Abstract
Background: Alzheimer’s disease (AD), the most threatening neurodegenerative disease, is characterized by the loss of memory and language function, an unbalanced perception of space, and other cognitive and physical manifestations. The pathology of AD is characterized by neuronal loss and the extensive distribution of senile plaques and neurofibrillary tangles (NFTs). The role of environment and the diet in AD is being actively studied, and nutrition is one of the main factors playing a prominent role in the prevention of neurodegenerative diseases. In this context, the relationship between dementia and wine use/abuse has received increased research interest, with varying and often conflicting results. Scope and Approach: With this review, we aimed to critically summarize the main relevant studies to clarify the relationship between wine drinking and AD, as well as how frequency and/or amount of drinking may influence the effects. Key Findings and Conclusions: Overall, based on the interpretation of various studies, no definitive results highlight if light to moderate alcohol drinking is detrimental to cognition and dementia, or if alcohol intake could reduce risk of developing AD.
1. Introduction
Throughout history, wine has often been used as a tool to manage health, and has been prescribed as medicine to treat symptoms or to promote health by preventing the most common ailments. In Mesopotamia, wine was used in various treatments and therapies, and was mixed with honey as a common therapy for cough treatment. The Egyptians used wine as a solvent, a remedy for weakness and injuries, or as an appetizer. The ancient Greek physician Hippocrates considered wine to be a key nutrient of a healthy diet and prescribed its use as a sedative and antiseptic [1]. Although many beneficial and negative effects of alcohol on health have been highlighted, controversies remain about the properties wine components and their cellular and molecular effects. The effect of wine on health is dose-dependent and the border between the quantity causing problems and the quantity that could be beneficial to health is low.
Drinking alcohol is a behavior that is increasing in popularity, especially among young people. Many people consume alcohol conscientiously and without health consequences, but the number of those who harmfully drink alcohol is growing. Many different kinds of alcoholic drinks exist with different alcohol contents. Although alcohol is legal and widely available, drinks with higher concentrations of alcohol risk causing health problems more quickly and in smaller doses.
Wine contains the products of the must or juice of the fruit of the species Vitis vinifera fermented by yeast. After fermentation, wine contains a complex mixture of different compounds affecting its quality. In alcoholic wine, phenolic compounds, polysaccharides, acids, volatile compounds, and water are present in varying concentrations. The principal polyphenols are flavanols, flavonols, anthocyanins, and resveratrol; flavonoids include catechin, epicatechin, proanthocyanidins, flavones, and anthocyanins [2]; and up to 60% of total phenolic compounds are represented by catechin and epicatechin, producing an antioxidant activity related to the inhibition of nuclear factor kappa-B (NF-κB) and inflammatory cytokines. Myricetin, kaempferol, rutin, and quercetin are flavonols, and quercetin provides ability to induce the activity of antioxidant enzymes such as heme oxygenase, glutathione S-transferase, and thioredoxin reductase to inhibit NF-κB translocation to the nucleus and to reduce the expression of toll-like receptors (TLR2 and TLR4), thus creating its anti-inflammatory activity [3]. Delphinidin-3-glucoside, cyanidin-3-glucoside, and malvidin-3-glucoside are the most commonly found anthocyanins in wines, with antioxidant and anti-inflammatory activities, along with resveratrol, which is able to induce or repress inflammatory cytokines, such as tumor necrosis factor-(TNF)-α, interleukin- (IL)-1β, and IL-6 modulating the inflammatory response, and inhibiting NF-κB and inflammatory enzymes, such as the inducible isoforms of nitric oxide synthase (iNOS) and cyclooxygenase-1 (COX-1) [4]. Tannin is present in the skins and seeds of grapes, which is another subgroup of phenols responsible for the quality of wine, contributing to color, bitterness, astringency, and structure of wine. Wine polyphenols, by activation of antioxidant and anti-inflammatory mechanisms, possess beneficial and therapeutic properties for prevention of neurodegeneration, cancer, metabolic disorders, and aging, as demonstrated by preclinical and in vitro studies. Polyphenols also play an important role in the treatment of pathogen infection, hypertension, and cardiovascular diseases [5,6,7,8]. The most abundant sugars present in grapes are glucose and fructose, whereas sucrose is only present in trace amounts. Ethanol and carbon dioxide are produced through the breakdown of sugars by yeast in the process of fermentation. The volatility of aromatic compounds is related to sugar concentrations. Sweetness, which is influenced by ethanol, acids, and tannins, is detected at levels higher than 1% (w/v) of overall sugars. Organic acids, such as tartaric, malic, and to a lesser extent, citric acids, are the most abundant solids present in wine and are responsible for the taste, wine stability, color, and pH. Sun et al. identified 2-O-feruloyl tartaric acid as potential phosphodiesterase 4D inhibitor (PDE4D). PDE4D alters the function of calcium channels in the central nervous system and is considered one of the causes of Alzheimer’s disease (AD) [9]. Low concentrations of proteins in wines are responsible for the clarity and stability of wines in association with factors of non-protein origin, such as polyphenols, pH, and polysaccharides. Nitrogenous compounds include ammonium cations and amino acids, peptides, and proteins are nutrients for yeast and lactic acid bacteria. The most important mineral compounds in the wine are potassium, sulfate, phosphates, sodium, iron, and chloride [10,11,12]. The composition of wine is summarized in Figure 1, and, with any natural biological material, the components may vary significantly.
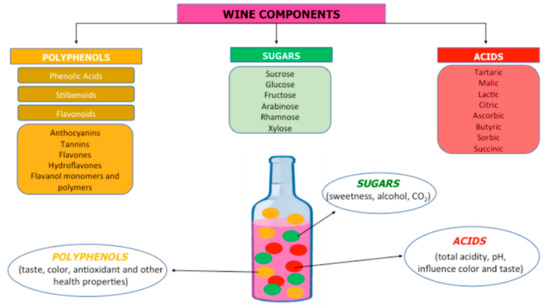
Figure 1.
The several components of the wine.
Consumption of wine is one component of the Mediterranean diet, and is increasingly associated with the promotion of human health and the prevention of diseases mainly associated with mental and heart health. However, possible health benefits may only exist with moderate drinking, i.e., “Up to one drink per day for women and up to two drinks per day for men and only for adults of legal drinking age” as reported by Dietary Guidelines of the United States (2015) [13]. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines moderate drinking as up to four alcoholic drinks for men and three for women in any single day and a maximum of 14 drinks for men and seven drinks for women per week. Moderate wine drinking is linked to higher blood levels of omega-3 fatty acids that protect against heart disease, metabolize glucose, and decrease cardiometabolic risk; increased levels of heme-oxygenase and prevention of blood clotting may protect the brain from stroke damage [14,15,16,17]. The risk of developing dementia and depression was suggested to be reduced by moderate wine drinking [18,19].
