Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge
Abstract
1. Introduction
2. Current Status of Knowledge
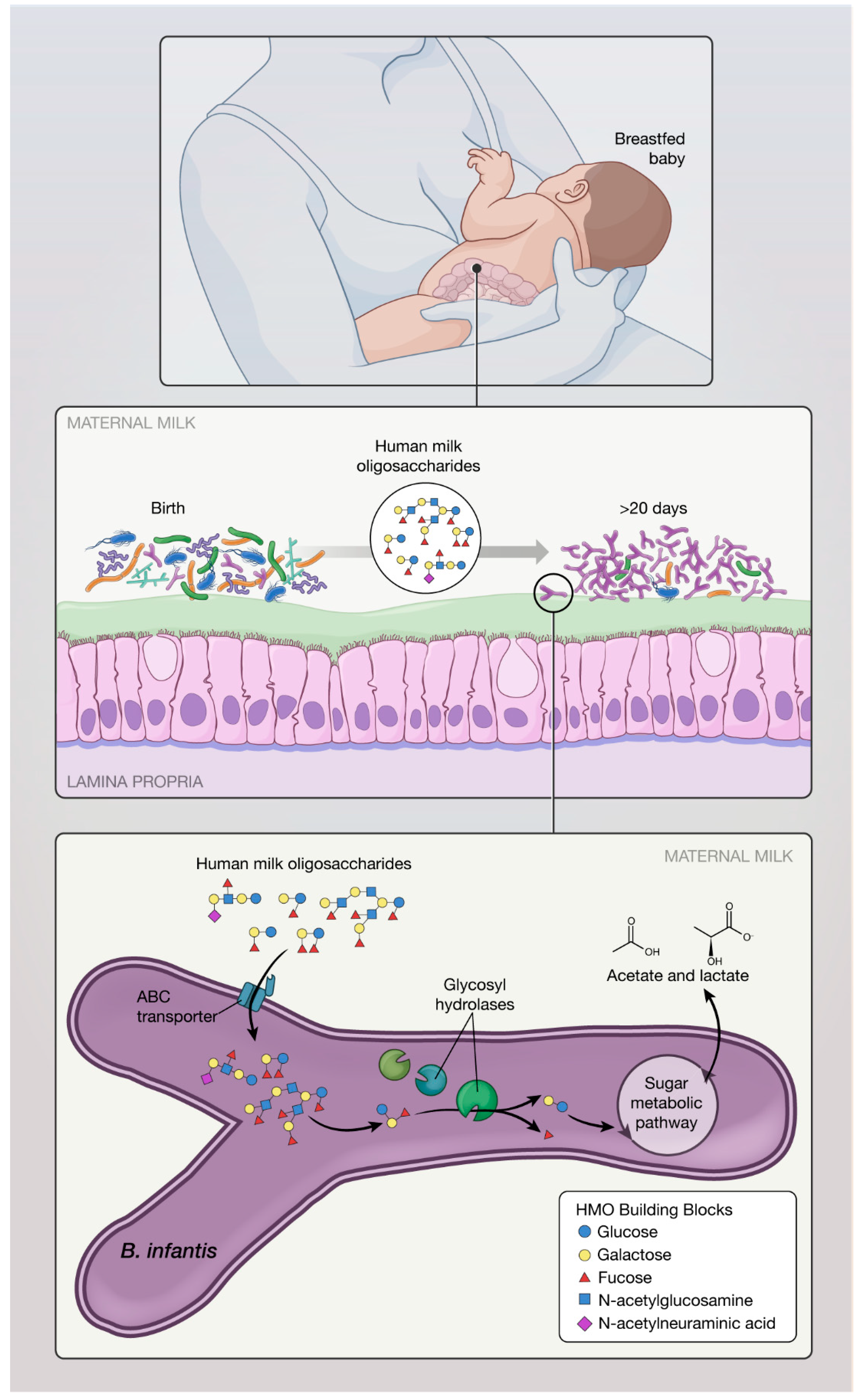
2.1. Carbohydrate Metabolism and Short Chain Fatty Acids (SCFA) Production
