Prevalence of Zinc Deficiency in Japanese Patients on Peritoneal Dialysis: Comparative Study in Patients on Hemodialysis
Abstract
:1. Introduction
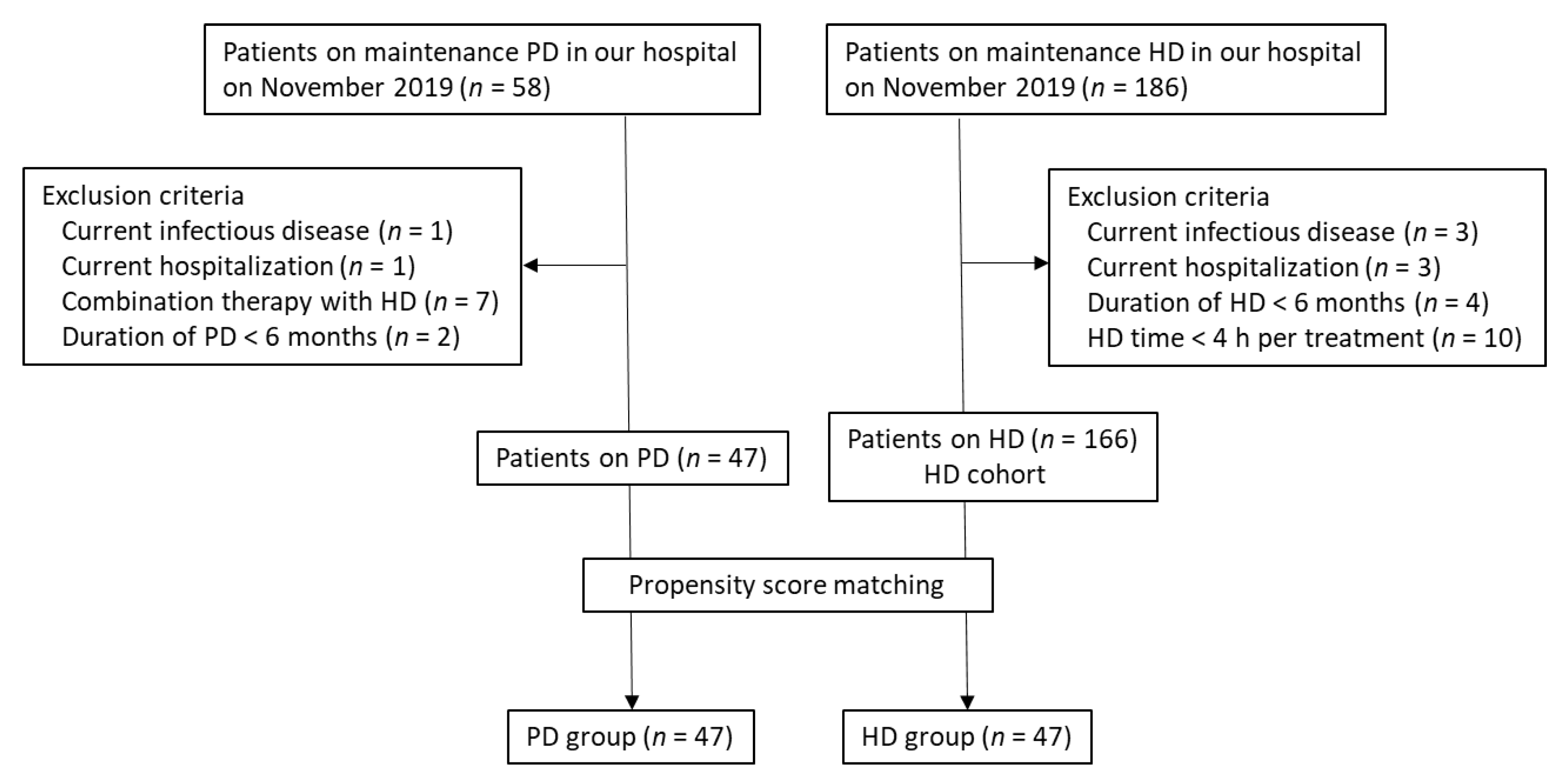
2. Subjects and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J.; The Alberta Kidney Disease Network. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Zinc in growth and development and spectrum of human zinc deficiency. J. Am. Coll. Nutr. 1988, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68 (Suppl. 2), 447S–463S. [Google Scholar] [CrossRef] [Green Version]
- Allon, M. Dialysis catheter-related bacteremia: Treatment and prophylaxis. Am. J. Kidney. Dis. 2004, 44, 779–791. [Google Scholar] [CrossRef]
- Mokrzycki, M.H.; Zhang, M.; Cohen, H.; Golestaneh, L.; Laut, J.M.; Rosenberg, S.O. Tunnelled haemodialysis catheter bacteraemia: Risk factors for bacteraemia recurrence, infectious complications and mortality. Nephrol. Dial. Transplant. 2006, 21, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Ishani, A.; Collins, A.J.; Herzog, C.A.; Foley, R.N. Septicemia, access and cardiovascular disease in dialysis patients: The USRDS Wave 2 study. Kidney. Int. 2005, 68, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Markovits, P.M.; Sankey, A.W.; James, D.K.; McCabe, R.; Mahomed, K.; Golding, J. Zinc taste test and postnatal depression. Br. J. Psychiatry 1990, 156, 451–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, R.M.; Requejo, A.M.; Andres, P.; Lopez-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Abe, M.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Zumkley, H.; Bertram, H.P.; Lison, A.; Knoll, O.; Losse, H. Aluminum, zinc and copper concentration in plasma in chronic renal insufficiency. Clin. Nephrol. 1979, 12, 18–21. [Google Scholar] [PubMed]
- Hosokawa, S.; Yoshida, O. Effect of erythropoietin on trace elements in patients with chronic renal failure undergoing hemodialysis. Nephron 1993, 65, 414–417. [Google Scholar] [CrossRef]
- Yonova, D.; Vazelov, E.; Tzatchev, K. Zinc status in patients with chronic renal failure on conservative and peritoneal dialysis treatment. Hippokratia 2012, 16, 356–359. [Google Scholar]
- Martín-del-Campo, F.; Batis-Ruvalcaba, C.; González-Espinoza, L.; Rojas-Campos, E.; Angel, J.R.; Ruiz, N.; González, J.; Pazarín, L.; Cueto-Manzano, A.M. Dietary micronutrient intake in peritoneal dialysis patients: Relationship with nutrition and inflammation status. Perit. Dial. Int. 2012, 32, 183–191. [Google Scholar] [CrossRef] [Green Version]
- The Japanese Society of Clinical Nutrition. Available online: http://www.jscn.gr.jp/pdf/aen20190423.pdf (accessed on 11 January 2020).