In the elderly, wine is a commonly consumed alcoholic beverage, especially during meals, whereas other alcoholic drinks are only drunk occasionally. Research has indicated that the positive effects of red wine on health are based on the presence of antioxidants that attack free radicals via different mechanisms. The antioxidant potential of red wine has been highlighted by the results of the French paradox, which describes how, in the French population, despite a relatively high dietary intake of saturated fats, the incidence of cardiovascular disease is relatively low, probably linked to the consumption of red wine [17]. The French paradox may have its basis in an environment containing various important molecules, and the advantages can be mainly linked to the joint, cumulative, or synergistic effects of alcohol with other components in wine.
Researchers are working to discover the relationship between the bioavailability of >200 phenolic compounds present in wine and their molecular and nutritional properties, environmental, social, or family factors that influence wine consumption, and the effects of alcohol on health. Although the benefits of polyphenols in fruit and vegetables are increasingly accepted, how wine, and in particular red wine with its abundant content of phenolic acids and polyphenols, provides further health benefits has not yet been fully clarified [20,21].
Many of the disorders due to alcohol intake are related to large amounts of consumed alcohol. About 10% of the population drinks 75% of the alcohol consumed in the United States [22]. Moderate wine drinking may have some health benefits; excessive alcohol consumption increases the risk of several cancers, heart disease, other chronic diseases, and mental health problems. Chronic alcohol consumption results in persistent changes in the brain, driving the reduction of behavioral control and difficulty avoiding negative consequences. Alcohol has been examined as a risk factor of dementia, and a relationship between alcohol consumption and dementia has been evidenced. Although chronic alcohol abuse results in significant activation of neurodegenerative processes [23] and the prevalence of alcohol-related dementia represents about 10% of all cases of dementia [24], whether light-to-moderate drinking has any health benefits remains to be determined [25]. The relationship between the amount of alcohol intake and cognitive outcomes is complicated by differing definitions of high levels of alcohol consumption, by the duration of abuse, the age at which alcohol consumption begins, sex, and alternation of binge and withdrawal periods.
Since a link between alcohol misuse and AD has been described, and alcohol dependence is a significant and independent predictor of AD [26], this review was designed to examine the association between wine intake and AD.
2. Neurodegeneration and Alzheimer’s Disease
Neurodegenerative diseases are age-dependent disorders with increasing prevalence, partly due to increased life length [27]. Neurodegenerative diseases are a group of diseases in which the loss of neuronal cells in certain areas of the brain is irreversible and progressive, related to loss of movement cognitive and behavioral function. The main neurodegenerative diseases include Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease, joined by some pathogenic and clinical characteristics. Dementia is responsible for the main burden of neurodegenerative diseases, and Alzheimer’s represents approximately 60–70% of dementia cases. According to the World Alzheimer’s Report 2016, 46.8 million people in 2015 were affected by dementia, and an increase of up to 131.5 million is expected by 2050. Based on the neuropsychiatric assessment of clinical features and memory deficits, diagnosing only probable AD is possible; certain diagnosis is only possible post-mortem with the examination of cerebral degeneration [28].
Several factors, including genetic and environmental factors, affect neurodegenerative diseases onset. Risk of AD development is associated with factors such as apolipoprotein-E (APO-E), diabetes mellitus, aging, smoking habits, and lower socio-economic status. Decreased risk of AD onset appears to be related with physically and cognitively stimulating activities, adherence to the Mediterranean diet, light-to-moderate alcohol consumption, and high level of education [29]. In the brain, the build-up of toxic proteins and a loss of mitochondrial function are the events that are precursors to progressive neuronal damage. Oxidative stress, linked to direct neuroinflammation, metal accumulation, and mitochondrial dysfunction, plays a crucial role in neurodegeneration [30,31]. The cascade of pathological events linked to neurodegeneration is characterized by a high production of free radicals that act on nucleic acids, proteins, and fats, as well as on glycosylation of DNA and proteins, inducing apoptosis and necrosis. Several proteins that undergo structural and functional changes responsible for degradation and misfolding, such as β-amyloid (Aβ) in Alzheimer’s disease and α-synuclein in Parkinson’s disease [32], may be present in small quantities in elderly subjects. Oxidative stress elicits lipid peroxidation, and derived products react with protein to form intracellular precipitates; oxidative stress also induces activation of glial cells that release pro-inflammatory cytokines strengthening the neurodegeneration.
AD, a progressive and neurodegenerative disease, affects more than 5% of the population over the age of 65 years. The neuropathology of AD is characterized by the presence intraneuronal deposits of neurofibrillary tangles, senile plaques around reactive microglia, deposits of amyloid β (Aβ), and progressive loss of neurons and brain functions [33]. Neuronal death may be responsible for memory failure, personality changes, and other manifestations. Brain inflammation is another hallmark of AD [34], being present from pre-clinical to terminal stages of the disease, as indicated by activated microglia and reactive astrocytes that edge plaques and secrete cytokine, thus increasing the neuro-inflammatory response [35]. The central role of inflammation in AD has been highlighted by many studies, confirming that AD pathogenesis is not restricted to the neuronal compartment, but also involves the central and peripheral immune systems. High levels of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, transforming growth factor (TGF)-β, and IL-18, have been found in the brain, cerebrospinal fluid, and peripheral blood, suggesting that AD may be associated with a more widespread inflammatory state [36,37,38].
Since the late 1990s, accumulation of Aβ and the deposition of neurofibrillary tangles have been considered the main causes of AD [39,40]. More recently, alternative explanations of the pathogenesis of AD have emerged, such as oxidative damage increasing production of reactive oxygen species (ROS) [41], loss of mitochondrial function [42], altered metal homeostasis, and reduced antioxidant defense [43], which could influence the production and accumulation of Aβ and hyperphosphorylated Tau protein, driving a vicious cycle that could worsen mitochondrial dysfunction and ROS production. Acetylcholinesterase (AChE) expression is substantially altered and its activity increases within and around the Aβ plaques but decreases in most brain regions of AD patients. Many studies are being performed to examine the relationship between obesity and neurodegenerative diseases and one of the major risk factors for dementia is metabolic syndrome and abdominal obesity, correlated with deregulation of adipokines [44,45].
AD is evidently a complex multifactorial syndrome and a general agreement exists that factors like smoking, physical exercise, lifestyle, diet, and education may play a central role in the AD.
3. Intake of Alcoholic Beverages: Risk or Protection of Alzheimer’s Disease?
Albeit through different mechanisms, both no consumption or excessive consumption of alcohol are both associated with an increased risk of dementia [46]. Deng et al. identified the relationship between drinking and risk of dementia, proving that elevated risk of cognitive impairment and high consumption of alcohol are related, and reduced risk of dementia is related to light-to-moderate alcohol drinking [47].