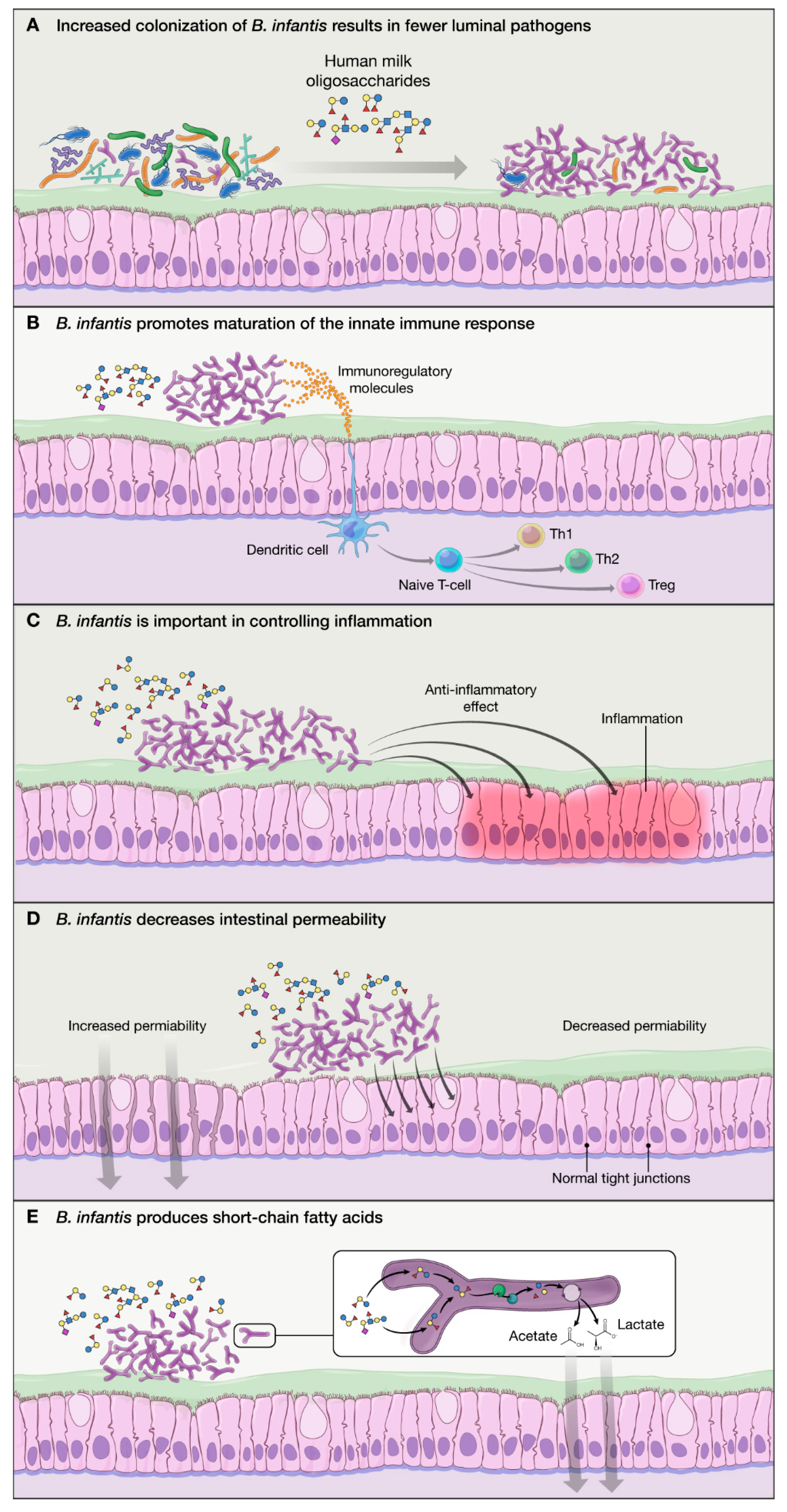
2.2. Proposed Mechanisms of Action of B. infantis Based on In Vitro Studies:
- B. infantis improves the intestinal barrier integrity through the production of tryptophan metabolite, indole-3-lactic acid [35].