- BMJ Best Practice 2018, Zinc Deficiency. Available online: https://bestpractice.bmj.com/topics/en-gb/1195 (accessed on 31 January 2020).
- Sriam, K.; Lonchyna, V.A. Micronutrient supplementation in adult nutrition therapy: Practical considerations. JPEN J. Parenter. Enteral. Nutr. 2009, 33, 548–562. [Google Scholar] [CrossRef] [Green Version]
- Working Group Committee for Preparation of Guidelines for Peritoneal Dialysis; Japanese Society for Dialysis Therapy. 2009 Japanese Society for Dialysis Therapy guidelines for peritoneal dialysis. Ther. Apher. Dial. 2010, 14, 489–504. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawanishi, H.; Suzuki, K.; Nakai, S.; Tsuchida, K.; Tabei, K.; Akiba, T.; Masakane, I.; Takemoto, Y.; Tomo, T.; et al. Japanese society for dialysis therapy clinical guideline for “Maintenance hemodialysis: Hemodialysis prescriptions”. Ther. Apher. Dial. 2015, 19 (Suppl. 1), 67–92. [Google Scholar] [CrossRef]
- Bentley, P.J.; Grubb, B.R. Experimental dietary hyperzincemia tissue disposition of excess zinc in rabbits. Trace. Elem. Med. 1991, 8, 202–207. [Google Scholar]
- Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; Henkin, R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986, 251, R398–R408. [Google Scholar] [CrossRef] [PubMed]
- Al-Timimi, D.J.; Sulieman, D.M.; Hussen, K.R. Zinc status in type 2 diabetic patients: Relation to the progression of diabetic nephropathy. J. Clin. Diagn. Res. 2014, 8, CC04–CC08. [Google Scholar] [CrossRef]
- Makhlough, A.; Makhlough, M.; Shokrzadeh, M.; Mohammadian, M.; Sedighi, O.; Faghihan, M. Comparing the levels of trace elements in patients with diabetic nephropathy and healthy individuals. Nephrourol. Mon. 2015, 7, e28576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.A.; Al Jameil, N.; Arjumand, S.; Khan, M.F.; Tabassum, H.; Alenzi, N.; Hijazy, S.; Alenzi, S.; Subaie, S.; Fatima, S. Comparative study of serum copper, iron, magnesium, and zinc in type 2 diabetes-associated proteinuria. Bio. Trace Elem. Res. 2015, 168, 321–329. [Google Scholar] [CrossRef]
- Kiziltas, H.; Ekin, S.; Erkoc, R. Trace element status of chronic renal patients undergoing hemodialysis. Biol. Trace Elem. Res. 2008, 124, 103–109. [Google Scholar] [CrossRef]
- Salame, C.; Eaton, S.; Grimble, G.; Davenport, A. Protein losses and urea nitrogen underestimate total nitrogen losses in peritoneal dialysis and hemodialysis patients. J. Ren. Nutr. 2018, 28, 317–323. [Google Scholar] [CrossRef]