Xu et al. reported a J-shaped relationship between alcohol intake and cognitive decline in patients with mild cognitive impairment, and showed that high consumption of alcoholic beverages as well as complete abstention increase the risk of dementia, and that light–moderate alcohol drinking may be associated with a decreased risk of dementia [48]. Many studies clarified if alcohol may be a risk or protective factor of developing AD, but the evidence is far from conclusive and the associations between abstinence, moderate, or heavy drinking and risk of dementia remain unclear.
In general, the literature examining the impact of alcohol on AD shows several limitations such as the validity of measurement of alcohol consumption, period of time (day, month), consumption (continuous or variable), and the cognitive assessments of drinkers. Environmental, socio-economic, and lifestyles factors, together with unknown causes of AD, can contribute to the uncertainty of the role of wine intake in AD. Cumulative evidence reveals that intermittent ethanol intake during adolescence enhances the weakness of the brain to both ethanol-induced neural cell death and cognitive impairments. Studies on human alcoholic brain found a correlation between the rate and amount of lifetime alcohol consumption and whole brain damage and reduction in the number of neurons. Most studies did not distinguish amongst wine, beer, or spirits, and studies that did distinguish reported no difference among the effects of these different types of alcohol. Thus, only further and in-depth studies will clarify the intricate relationship between alcohol consumption and the onset of AD and clarify the risks or benefits involved in alcohol consumption, and if and how diet and ingestion of wine in particular may help slow the cognitive decline and protect against AD regardless of other risk factors.
3.1. Moderate Alcohol Consumption
Evidence exists that moderate consumption of wine positively affects organs and systems. Using rodent models, cardiac myocytes, and endothelial cells, the results of several studies indicated that moderate alcohol intake can support anti-inflammatory processes involving adenosine receptors, protein kinase C (PKC), and nitric oxide synthase, which could drive cardioprotection. Collis et al. reported that the modifications to alcohol-related anti-inflammatory heat shock protein and protein kinase in the brain have similarities with those observed in the heart [49], highlighting that dementia and cardiovascular disease share several common risk factors. These observations have encouraged studies on the association of alcohol intake with dementia. An inverse correlation between cardiovascular risk and moderate alcohol intake has been highlighted, which is nullified when consumption is high. Several systemic reviews were conducted to answer the question “Is alcohol consumption a protective factor against AD?” Taken together, the literature does not provide adequate evidence for a conclusive answer. A study reported that light-to-moderate/regular drinking have a protective effect against AD [47], whereas other studies reported a protective effect of moderate-to-high levels of drinking, but many variables, such as age and sex, ethnicity, measures of alcohol, clinical evaluation, and use of standardized cognitive assessments, do not allow drawing adequate conclusions [50,51,52,53,54,55]. Zuccalà et al. showed that cognitive dysfunction is proportionally associated with high or moderate alcohol consumption and sex [56]. A longitudinal study examined the association between alcohol consumption and dementia and showed that middle-aged non-drinkers have a greater risk of developing dementia than those who drink moderate amounts, with more pronounced effects particularly evident in people who drink wine. Cohort studies have indicated that light or moderate alcohol consumption may reduce or not significantly change the risk for AD due to the presence of resveratrol in red wine [57,58]. No evidence exists that consumption of alcohol between 1 and 14 units/week (1 unit = 10 mL or 8 g pure alcohol) increases the risk of dementia, whereas >14 units/week linearly increases the risk of dementia with age. Thus, risk of developing dementia progressively increases as alcohol drinking increases. Even though men and women qualitatively and quantitatively consume different amounts of alcohol, no sex differences in alcohol effects on cognition were observed [59]. The Rotterdam study reported that in individuals aged from 55 years and older, drinking a light to moderate amount of alcohol (1–3 drinks per day) is correlated with a lower risk of dementia and that this outcome is not especially confined to any beverage type [60]. Others European investigations reported that a modest alcohol intake is correlated with a reduced risk of dementia [61,62].
In several studies, drinkers have been compared with non-drinkers, and results showed that those who drink a light-to-moderate amount of wine exhibited lower lifetime risk for AD than those who abstained, and that both non-heavy drinkers and abstainers exhibited a decreased risk of AD [63]. The effect of moderate alcohol consumption for cognition was found in both men and women, although the amount and timing of drinking is different between men and women. Overall, in younger subjects, cognition does not appear to be compromised by light to moderate drinking whereas in older subjects, mild to moderate drinking seems to reduce the risk of dementia and cognitive decline.
The study of a cohort of individuals aged 65 and over showed that light or moderate alcohol drinking is associated with a lower risk of dementia and AD, whereas beer and liquor drinking is not associated with risk of dementia. The analyses were stratified according to the presence of Apolipoprotein E (APOE)-4 and showed that the association between the light to moderate wine intake and A lower risk of AD was limited to subjects without the APOE-4 allele. Thus, reduced cognitive risk effect of moderate drinking was annulled by the presence of the APOE-4 allele [58]. A study on binge Finnish drinkers without the APOE-4 allele reported an insignificantly reduced risk of dementia, whereas infrequent binge drinkers with the APOE-4 allele displayed significant increase in the risk of dementia [64]. In a study conducted to analyze the effect of alcohol and APOE-4 on the age of onset of AD, the absence of the APOE-4 allele in drinkers was found to be associated with an earlier onset of AD [65]. Women who drink moderately had a significantly reduced risk of cognitive decline independent of APOE-4 [66]. The question of whether the APOE-4 allele influences the protective effect of moderate ethanol intake on cognitive risk has not yet been resolved [58]. A glymphatic system [67] formed by astroglial cells was discovered in the central nervous system, which may eliminate soluble proteins and metabolites and plays a key role in the clearance of proteins that are potentially neurotoxic, such as Aβ18 and Tau23. The effect of acute and chronic exposure to ethanol on glymphatic function was investigated. A reduction in glymphatic function resulting from acute and chronic binge alcohol intake and, surprisingly, in mice treated with a low acute dose or a low chronic dose of alcohol, an increased glymphatic activity was observed [68]. Thus, the increase in glymphatic function combined with the reduction of glial fibrillary acidic protein expression could play an important role in the reduction of risk of dementia in individuals who habitually intake low amounts of alcohol.
All studies confirm that when light-to-moderate wine drinking is associated with a healthy lifestyle and habits, such as the adoption of the Mediterranean diet and physical activity, the positive effects are even more pronounced.
Animal models have shown that the synapse damage induced by amyloid-β and α-synuclein may be counteracted by low concentrations of alcohol [69], and polyphenols, due to their antioxidant properties, might provide neuroprotection.