2.3. Clinical Evidence: Safety and Efficacy of Select Commercialized Strains of B. infantis Used in Pediatric Populations
2.3.1. B. infantis M63
2.3.2. B. infantis ATCC 15697
2.3.3. B. infantis UCD272
2.3.4. B. infantis EVC001
2.3.5. B. infantis CECT 7210
2.3.6. B. infantis BB02
2.3.7. B. infantis R0033
2.3.8. B. infantis BT1
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, I.; Schofield, Z.; Hall, L.J. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg. Top. Life Sci. 2017, 1, 333–349. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of caco-2 monolayers and in a rat NEC model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Makino, H.; Martin, R.; Ishikawa, E.; Gawad, A.; Kubota, H.; Sakai, T.; Oishi, K.; Tanaka, R.; Ben-Amor, K.; Knol, J.; et al. Multilocus sequence typing of bifidobacterial strains from infant’s faeces and human milk: Are bifidobacteria being sustainably shared during breastfeeding? Benef. Microbes 2015, 6, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Lugli, G.A.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef]
- Chen, J.; Cai, W.; Feng, Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin. Nutr. 2007, 26, 559–566. [Google Scholar] [CrossRef]
- Wampach, L.; Heintz-Buschart, A.; Hogan, A.; Muller, E.E.L.; Narayanasamy, S.; Laczny, C.C.; Hugerth, L.W.; Bindl, L.; Bottu, J.; Andersson, A.F.; et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front. Microbiol. 2017, 8, 738. [Google Scholar] [CrossRef]
- Grönlund, M.M.; Lehtonen, O.P.; Eerola, E.; Kero, P. Fecal microflora in healthy infants born by different methods of delivery: Permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 19–25. [Google Scholar] [CrossRef]
- Ly, N.P.; Litonjua, A.; Gold, D.R.; Celedón, J.C. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 2011, 127, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Hutton, A.A.; Palumbo, M.C.; Casaburi, G.; Mitchell, R.D.; Underwood, M.A.; Smilowitz, J.T.; Frese, S.A. Elevated fecal ph indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Ann. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.E.; Ninonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- LoCascio, R.G.; Niñonuevo, M.R.; Kronewitter, S.R.; Freeman, S.L.; German, J.B.; Lebrilla, C.B.; Mills, D.A. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol. 2009, 2, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Garrido, D.; Lerno, L.; Wu, S.; Tan, K.; Eom, H.J.; Joachimiak, A.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl. Environ. Microbiol. 2012, 78, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Li, Y.; Lerno, L.; Wu, S.; Marcobal, A.M.; German, J.B.; Chen, X.; Lebrilla, C.B.; Mills, D.A. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 2011, 286, 11909–11918. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- LoCascio, R.G.; Desai, P.; Sela, D.A.; Weimer, B.; Mills, D.A. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl. Environ. Microbiol. 2010, 76, 7373–7381. [Google Scholar] [CrossRef]
- Ninonuevo, M.R.; Perkins, P.D.; Francis, J.; Lamotte, L.M.; LoCascio, R.G.; Freeman, S.L.; Mills, D.A.; German, J.B.; Grimm, R.; Lebrilla, C.B. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem. 2008, 56, 618–626. [Google Scholar] [CrossRef]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Underwood, M.A.; Arriola, J.; Gerber, C.W.; Kaveti, A.; Kalanetra, K.M.; Kananurak, A.; Bevins, C.L.; Mills, D.A.; Dvorak, B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: Alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014, 76, 326. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2007, 91, 915–923. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, L.; Puyal, J.; Chatton, J.Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 2013, 8, e71721. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.R.; Liu, S.X.L.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am. J. Pathol. 2013, 182, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M.; et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere 2017, 2. [Google Scholar] [CrossRef]
- EFSA. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the Scientific Committee. EFSA J. 2007, 5, 587. [Google Scholar] [CrossRef]
- Dupont, C.; Rivero, M.; Grillon, C.; Belaroussi, N.; Kalindjian, A.; Marin, V. α-Lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: Growth and gastrointestinal tolerance. Eur. J. Clin. Nutr. 2010, 64, 765. [Google Scholar] [CrossRef]