- Shimizu, S.; Takashima, H.; Tei, R.; Furukawa, T.; Okamura, M.; Kitai, M.; Nagura, C.; Maruyama, T.; Higuchi, T.; Abe, M. Prevalence of carnitine deficiency and decreased carnitine levels in patients on peritoneal dialysis. Nutrients 2019, 11, 2645. [Google Scholar] [CrossRef] [Green Version]
- Panorchan, K.; Davenport, A. Incidence and predictors of zinc deficiency in stable peritoneal dialysis patients. Perit. Dial. Int. 2015, 35, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Wallaeys, B.; Cornelis, R.; Mees, L.; Lameire, N. Trace elements in serum, packed cells, and dialysate of CAPD patients. Kidney Int. 1986, 30, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Beerbower, K.S.; Raess, B.U. Erythrocyte, plasma, urine and dialysate zinc levels in patients on continuous ambulatory peritoneal dialysis. Am. J. Clin. Nutr. 1985, 41, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar] [PubMed]
- Abu-Hamdan, D.K.; Mahajan, S.K.; Migdal, S.D.; Prasad, A.S.; McDonald, F.D. Zinc tolerance test in uremia: Effect of ferrous sulfate and aluminum hydroxide. Ann. Intern. Med. 1986, 104, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Kogirima, M.; Kurasawa, R.; Kubori, S.; Sarukura, N.; Nakamori, M.; Okada, S.; Kamioka, H.; Yamamoto, S. Ratio of low serum zinc levels in elderly Japanese people living in the central part of Japan. Eur. J. Clin. Nutr. 2007, 61, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogeveen, E.K.; Halbesma, N.; Rothman, K.J.; Stijnen, T.; van Dijk, S.; Dekker, F.W.; Boeschoten, E.W.; de Mutsert, R.; Netherlands Cooperative Study on the Adequacy of Dialysis-2 (NECOSAD) Study Group. Obesity and mortality risk among younger dialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Kato, A.; Masakane, I.; Kanno, Y. A new nutritional risk index for predicting mortality in hemodialysis patients: Nationwide cohort study. PLoS ONE 2019, 14, e0214524. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.N.; Ibrahim, S.A.; El-Mashad, G.M.; Sabry, J.H.; Sherbini, N.S. Effect of zinc supplementation on body mass index and serum levels of zinc and leptin in pediatric hemodialysis patients. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 159–163. [Google Scholar]
- Abdollahi, S.; Toupchian, O.; Jayedi, A.; Meyre, D.; Tam, V.; Soltani, S. Zinc supplementation and body weight: A systematic review and dose–response meta-analysis of randomized controlled trials. Adv. Nutr. 2019. [Google Scholar] [CrossRef]
- Argani, H.; Mahdavi, R.; Ghorbani-haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol. 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Jern, N.A.; VanBeber, A.D.; Gorman, M.A.; Weber, C.G.; Liepa, G.U.; Cochran, C.C. The effects of zinc supplementation on serum zinc concentration and protein catabolic rate in hemodialysis patients. J. Ren. Nutr. 2000, 10, 148–153. [Google Scholar] [CrossRef]