3.2. High Wine Consumption
Chronic intake of alcohol is linked, other than to cardiac and liver problems, to cognitive impairments and brain damage. The characteristics of dementia due to excessive alcohol drinking have received increased interest, and both neuropathological and imaging studies have suggested that excessive and prolonged use of alcohol may be responsible for structural and functional brain damage [70,71,72]. Chronic or excessive alcohol consumption may cause damage to the temporal lobe similar to that observed in AD [73]. Loss of white matter in the prefrontal cortex, corpus callosum, and cerebellum, and neuronal loss in the hypothalamus and cerebellum was observed [74].
Cholinergic dysfunction and neuroinflammation are characteristic hallmarks of dementia, as confirmed by the ability of acetylcholine (ACh) receptor agonists or AChE inhibitors to improve cognitive functions and decrease the levels of inflammatory cytokines in AD patients. Ethanol intake reduces expression of Choline acetyltransferase (ChAT), induces loss of ChAT+ neurons, upregulates neuroimmune signaling, such as proinflammatory cytokines and their receptors, and increases of NF-κB p65 (pNF-κB p65) phosphorylation, in association with increased neuroimmune signaling [75]. Chronic alcohol use has been linked with degeneration of cholinergic neurons, as confirmed by the positive effect of pharmacological manipulation of the neuronal cholinergic pathway [76]. Alcohol may affect cognition by modulating the synthesis and release of acetylcholine in hippocampus, and may induce muscarinic and benzodiazepine receptor loss contributing to the cognitive deficits in AD [77,78]. Thus, one mechanism through which alcohol intake could be linked to AD is alcohol’s effect on cholinergic system.
In AD, most risk loci are located in or near genes that are predominantly expressed in microglia, confirming the hypothesis that microglia play a decisive role in AD progression. Microglial activation was detected in the brains of human alcoholics, but the debate whether microglial activation is the cause or consequence of alcohol-induced neurodegeneration remains open [79]. Toll-like receptors (TLRs), high-mobility group box 1 (HMGB1), microRNAs, and pro-inflammatory cytokines and their receptors are involved in signaling between microglia, innate immune cells of the brain, and neurons in response to alcohol. Ethanol, at high concentrations, is capable of activating TLR4 signaling in astrocytes and microglia [80,81], and triggering the production of inflammatory mediators induces neuronal death. Ethanol can activate microglial cells, altering their morphology, phagocytic response, and production of inflammatory cytokines, such as TNF-α and IL-1β, or inflammatory mediators such as nitric oxide (NO), driving neurodegeneration. A study reported that ethanol, at relevant concentrations, induces microglia activation and secretion of cytokines and inflammatory mediators [81]. Chronic ethanol treatment increases the production of cytokines and inflammatory mediators and induces neural cell death [82]. Several studies have demonstrated that a conditioned medium of ethanol-treated microglia induces apoptosis in cultured neurons, in agreement with observations that high levels of inflammatory mediators produced by activated microglia are deleterious for neurons [83,84,85]. Ethanol induces upregulation of COX-2 and iNOS expression NO production in glial cells, [80,86], and triggers signaling pathways that prime the production and expression of cytokines and inflammatory mediators and cell death in the brain [82].
Taken together, the results of numerous studies indicate that neuroimmune signaling plays a key role in the development of alcohol-related brain disorders. Chronic ethanol sensitizes both systemic and brain responses to the neuroimmune-gene, resulting in the hypothalamic-pituitary-adrenal (HPA)-mediated enhancement of peripheral cytokines, which further exacerbates the neuroimmune response, which increases neurodegeneration [87,88,89,90]. In addition to neuroimmune signaling, glutamate excitotoxicity also is linked to alcoholic neurodegeneration. Neuronal degeneration observed in the adult brain after chronic alcohol exposure gave rise to the neurotoxicity hypothesis, according to which chronic intake of alcohol can cause glutamate excitotoxicity and oxidative stress with neuronal loss [91]. If excitotoxicity is fundamental for neuro-destruction in adult models of chronic alcoholism is only speculative, further studies are needed [92].
The effect of alcohol is also related to age; subjects who begin to consume alcohol before the age of 20 years demonstrated more serious deficits on multiple memory tasks than those who started drinking after the age of 20 [93]. Heavy drinking during adolescence is associated with damage to the prefrontal cortex and hippocampal area, and with neurocognitive dysfunctions as well as in visuospatial, verbal, and attention functions [94,95]. Pascual et al. showed that, during adolescence, intermittent ethanol intake induces inflammation and cell death in the neocortex, hippocampus, and cerebellum, and cognitive impairment, supporting the role of inflammation in ethanol-induced brain damage [96]. Overall, alcohol may support the generation and sustenance of AD pathology via neuroinflammation.
Studies on animals demonstrated that prolonged intake of alcohol induces alteration in different areas of brain such as the hippocampus, hypothalamus, and cerebellum, with impairment of cholinergic neurotransmission, which plays a key role in attention, learning, and memory [97]. The effects of chronic alcohol intake on both basal forebrain cholinergic nuclei and on the brainstem cholinergic nuclei have been studied. The results showed a reduction in the expression of choline acetyltransferase (ChAT), which is involved in acetylcholine biosynthesis [98,99]. Several studies showed that reduction of ChAT is correlated with memory and cognitive impairments in patients with AD and other neurodegenerative diseases [100,101]. A critical role of the TLR4 response was confirmed by observation of microglia activation and neuroinflammatory damage induced by ethanol, and by microglia activation (CD11b+ cells) in cerebral cortex of TLR4+/+mice, but not in TLR4-deficient mice after acute ethanol administration. Fernandez-Lizarbe et al. [81] demonstrated that a conditioned medium of ethanol-treated microglia induces apoptosis in cultured neurons. Growing evidence suggests that, in rats, alcohol-intake-related alterations in brain circuitry are linked to neuroimmune signaling via TLRs [102]. Qin et al. showed that both 10 daily doses and an acute dose of ethanol induce a persistent increase in proinflammatory cytokines and microglial activation in the mouse brain [103].
4. Effects of Components of Wine on AD Molecular Targets
Although most studies reported an insignificant association between risk of AD and wine intake, this does not necessarily mean that alcohol has no effect. The mechanisms through which wine intake may influence the risk of developing AD are not completely understood.
Whether the effect of wine on health is attributed to ethanol, micro-components of wine, or their synergistic effect is not yet known. Distinguishing the action of one from that of the other micro-components of wine is difficult. Even the use of alcohol-free wine confounds this issue because the bioavailability of the wine compounds can change in the absence of ethanol.