- Rozé, J.C.; Barbarot, S.; Butel, M.J.; Kapel, N.; Waligora-Dupriet, A.J.; De Montgolfier, I.; Leblanc, M.; Godon, N.; Soulaines, P.; Darmaun, D.; et al. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: A multicentre, double-blind, randomised trial. Br. J. Nutr. 2011, 107, 1616–1622. [Google Scholar] [CrossRef]
- Russo, M.; Giugliano, F.P.; Quitadamo, P.; Mancusi, V.; Miele, E.; Staiano, A. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Ital. J. Pediatr. 2017, 43, 24. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, E.; Maglione, M.; Alessandrella, A.; Strisciuglio, C.; De Giovanni, D.; Campanozzi, A.; Miele, E.; Staiano, A. A Mixture of 3 Bifidobacteria Decreases Abdominal Pain and Improves the Quality of Life in Children With Irritable Bowel Syndrome: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Clin. Gastroenterol. 2017, 51, e5–e10. [Google Scholar] [CrossRef] [PubMed]
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration. Anaerobe 2013, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Kalanetra, K.M.; Bokulich, N.A.; Lewis, Z.T.; Mirmiran, M.; Tancredi, D.J.; Mills, D.A. A comparison of two probiotic strains of bifidobacteria in premature infants. J. Pediatr. 2013, 163, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.T.; Borghese, R.A.; Kalanetra, K.M.; Mirmiran, M.; Mills, D.A.; Underwood, M.A. Probiotic administration in infants with gastroschisis: A pilot randomized placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 852–857. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Moya, J.; Breck, M.A.; Cook, C.; Fineberg, A.; Angkustsiri, K.; Underwood, M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017, 17, 133. [Google Scholar] [CrossRef]
- Casaburi, G.; Vance, D.; Duar, R.; Frese, S.; Smilowitz, J.; Underwood, M. Targeted probiotic supplementation reduces antibiotic resistance gene carriage in breastfed infants. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 874. [Google Scholar]
- Karav, S.; Casaburi, G.; Frese, S.A. Reduced colonic mucin degradation in breastfed infants colonized by Bifidobacterium longum subsp. infantis EVC001. FEBS Open Bio 2018, 8, 1649–1657. [Google Scholar] [CrossRef]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef]
- Escribano, J.; Ferré, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoñer, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018, 83, 1120. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.L.; Bulach, D.M.; Murray, G.L.; Jacobs, S.E.; Tabrizi, S.N.; Garland, S.M.; ProPrems Study, G. Gut microbiota of preterm infants supplemented with probiotics: Sub-study of the ProPrems trial. BMC Microbiol. 2018, 18, 184. [Google Scholar] [CrossRef]
- Manzano, S.; Andrés, J.D.; Castro, I.; Rodríguez, J.M.; Jiménez, E.; Espinosa-Martos, I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef. Microbes 2017, 8, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.D.; Manzano, S.; García, C.; Rodríguez, J.M.; Espinosa-Martos, I.; Jiménez, E. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef. Microbes 2018, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Pham-Thi, N.; Kerihuel, J.C.; Durand, H.; Bohbot, S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: A randomized, double-blind, placebo-controlled pilot study. Ther. Adv. Respir. Dis. 2010, 4, 271–278. [Google Scholar] [CrossRef]
- Bazanella, M.; Maier, T.V.; Clavel, T.; Lagkouvardos, I.; Lucio, M.; Maldonado-Gòmez, M.X.; Autran, C.; Walter, J.; Bode, L.; Schmitt-Kopplin, P.; et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 2017, 106, 1274–1286. [Google Scholar] [CrossRef]
- Wessel, M.A.; Cobb, J.C.; Jackson, E.B.; Harris, G.S.; Detwiler, A.C. Paroxysmal fussing in infancy, sometimes called “colic”. Pediatrics 1954, 14, 421–435. [Google Scholar]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nature Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef]
| B. infantis Strain (Manufacturer) | Trial ID | Site | Enrollment | Feeding Period | Study Design/ Study Groups | Study Outcomes | Conclusions | Year |
|---|---|---|---|---|---|---|---|---|
| M63 (Morinaga) | n/a | France | 66 infants | 1 month | Term infants identified with colic a at enrollment (3 w to 3 months) multicenter double-blind randomized controlled trial (DBRCT)