- Chevalier, C.A.; Liepa, G.; Murphy, M.D.; Suneson, J.; Vanbeber, A.D.; Gorman, M.A.; Cochran, C. The effects of zinc supplementation on serum zinc and cholesterol concentrations in hemodialysis patients. J. Ren. Nutr. 2002, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kasarskis, E.J.; Schuna, A. Serum alkaline phosphatase after treatment of zinc deficiency in humans. Am. J. Clin. Nutr. 1980, 33, 2609–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, R.T.; Hamed, A.I.; Sallam, M.T. Effect of zinc supplementation on growth hormone insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012, 38, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Wu, M.L.; Chou, Y.Y.; Li, S.Y.; Deng, J.F.; Yang, W.C.; Ng, Y.Y. Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin. Nutr. 2012, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]

| Variable | PD Group | HD Group | p-Value |
|---|---|---|---|
| Patients, n (male %) | 47 (72.3) | 47 (70.2) | 0.819 † |
| Age, years | 61.3 ± 14.6 | 62.3 ± 13.9 | 0.724 |
| Duration of dialysis (months) | 22 (9–46) | 19 (9–45) | 0.893 |
| Comorbid CVD, n (%) | 5 (10.6) | 8 (17.0) | 0.368 * |
| Smoking, n (%) | 3 (6.4) | 5 (10.6) | 0.457 * |
| Alcohol use n (%) | 5 (10.6) | 8 (17.0) | 0.370 * |
| Body mass index, kg/m2 | 23.7 ± 4.0 | 22.4 ± 3.6 | 0.107 |
| Cause of ESKD, n (%) | |||
| Diabetic nephropathy | 14 (29.8) | 15 (31.9) | 0.823 † |
| Chronic glomerular nephritis | 8 (17.0) | 12 (25.6) | 0.313 † |
| Hypertension | 23 (48.9) | 19 (40.4) | 0.406 † |
| Other | 2 (4.3) | 1 (2.1) | 0.557 * |
| Anuria, n (%) | 14 (29.8) | 22 (46.8) | 0.089 † |
| CAPD, n (%) | 22 (46.8) | ||
| APD, n (%) | 25 (53.2) | - | |
| PD treatment time, h/day | 16.9 ± 4.6 | - | |
| PD fluid volume, L/day | 8.0 ± 2.8 | - | |
| PD solution bag exchanges,/day | 4.3 ± 1.4 | - | |
| Systolic BP, mmHg | 144 ± 19 | 144 ± 13 | 0.951 |
| Diastolic BP, mmHg | 82 ± 10 | 81 ± 9 | 0.784 |
| Heart rate, (bpm) | 75 ± 11 | 74 ± 9 | 0.816 |
| Medications, n (%) | |||
| RAS inhibitor | 40 (85.1) | 39 (83.0) | 0.778 † |
| Statin | 22 (46.8) | 20 (42.6) | 0.678 † |
| VDRA | 33 (70.2) | 38 (80.9) | 0.230 † |
| Phosphate binder | 41 (87.2) | 45 (95.7) | 0.139 † |
| Calcium carbonate | 19 (40.4) | 18 (38.3) | 0.832 † |
| Lanthanum carbonate | 20 (42.5) | 23 (48.9) | 0.534 † |
| Sevelamer hydrochloride | 1 (2.1) | 3 (6.4) | 0.306 * |
| Ferric citrate | 11 (23.4) | 9 (19.1) | 0.614 † |