Alcohol’s possible beneficial effects are attributed principally to polyphenols. Useful outcomes of polyphenols on AD have been principally reported in investigations with animal models for AD, considering the effects of fortified diets in specific polyphenols such as resveratrol, epigallocatechin-3-gallate, and quercetin [104,105,106] or in a mixture of polyphenols viz. grape seed extracts and red wine [107,108,109,110]. Dietary polyphenols including those present in grape products are considered promising neuromodulatory agents to fight AD because they have the ability to cross the blood–brain barrier, to protect neurons from neurotoxin-induced lesions, to weaken the signaling cascade of oxidative stress and inflammatory response in the brain, to interact with misfolded proteins and polypeptides preventing the formation of toxic aggregates, and to improve cognitive and memory skills (Figure 2) [111].
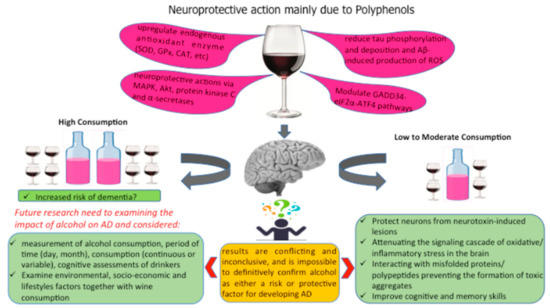
Figure 2.
Different components of wine that may protect the brain.
Several studies showed that the suggested health benefits from red wine consumption are due to resveratrol, whose amount in red wine can vary widely. A neuroprotective effect of resveratrol was suggested on neuron cell death, induced by ethanol and other oxidative agents [112], as well as a powerful neuroprotective activity in focal cerebral ischemia [113] and antioxidant and ion channel regulation (Ca2þ channels). Antonio et al. reported that resveratrol protects against ethanol-induced neurotoxicity [114]. Resveratrol has several effects on AD pathogenesis, targeting many molecular mechanisms of the amyloid cascade such as inhibition of Aβ fibrils formation [115,116], reduction of Aβ production through sirtuin-dependent activation of a disintegrin, and metalloproteinase domain-containing protein 10 [117], and autophagic and lysosomal Aβ degradation [118].
Resveratrol may interrupt the amyloid cascade acting as antioxidant and anti-inflammatory agent, reducing tau phosphorylation and deposition as well as Aβ-induced production of reactive oxygen species (ROS) [119]. Other than the free-radical scavenging abilities [120], resveratrol shows the ability to upregulate endogenous antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and heme oxygenase, and to downregulate enzymes involved in the production of ROS, such as xanthine oxidase [121]. Resveratrol is able to counteract the production of mitochondrial ROS and effectively eliminate hydroxyl, superoxide, and metal-induced radicals, with resulting strengthening of mitochondrial activity and biogenesis by the activation of the Sirtuin1- Peroxisome proliferator-activated receptor gamma, coactivator 1 α (SIRT1-PGC-1α) pathway, thus intensifying mitochondrial bioenergetic productivity [122,123,124]. Sirtuin-1 (SIRT1), activated by resveratrol, induces direct deacetylation of tau to acetylate, helping its proteasomal degradation [125]. Phospho-tau toxicity (induced by cyclin-dependent kinase 5-p25-dependent tau phosphorylation) may decrease; thus, the deacetylation of (PGC-1α, and p53 is promoted.
Resveratrol showed the ability to reduce the inflammatory status [126,127] in in vitro and in vivo settings of neuroinflammation by inhibiting both IL-8 and granulocyte-macrophage colony-stimulating factor release, cytokine-stimulated iNOS expression, and the development of cytokine-producing CD4+ and CD8+ T cells by peripheral blood mononuclear cells [128,129].
Resveratrol interacts with several proteins and pathways involved in the pathogenesis of obesity, such as mitochondrial ATP synthase and complex III, fatty acid synthase, protein kinase C, p53, a protein kinase activated by mitogen 1, TNF-α, and NF-κB. Milne et al. reported that various resveratrol analogs are able to reduce insulin resistance by improving energy homeostasis [130]. In diabetic mice, the activation of AMP kinase by resveratrol protects against atherosclerosis and liver damage, in agreement with the observations of De La Lastra et al. [131]. Aruoma et al. [132] reported that oligonol, a low-molecular-weight proanthocyanidin dietary biofactor, exhibited neuroprotective effects by modulating oxidative stress and additional factors [133].
Using the 3xTg-AD mouse model of AD, characterized by learning and memory deficits, the administration of a low dose of a selected pool of polyphenolic compounds of wine slowed disease progression without undesired side effects on healthy controls, as suggested by the results obtained with non-Tg mice. Starting from the observation that polyphenolic-enriched nutrition may have the potential to benefit AD patients by modulating multiple disease-modifying mechanisms, the development of polyphenolic compounds may provide an alternative strategy for treatment and/or prevention of AD [134].
Quercetin is found in wine, affecting its color and taste. In addition to this function, quercetin is a molecule representative of flavonols, which have many beneficial effects on human health such as reduction of the risk of atherosclerosis by reducing low-density lipoprotein, and reduction of IL-1β, C-reactive protein, and monocyte chemotactic protein-1 levels. Quercetin has been identified as one of the potent antioxidants, and AD can be benefited by the effective removal of ROS. Quercetin induces a decrease in oxidative stress by increasing glutathione (GSH) levels in astrocytes and neurons, which is probably responsible for the reduction of Aβ and τ levels. In cell-free, cell-based and in silico studies, quercetin showed the ability to suppresses Aβ synthesis [135].
Although quercetin is able to inhibit Aβ toxicity in vitro and in vivo, the detailed mechanisms are still elusive. Other than Aβ synthesis, quercetin induces inhibition of the formation and extension of Aβ fibrils, and also stimulates and destabilizes the preformed Aβ fibrils [136,137]. The initial protein–protein interaction of Aβ40 and Aβ42, which has been proven to be necessary for Aβ oligomerization, occurs with the interference of quercetin-3-O-glucuronide [138]. Quercetin exerts neuroprotective effects against toxic molecules [139], modulating the mechanisms of cell death, increasing the resistance of neurons to oxidative stress and excitotoxicity [140], inhibiting iNOS, regulating the expression of COX-2, and exerting anti-inflammatory activity [141].
Studies have reported that several signaling pathways that participate in AD pathogenesis, such as cAMP-response element binding protein (CREB), c-Jun N-terminal kinases, the mitogen-activated protein, macroautophagy, calcium homeostasis, proteasomal degradation, and GADD34-eIF2α-ATF4 pathways, may be modulated by quercetin and its metabolites [139,142]. Quercetin also plays a role as a sirtuin-1 (SIRT-1) agonist and AChE inhibitor to ameliorate AD phenotypes [143]. Quercetin, in acting as an antioxidant, may produce a protective effect in AD and oxidative stress-related neurodegenerative diseases.
The literature shows that epigallocatechin-3-gallate (EGCG) may reduce the risk of various neurodegenerative diseases. EGCG produces neuroprotective activity by modulating mitogen-activated protein kinase (MAPK), Akt, protein kinase C, and α-secretases [144], and affecting amyloid precursor protein (APP) processing through action on the non-amyloidogenic α-secretase and the β-secretase pathways [145].