|
|
| 2010 [38] |
| ClinicalTrials.gov NCT00920166 | France | 97 infants | 6 months | Term infants (<postnatal day [PND] 3 at enrollment. Multicenter DBRCT
Stool samples collected at 1 and 6 months. | Primary
|
| 2011 [39] | |
| n/a | Italy | 55 children (4–12 years old [yo]) with functional constipation. | 8 weeks | Prospective, placebo-controlled, randomized trial
|
|
| 2017 [40] | |
| ClinicalTrials.gov NCT02807064 | Italy | 40 children (9 yo) with allergic rhinitis and asthma. | 4 weeks | Prospective, placebo-controlled, randomized trial
| Primary
|
| 2017 [41] | |
| ClinicalTrials.gov NCT02566876 | Italy | 73 children (8–16 yo) with abdominal pain (AP)-associated functional GI disorders (FGID). | 6 weeks | Prospective, placebo-controlled, randomized trial
| Primary
|
| 2017 [42] | |
| not known | Japan | 44 infants (low-birthweight). | 6 weeks |
|
|
| 2013 [43] | |
| ATCC 15697 | ClinicalTrials.gov NCT00810160 | US | 12 premature infants. | 5 weeks |
|
|
| 2013 [44] |
| ClinicalTrials.gov NCT01316510 | US | 24 infants with gastroschisis. | 6 weeks (or hospital discharge) |
| Primary
|
| 2016 [45] | |
| UCD272 (Culture Systems Inc) | ClinicalTrials.gov NCT02086110 (listed as SC268) | US | 11 children (2–11 yo) with ASD | 12 weeks | DBRC
| Primary
|
| 2019 [46] |
| EVC001 (Evolve Biosystems) | ClinicalTrials.gov NCT02457338 | US | 80 mother/ infant dyads | 21 days | Randomized, parallel assignment
stool samples collected through PND 60. |
|
| 2017 [47] |
| In infants who received EVC001:
| 2017 [36] | ||||||
|
| 2018 [48] | ||||||
|
| 2018 [49] | ||||||
|
| 2019 [50] | ||||||
| CECT 7210 (IM-1®, Ordesa S.L.) | Clinicaltrials.gov NCT02096302 | Spain | 151 term infants | 12 weeks | Multicenter DBRCT
| Primary
| In CECT 7210 group:
| 2018 [51] |
| BB-02 (Chr. Hansen) | Australia and New Zealand Clinical Trials Register, ACTRN012607000144415 | Australia/New Zealand | 1099 preterm infants | Through hospital discharge or term corrected age | Multicenter DBRCT
| Primary
| Incidence of NEC was significantly reduced in infants receiving the probiotic combination but not definite late-onset sepsis or mortality. | 2013 [52] |
| Higher levels of Bifidobacterium spp. found in infants who received the probiotics; Enterococcus reduced in infants receiving the probiotic mix during the supplementation period | 2018 [53] | ||||||
| R0033 (Lallemand) | Clinicaltrials.gov NCT02215304 | Spain | 221 infants | 8 weeks | Multicenter DBRCT
| Primary
| Use of R0033 was safe and well tolerated. No impact on growth (weight, height, and head circumference), adverse events, or serious adverse events. Increased ratio of IL-10/IL-12 and significant reduction in Collinsella, Enterococcus, and Klebsiella genera in infants receiving R0033. | 2017 [54] 2018 [55] |
| 135 children (3–7 yo) | 3 months | Multicenter DBRCT
| Percentage of children free of any episodes of ear, nose and throat, respiratory tract, or gastrointestinal illness | Synbiotic preparation decreased the risk of occurrence of common infectious diseases. No side effects were detected in either group. | 2010 [56] | |||
| BT1 | germanctr.de (DRKS00003660) | Germany | 106 infants | 12 months | Double-blind, randomized, placebo-controlled study
| Composition of the fecal microbiota (16s rRNA sequencing) and fecal metabolome (HPLC). | Probiotic formula modulated the infant stool microbiome (e.g., Bacteroides) and metabolome (e.g., lipids) at very early stages of life, with no detectable long-term consequences. | 2017 [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chichlowski, M.; Shah, N.; Wampler, J.L.; Wu, S.S.; Vanderhoof, J.A. Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients 2020, 12, 1581. https://doi.org/10.3390/nu12061581
Chichlowski M, Shah N, Wampler JL, Wu SS, Vanderhoof JA. Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients. 2020; 12(6):1581. https://doi.org/10.3390/nu12061581
Chicago/Turabian StyleChichlowski, Maciej, Neil Shah, Jennifer L. Wampler, Steven S. Wu, and Jon A. Vanderhoof. 2020. "Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge" Nutrients 12, no. 6: 1581. https://doi.org/10.3390/nu12061581
APA StyleChichlowski, M., Shah, N., Wampler, J. L., Wu, S. S., & Vanderhoof, J. A. (2020). Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients, 12(6), 1581. https://doi.org/10.3390/nu12061581