| Sucroferric oxyhydroxide | 6 (12.8) | 8 (17.0) | 0.773 † |
| Variable | CAPD | APD |
|---|---|---|
| n (%) | 22 (46.8) | 25 (53.2) |
| PD treatment time, h/day | 15 (13.5–20) | 18 (14–21) |
| During the night for APD, h/day | - | 8 (7–8) |
| During the day for APD, h/day | - | 10 (6.5–12.8) |
| PD fluid volume, L/day | 6.4 ± 1.7 | 9.3 ± 2.9 |
| PD solution bag exchanges/day | 3.5 (3–4) | 5 (4–6) |
| 1.5% glucose dialysate, L/day | 3 (2–4.6) | 5.6 ± 3.4 |
| 2.5% glucose dialysate, L/day | 1.5 (0–2) | 2 (0–4) |
| Icodextrin, L/day | 2 (1.5–2) | 2 (1.5–2) |
| Variable | PD Group | HD Group | P-Value |
|---|---|---|---|
| Serum urea nitrogen, mg/dL | 54 ± 15 | 56 ± 12 | 0.432 |
| Creatinine, mg/dL | 10.2 ± 3.8 | 9.9 ± 3.0 | 0.630 |
| Total protein, g/dL | 6.3 ± 0.7 | 6.6 ± 0.5 | 0.012 |
| Albumin, g/dL | 3.3 ± 0.5 | 3.6 ± 0.5 | 0.008 |
| Sodium, mEq/L | 137 ± 3.5 | 140 ± 2.8 | <0.0001 |
| Potassium, mEq/L | 4.3 ± 0.6 | 4.8 ± 0.6 | 0.0003 |
| Corrected calcium, mg/dL | 9.1 ± 0.5 | 9.4 ± 0.7 | 0.057 |
| Phosphate, mg/dL | 5.6 ± 1.4 | 5.2 ± 1.2 | 0.106 |
| Alkaline phosphatase, U/L | 266 (217–394) | 232 (191–320) | 0.020 |
| Intact PTH, pg/mL | 230 (119–282) | 162 (83–207) | 0.009 |
| Total cholesterol, mg/dL | 177 ± 39 | 156 ± 32 | 0.004 |
| HDL cholesterol, mg/dL | 49 ± 36 | 38 ± 15 | 0.035 |
| Triglycerides, mg/dL | 94 (71–138) | 98 (73–155) | 0.549 |
| Postprandial plasma glucose, mg/dL | 138 ± 30 | 140 ± 28 | 0.689 |
| C-reactive protein, mg/dL | 0.11 (0.1–0.6) | 0.17 (0.05–0.54) | 0.378 |
| Hemoglobin, g/dL | 11.0 ± 1.2 | 10.7 ± 0.8 | 0.195 |
| Iron, μg/dL | 82 ± 34 | 82 ± 30 | 0.979 |
| Transferrin saturation (%) | 33 ± 14 | 35 ± 14 | 0.519 |
| Ferritin, ng/mL | 112 (37–174) | 82 (50–147) | 0.898 |
| ESA, μg/m | 120 (75–180) | 100 (80–160) | 0.354 |
| β2-microglobulin, mg/L | 26 ± 11 | 24 ± 7 | 0.260 |
| Renal Kt/V | 0.13 (0.05–0.91) | - | - |
| PD Kt/V | 1.13 ± 0.4 | - | - |
| Total Kt/V | 1.67 ± 0.7 | - | - |
| spKt/V | - | 1.33 ± 0.2 | - |
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-Value | Estimate | SE | 95% CI | p-Value | ||
| Age | −0.36 | 0.07 | <0.0001 | −0.19 | 0.06 | −0.335 | −0.063 | 0.005 |
| Female sex | 0.20 | 2.97 | 0.945 | |||||
| Duration of dialysis | −0.01 | 0.05 | 0.862 | |||||
| Body mass index | 1.48 | 0.22 | <0.0001 | 0.84 | 0.23 | 0.364 | 1.331 | 0.001 |
| Urine volume | 1.56 | 1.63 | 0.341 | |||||
| Serum urea nitrogen | 0.02 | 0.08 | 0.815 | |||||
| Creatinine | 0.06 | 0.34 | 0.841 | |||||
| Hemoglobin | −0.001 | 1.08 | 0.998 | |||||
| Albumin | 4.78 | 1.63 | 0.004 | 4.69 | 1.79 | 1.065 | 8.326 | 0.012 |