Aβ-induced cytotoxicity could be overcome by either the activation of the Akt signaling pathway [146] or by increasing the levels of acetylcholine in the presence of EGCG, which behaves as an acetylcholinesterase inhibitor. Neuroprotection upon Aβ-induced neuronal apoptosis could be achieved by effective removal of ROS. The rescue of the neuronal cells from τ-induced neurotoxicity is possible as EGCC has the capacity to remold existing oligomers to an unfolded monomeric state [147,148].
Dietary intake of EGCG, due to biological activities and mostly to antioxidant properties, has been extensively studied for its potential beneficial effects in AD. Oligonol, composed of catechin-type monomers and proanthocyanidin oligomers, is a polyphenolic compound derived from grape seed or lychee fruit with antioxidant and anti-inflammatory activities. Studies have suggested that the antioxidant effects of oligonol are directly or indirectly associated with the activation of SIRT1. Oligonol may downregulate mRNA expression related to iNOS, COX-2, NF-κBp65, and oxidative stress. Since oxidative stress and inflammatory processes are mainly associated with many neurodegenerative diseases, oligonol may have a protective effect for neurodegeneration through its activity upon oxidative-stress-induced inflammation [148].
5. Conclusions
AD is a common disease among aging individuals, being the sixth leading cause of all death and one of the most common causes of impairment, and about 60–80% of cases of dementia are caused by this disease. One possible method of delaying and/or preventing the onset of AD is by acting on its modifiable risk factors, amongst which diet plays an important role.
Since the rate of wine consumption is constantly increasing, numerous studies have been conducted to evaluate if it might represent a modifiable risk factor for cognitive impairment, but the results have been conflicting. Excessive wine consumption, associated with adverse brain outcomes, increases the risk of dementia by direct neurotoxic effects; however, light to moderate wine consumption seems to reduce the risk of dementia and cognitive decline in an age-dependent manner. An emerging body of literature contends that wine consumption may serve as a protective factor for cognitive decline and has associated the health properties of wine with polyphenolic content and their antioxidant properties. The increase in wine consumption is associated with factors that, in turn, promote the onset of dementia, such as hypertension and diabetes. Thus, the protection, attenuation, or intensification of AD may be based on the amount and frequency of wine consumption, individual characteristics, and individual lifestyles. Thus, further research is needed to clarify and comprehensively understand the effect of wine consumption on AD.
Author Contributions
Conceptualization, M.R.; methodology, S.J.; software, T.B.; validation, M.R., E.C. and A.C.; data curation, M.R. and H.K.; writing—original draft preparation, M.R.; writing—review and editing, H.K. and M.R.; visualization, T.B.; supervision, M.R.; project administration, M.R.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Norrie, P. The History of Wine as a Medicine: From Its Beginnings in China to the Present Day; Cambridge Scholars Publishing: Cambridge, UK, 2018. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 17, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; IvanaBeara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Front. Pharmacol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Sun, Z.-K.; Yang, H.-Q.; Chen, S.-D. Traditional Chinese medicine: A promising candidate for the treatment of Alzheimer’s disease. Transl. Neurodegener. 2013, 2, 6. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Burin, V.M.; Gomes, T.M.; Caliari, V.; Rosier, J.P.; Bordignon Luiz, M.T. Establishment of influence the nitrogen content in musts and volatile profile of white wines associated to chemometric tools. Microchem. J. 2015, 122, 20–28. [Google Scholar] [CrossRef]
- Buglass, A.J. Chemical Composition of Beverages and Drinks. In Handbook of Food Chemistry; Cheung, P., Mehta, B., Eds.; Springer: Berlin, Heidelberg, 2014. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans, 2015–2020, 8th ed.; U.S. Department of Health and Human Services/U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 12 January 2020).
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Boucher, F.; de Leiris, J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: A fish-like effect of moderate wine drinking. Am. Heart J. 2008, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Krenz, M.; Korthuis, R.J. Moderate ethanol ingestion and cardiovascular protection: From epidemiologic associations to cellular mechanisms. J. Mol. Cell. Cardiol. 2012, 52, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate Red Wine Consumption and Cardiovascular Disease Risk: Beyond the “French Paradox”. Semin. Thrombosis Hemostasis 2010, 36, 59–70. [Google Scholar] [CrossRef]
- Nooyens, A.C.; Bueno-de-Mesquita, H.B.; van Gelder, B.M.; van Boxtel, M.P.; Verschuren, W.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br. J. Nutr. 2014, 111, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L. Risk of dementia and alcohol and wine consumption: A review of recent results. Biol. Res. 2004, 37, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Intern. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Stockley, C.S. Wine consumption, cognitive function and dementias—A relationship? Nutr. Aging 2015, 3, 125–137. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Yaxley, R.; Paniagua, B.; Johnson, G.A.; Crews, F.T. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addict. Biol. 2017, 22, 712–723. [Google Scholar] [CrossRef]
- Gupta, S.; Warner, J. Alcohol-related dementia: A 21st-century silent epidemic? Br. J. Psychiatry 2008, 193, 351–353. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Tomasi, D.; Wiers, C.E.; Wang, G.-J.; Volkow, N.D. Alcohol affects brain functional connectivity and its coupling with behavior: Greater effects in male heavy drinkers. Mol. Psychiatry 2017, 22, 1185. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Kalk, N.; Sewell, G.; Ritchie, C.W.; Lingford-Hughes, A. Alcohol and Alzheimer’s Disease-Does Alcohol Dependence Contribute to Beta-Amyloid Deposition, Neuroinflammation and Neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017, 52, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Heemels, M.-T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 2017, 61, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.A.; Kamal, M.; Velluto, L.; Gambi, D.; Di Nicola, M.H.; Greig, N. Relationship between inflammatory mediators, Aβ levels and ApoE genotype in Alzheimer disease. Curr. Alzheimer Res. 2012, 9, 447–457. [Google Scholar] [CrossRef]
- Regen, F.; Hellmann-Regen, J.; Costantini, E.; Reale, M. Neuroinflammation and Alzheimer’s disease: Implications for microglial activation. Curr. Alzheimer Res. 2017, 14, 1140–1148. [Google Scholar] [CrossRef]
- Soto, C.; Estrada, L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008, 65, 184–189. [Google Scholar] [CrossRef]
