae Without Live Bacteria. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 392–397. [Google Scholar] [CrossRef]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O. Partly Fermented Infant Formulae With Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e43–e53. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Ludwig, T.; Bouritius, H.; Alliet, P.; Forde, D.; Peeters, S.; Huet, F.; Hourihane, J. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 2017, 106, 1150–1158. [Google Scholar] [CrossRef]
- Mischke, M.; Vincent, A.; Duchene, B.; Knol, J.; Van Seuningen, I.; Renes, I. Combination of Specific Pre-and Postbiotics in Infant Formula Induces Gut Barrier Maturation Closer to Mother’s Milk and Supports Gut Functionality in Mice. J. Pediatr. Gastroenterol. Nutr. 2019, 68, N-O-011. [Google Scholar]
- Sureda, E.A.; Gidlund, C.; Weström, B.; Prykhodko, O. Induction of precocious intestinal maturation in T-cell deficient athymic neonatal rats. World J. Gastroenterol. 2017, 23, 7531–7540. [Google Scholar] [CrossRef]
- Brunser, O.; Araya, M.; Espinoza, J.; Guesry, P.R.; Secretin, M.C.; Pacheco, I. Effect of an Acidified Milk on Diarrhoea and the Carrier State in Infants of Low Socio-Economic Stratum. Acta Paediatr. 1989, 78, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, P.A.; Oozeer, R.; Martín, R.; Ben Amor, K.; Knol, J. The Early Settlers: Intestinal Microbiology in Early Life. Annu. Rev. Food Sci. Technol. 2012, 3, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.N.M.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.-F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.-B.; Mulli, C. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of Long-term Consumption of a Fermented Infant Formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on Acute Diarrhea in Healthy Infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a Fermented Formula on Thymus Size and Stool pH in Healthy Term Infants. Pediatr. Res. 2007, 62, 98–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.-J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.-A.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2010, 65, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tims, S.; Roeselers, G.; Knol, K. Gut Microbiota Composition Modulation by Partly Fermented Infant Formulae Supplemented with Prebiotics, scGOS/lcFOS. J. Pediatr. Gastroenterol. Nutr. 2018, 66, N-eP-029. [Google Scholar]

| HMO | Technology | Application | Ref |
|---|---|---|---|
| 2′-FL and LNnT | Isolation from human milk | For structural identification and fundamental research applications only | [40,82,83,84] |
| Chemo-enzymatic synthesis using recombinantly expressed glycosyltransferases matched with nucleotide-activated donor substrates and acceptors | For generating libraries of asymmetrical multi-antennary HMOs for research purposes only | [85] | |
| Chemical synthesis from L-fucose, D-galactose, N-acetyl-D-glucosamine, and D-lactose, respectively | Prohibitively expensive for large-scale nutrition applications due to complexity, number of reaction steps, limited availability, and high cost of raw materials | [86,87,88,89] | |
| Coupling of the bacterial homogenates of two or more recombinant microbial cells overexpressing genes for HMO synthesis | For the industrial manufacturing of LNnT and fucosylated oligosaccharides | [90,91] | |
| Microbial fermentation of engineered E. coli strains for fucosylation reaction (complete removal of the strain and all other non-desired biomolecules after fermentation) | Used for both research and viable commercial production with high titers of 2′-FL and LNnT | [92] | |
| 3′-GL | Milk fermentation process using Bifidobacterium breve C50 and Streptococcus thermophilus 065 and providing 3′-GL as a metabolic by-product at levels of ~250 µg/mL | Proprietary fermentation process (LactofidusTM) for large-scale production of fermented infant formula | [12] |
| Infants | Duration (and Start) of Diet | Diet (No. of Infants) | Impact (Fermented vs. Standard Formula Group) | Ref |
|---|---|---|---|---|
| Impact on immune parameters | ||||
| Healthy infants | 4 m (from birth) | Fermented (11) vs. standard formula (9) | Higher faecal IgA response to polio vaccine | [132] |
| Healthy infants | 5 m (from 4–6 m of age) | Fermented (464) vs. standard formula (449) | Reduced severity of acute diarrhoea (no effect on incidence and duration of diarrhoea episodes and number of hospital admissions) | [133] |
| Healthy infants | 4 m (from birth) | Fermented (30) vs. standard formula (30); HM (30) | Enhanced thymus size (fermented formula group closer to HM) | [134] |
| Preterm infants (GA < 35 w) | 2–5 w (from birth) | Fermented (21) vs. standard formula (31) | Lower faecal calprotectin and higher secretory IgA (no effect on TNF-α) | [135] |
| Infants at high risk of atopy | 12 m (from birth) | Fermented (66) vs. standard formula (63) | Less positive SPT to cow’s milk (no effect on CMA incidence) and lower incidence of digestive and respiratory potentially allergic AEs | [136] |
| Impact on gut parameters (fermented formula 1 additionally contained prebiotic scGOS/lcFOS (9:1)) | ||||
| Healthy infants | From 0–28 d until 17 w of life | Fermented (77) vs. standard formula (86); HM (90) | Softer stool consistency (fermented formula group closer to HM) No differences regarding parent-reported GI (and related) symptoms and investigator-reported AEs (except lower incidence of infantile colic) | [12] |
| Subset of 30 infants per study arm | Stools: lower pH, higher levels of acetic acid and sIgA, increased Bifidobacterium sp, and decreased Clostridium difficile occurrence (gut microbiota composition of fermented formula group closer to HM) | [137] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. https://doi.org/10.3390/nu12071952
Salminen S, Stahl B, Vinderola G, Szajewska H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients. 2020; 12(7):1952. https://doi.org/10.3390/nu12071952
Chicago/Turabian StyleSalminen, Seppo, Bernd Stahl, Gabriel Vinderola, and Hania Szajewska. 2020. "Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives" Nutrients 12, no. 7: 1952. https://doi.org/10.3390/nu12071952