| Sodium | −0.12 | 0.38 | 0.750 | |||||
| Calcium | −2.10 | 2.52 | 0.409 | |||||
| Phosphate | 0.13 | 0.94 | 0.888 | |||||
| Alkaline phosphatase | −0.01 | 0.01 | 0.241 | |||||
| Plasma glucose | −0.06 | 0.04 | 0.713 | |||||
| C-reactive protein | −1.19 | 4.27 | 0.781 | |||||
| Ferritin | 0.01 | 0.01 | 0.348 | |||||
| HDL-cholesterol | 0.09 | 0.08 | 0.266 | |||||
| β2-microglobulin | −0.22 | 0.11 | 0.055 | −0.15 | 0.07 | −0.304 | 5.890 | 0.050 |
| Total Kt/V | 1.14 | 1.97 | 0.565 | |||||
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-Value | Estimate | SE | 95% CI | p-Value | ||
| Age | −0.19 | 0.07 | 0.010 | −0.02 | 0.05 | −0.138 | 0.102 | 0.760 |
| Female sex | 4.44 | 2.31 | 0.060 | |||||
| Duration of dialysis | −0.16 | 0.03 | <0.0001 | −0.09 | 0.03 | −0.167 | −0.031 | 0.005 |
| Body mass index | 1.20 | 0.21 | <0.0001 | 0.79 | 0.22 | 0.346 | 1.239 | 0.0009 |
| Urine volume | −0.88 | 2.81 | 0.754 | |||||
| Serum urea nitrogen | 0.01 | 0.09 | 0.928 | |||||
| Creatinine | −0.25 | 0.36 | 0.484 | |||||
| Hemoglobin | −1.46 | 1.26 | 0.253 | |||||
| Albumin | 7.59 | 2.02 | 0.0005 | 3.52 | 1.88 | −0.282 | 7.336 | 0.068 |
| Sodium | −0.35 | 0.38 | 0.360 | |||||
| Calcium | 0.13 | 1.41 | 0.922 | |||||
| Phosphate | −0.53 | 0.93 | 0.571 | |||||
| Alkaline phosphatase | −0.001 | 0.01 | 0.935 | |||||
| Plasma glucose | 0.02 | 0.04 | 0.684 | |||||
| C-reactive protein | −3.17 | 2.04 | 0.127 | |||||
| Ferritin | −0.001 | 0.01 | 0.913 | |||||
| HDL-cholesterol | −0.07 | 0.06 | 0.291 | |||||
| β2-microglobulin | −0.18 | 0.14 | 0.187 | |||||
| spKt/V | 10.8 | 5.32 | 0.047 | 9.59 | 3.70 | 2.118 | 17.06 | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, S.; Tei, R.; Okamura, M.; Takao, N.; Nakamura, Y.; Oguma, H.; Maruyama, T.; Takashima, H.; Abe, M. Prevalence of Zinc Deficiency in Japanese Patients on Peritoneal Dialysis: Comparative Study in Patients on Hemodialysis. Nutrients 2020, 12, 764. https://doi.org/10.3390/nu12030764
Shimizu S, Tei R, Okamura M, Takao N, Nakamura Y, Oguma H, Maruyama T, Takashima H, Abe M. Prevalence of Zinc Deficiency in Japanese Patients on Peritoneal Dialysis: Comparative Study in Patients on Hemodialysis. Nutrients. 2020; 12(3):764. https://doi.org/10.3390/nu12030764
Chicago/Turabian StyleShimizu, Satoshi, Ritsukou Tei, Masahiro Okamura, Nobuteru Takao, Yoshihiro Nakamura, Hidetaka Oguma, Takashi Maruyama, Hiroyuki Takashima, and Masanori Abe. 2020. "Prevalence of Zinc Deficiency in Japanese Patients on Peritoneal Dialysis: Comparative Study in Patients on Hemodialysis" Nutrients 12, no. 3: 764. https://doi.org/10.3390/nu12030764