- Lavretsky, H.; Siddarth, P.; Kepe, V.; Ercoli, L.M.; Miller, K.J.; Burggren, A.C.; Bookheimer, S.Y.; Huang, S.-C.; Barrio, J.R.; Small, G.W. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am. J. Geriatr. Psychiatry 2009, 17, 493–502. [Google Scholar] [CrossRef]
- Akiyama, H. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Reale, M.; Brenner, T.; Greig, N.H.; Inestrosa, N.; Paleacu, D. Neuroinflammation, AD, and Dementia. Int. J. Alzheimers Dis. 2010, 2010, 974026. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Iarlori, C.; Gambi, F.; Lucci, I.; Salvatore, M. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp. Gerontol. 2005, 40, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Iarlori, C.; Gambi, D.; Gambi, F.; Lucci, I.; Feliciani, C.; Salvatore, M.; Reale, M. Expression and production of two selected beta-chemokines in peripheral blood mononuclear cells from patients with Alzheimer’s disease. Exp. Gerontol. 2005, 40, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease. In the beginning. Nature 1991, 354, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. β-secretase inhibitors for Alzheimer’s disease: Heading in the wrong direction? Lancet Neurol. 2019, 18, 624–626. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, KM.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Parimisetty, A.; Dorsemans, A.-C.; Awada, R.; Ravanan, P.; Diotel, N.; d’Hellencourt, C.L. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflamm. 2016, 13, 67–73. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. Neuromol. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Dugravot, A.; Akbaraly, T.; Britton, A.; Kivimäki, M.; Singh-Manoux, A. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. Br. Med. J. 2018, 362, k2927. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, D.H.; Li, J.; Wang, Y.J.; Gao, C.; Chen, M.E. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin. Neurol. Neurosurg. 2006, 108, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, X.; Yin, Q.; Zhu, W.; Zhang, R.; Fan, X. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin. Neurosci. 2009, 63, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.A.; Neafsey, E.J.; Mukamal, K.J.; Gray, M.O.; Parks, D.A.; Das, D.K.; Korthuis, R.J. Alcohol in moderation, cardioprotection, and neuroprotection: Epidemiological considerations and mechanistic studies. Alcohol. Clin. Exp. Res. 2009, 33, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Piazza-Gardner, A.K.; Gaffud, T.J.; Barry, A.E. The impact of alcohol on Alzheimer’s disease: A systematic review. Aging Ment. Health 2013, 17, 133–146. [Google Scholar] [CrossRef]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2014, 35, 155–173. [Google Scholar]
- Anstey, K.J.; Peters, R. Alcohol and dementia: Risk or protective factor? Nat. Rev. Neurol. 2018. [Google Scholar] [CrossRef]
- Anstey, K.J.; Mack, H.A.; Cherbuin, N. Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry. 2009, 17, 542–555. [Google Scholar] [CrossRef]
- Benton, S.L.; Schmidt, J.L.; Newton, F.B.; Shin, K.; Benton, S.A.; Newton, D.W. College student protective strategies and drinking consequences. J. Stud. Alcohol. 2004, 65, 115–121. [Google Scholar] [CrossRef]
- Neafsey, E.J.; Collins, M.A. Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 2011, 7, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Zuccalà, G.; Onder, G.; Pedone, C.; Cesari, M.; Landi, F.; Bernabei, R.; Cocchi, A. Dose-Related Impact of Alcohol Consumption on Cognitive Function in Advanced Age: Results of a Multicenter Survey. Alcohol. Clin. Exp. Res. 2001, 25, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.P.; Seltzer, B. Alcohol abuse and Alzheimer’s disease. Hosp. Community Psychiatry 1994, 45, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Siddiqui, M.; Shea, S.; Mayeux, R. Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 2004, 52, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mehlig, K.; Skoog, I.; Guo, X.; Schütze, M.; Gustafson, D.; Waern, M.; Östling, S.; Björkelund, C.; Lissner, L. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Göteborg. Am. J. Epidemiol. 2008, 167, 684–691. [Google Scholar] [CrossRef]
- Ruitenberg, A.; van Swieten, J.C.; Witteman, J.C.; Mehta, K.M.; van Duijn, C.M.; Hofman, A.; Breteler, M.M. Alcohol consumption and risk of dementia: The Rotterdam Study. Lancet 2002, 359, 281–286. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, C.; Winblad, B.; Fratiglioni, L. Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. J. Clin. Epidemiol. 2002, 55, 959–964. [Google Scholar] [CrossRef]
- Truelsen, T.; Thudium, D.; Grønbæk, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef]
- Weyerer, S.; Schäufele, M.; Wiese, B.; Maier, W.; Tebarth, F.; van den Bussche, H.; Pentzek, M.; Bickel, H.; Luppa, M.; Riedel-Heller, S.G. Current alcohol consumption and its relationship to incident dementia: Results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 2011, 40, 456–463. [Google Scholar] [CrossRef]
- Anttila, T.; Helkala, E.L.; Viitanen, M.; Kåreholt, I.; Fratiglioni, L.; Winblad, B.; Soininen, H.; Tuomilehto, J.; Nissinen, A.; Kivipelto, M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: A prospective population based study. Br. Med. J. 2004, 329, 539. [Google Scholar] [CrossRef]
- Harwood, D.G.; Kalechstein, A.; Barker, W.W.; Strauman, S.; St. George-Hyslop, P.; Iglesias, C.; Loewenstein, D.; Duara, R. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2010, 25, 511–518. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Jessen, NA.; Munk, AS.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Wang, W.; Eberhardt, A.; Vinitsky, HS.; Reeves, BC.; Peng, S.; Lou, N.; Hussain, R.; Nedergaard, M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci. Rep. 2018, 8, 2246. [Google Scholar] [CrossRef] [PubMed]
- Bate, C.; Williams, A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology 2011, 61, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The association between alcohol use and the progression of Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 1356–1362. [Google Scholar] [CrossRef]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults with or Without Mild Cognitive Impairment. JAMA Netw. Open. 2019, 2, e1910319. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Savage, L.M. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience 2017, 361, 129–143. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Lim, K.O.; Zipursky, R.B.; Mathalon, D.H.; Rosenbloom, M.J.; Lane, B.; Ha, C.N.; Sullivan, E.V. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol. Clin. Exp. Res. 1992, 16, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.; Matsumoto, I. Ethanol and brain damage. Curr. Opin. Pharmacol. 2005, 5, 73–78. [Google Scholar] [CrossRef]
- Ökvist, A.; Johansson, S.; Kuzmin, A.; Bazov, I.; Merino-Martinez, R.; Ponomarev, I.; Mayfield, R.D.; Adron Harris, R.; Sheedy, D.; Garrick, T.; et al. Neuroadaptations in Human Chronic Alcoholics: Dysregulation of the NF-κB System. PLoS ONE 2007. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J. Neural. Transm. 1994, 44, 173–187. [Google Scholar]
- Tyas, S.L. Alcohol use and the risk of developing Alzheimer’s disease. Alcohol. Res. Health 2001, 25, 299–306. [Google Scholar]
- Freund, G.; Ballinger, W.E. Alzheimer’s disease and alcoholism: Possible interactions. Alcohol 1992, 9, 233–240. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Kelso, M.L.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013, 54, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Valles, S.L.; Pascual, M.; Guerri, C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J. Immunol. 2005, 175, 6893–6899. [Google Scholar] [CrossRef]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef]
- Valles, S.L.; Blanco, A.M.; Pascual, M.; Guerri, C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004, 14, 365–371. [Google Scholar] [CrossRef]
- Boyadjieva, N.I.; Sarkar, D.K. Role of microglia in ethanol’s apoptotic action on hypothalamic neuronal cells in primary cultures. Alcohol. Clin. Exp. Res. 2010, 34, 1835–1842. [Google Scholar] [CrossRef]
- Boyadjieva, N.I.; Sarkar, D.K. Microglia play a role in ethanol-induced oxidative stress and apoptosis in developing hypothalamic neurons. Alcohol. Clin. Exp. Res. 2013, 37, 252–262. [Google Scholar] [CrossRef]
- Kawabori, M.; Yenari, M.A. The role of the microglia in acute CNS injury. Metab. Brain Dis. 2015, 30, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Pascual, M.; Valles, S.L.; Guerri, C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport 2004, 15, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol. Res. 2015, 37, 331–341, 344–351. [Google Scholar] [PubMed]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G., Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Gano, A.; Doremus-Fitzwater, T.L.; Deak, T. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res. 2016, 1646, 62–72. [Google Scholar] [CrossRef]
- Noor, S.; Milligan, E.D. Lifelong Impacts of Moderate Prenatal Alcohol Exposure on Neuroimmune Function. Front. Immunol. 2018, 9, 1107. [Google Scholar] [CrossRef]
- Bates, M.E.; Bowden, S.C.; Barry, D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp. Clin. Psychopharmacol. 2002, 10, 193–212. [Google Scholar] [CrossRef]
- Collins, M.; Neafsey, E. Alcohol, Excitotoxicity and Adult Brain Damage: An Experimentally Unproven Chain-of-Events. Front. Mol. Neurosci. 2016, 9, 8. [Google Scholar] [CrossRef]
- Salas-Gomez, D.; Fernandez-Gorgojo, M.; Pozueta, A.; Diaz-Ceballos, I.; Lamarain, M.; Perez, C.; Sanchez-Juan, P. Binge Drinking in Young University Students Is Associated with Alterations in Executive Functions Related to Their Starting Age. PLoS ONE 2016, 11, e0166834. [Google Scholar] [CrossRef]
- Brown, S.A.; Tapert, S.F.; Granholm, E.; Delis, D.C. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcohol. Clin. Exp. Res. 2000, 24, 164–171. [Google Scholar] [CrossRef]
- White, A.M.; Swartzwelder, H.S. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev. Alcohol. 2005, 17, 161–176. [Google Scholar] [PubMed]
- Pascual, M.; Blanco, A.M.; Cauli, O.; Minarro, J.; Guerri, C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007, 25, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Collins, M.A.; Dlugos, C.; Littleton, J.; Wilkins, L.; Neafsey, E.J.; Pentney, R.; Snell, L.D.; Tabakoff, B.; Zou, J. Alcohol-induced neurodegeneration: When, where and why? Alcohol. Clin. Exp. Res. 2004, 28, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Henning, D.; Gray, J.A.; Marchbanks, R. Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Res. Bull. 1988, 21, 563–570. [Google Scholar] [CrossRef]
- Pereira, P.A.; Gonçalves, E.; Silva, A.; Millner, T.; Madeira, M.D. Effects of chronic alcohol consumption and withdrawal on the cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei of the rat: An unbiased stereological study. Neurotoxicology 2020, 7, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Mufson, E.J.; Wuu, J.; Bennett, D.A.; DeKosky, S.T. Reduction of choline acetyltransferase activity in primary visual cortex in mild to moderate Alzheimer’s disease. Arch. Neurol. 2005, 62, 425–430. [Google Scholar] [CrossRef]
- Di Bari, M.; Reale, M.; Di Nicola, M.; Orlando, V.; Galizia, S.; Porfilio, I.; Costantini, E.; D’Angelo, C.; Ruggieri, S.; Biagioni, S.; et al. Dysregulated Homeostasis of Acetylcholine Levels in Immune Cells of RR-Multiple Sclerosis Patients. Int. J. Mol. Sci. 2016, 17, 2009. [Google Scholar] [CrossRef]
- Randall, P.A.; Vetreno, R.P.; Makhijani, V.H.; Crews, F.T.; Besheer, J. The Toll-Like Receptor 3 Agonist Poly (I:C) Induces Rapid and Lasting Changes in Gene Expression Related to Glutamatergic Function and Increases Ethanol Self-Administration in Rats. Alcohol. Clin. Exp. Res. 2019, 43, 48–60. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimer’s Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Ho, L. Role of grape seed polyphenols in Alzheimer’s disease neuropathology. Nutr. Diet. 2010, 2, 97–103. [Google Scholar]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2000, 46, 78–83. [Google Scholar] [CrossRef]
- Antonio, A.M.; Druse, M.J. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008, 1204, 16–23. [Google Scholar] [CrossRef]
- Donmez, G.; Wang, D.; Cohen, D.; Guarente, L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM. Cell 2010, 142, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C.; Richard, T.; Quentin, L.; Krisa, S.; Mérillon, J.M.; Monti, J.P. Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007, 15, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Bitan, G. Modulating self-assembly of amyloidogenic proteins as a therapeutic approach for neurodegenerative diseases: Strategies and mechanisms. Chem. Med. Chem. 2012, 7, 359–374. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, M.; Zeng, X.; Yang, J.; Deng, H.; Yi, L.; Mi, M. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014, 5, e1576. [Google Scholar] [CrossRef]
- Soares, D.G.; Andreazza, A.C.; Salvador, M. Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J. Agric. Food Chem. 2003, 51, 1077–1080. [Google Scholar] [CrossRef]
- Gerszon, J.; Rodacka, A.; Puchała, M. Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. Adv. Cell Biol. 2014, 4, 97–117. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Gueguen, N.; Leman, G.; Baron, S.; Nivet-Antoine, V.; Chupin, S.; Chevrollier, A.; Vessières, E.; Ayer, A.; Ferré, M. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J. Biol. Chem. 2013, 288, 36662–36675. [Google Scholar] [CrossRef]
- Donmez, G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol. Sci. 2012, 33, 494–501. [Google Scholar] [CrossRef]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Chen, M.I.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.Y. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not cox-2 a mechanistic approach to the design of cox-1 selective agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care. 2007, 10, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712. [Google Scholar] [CrossRef] [PubMed]
- De La Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Sun, B.; Fujii, H.; Neergheen, V.S.; Bahorun, T.; Kang, K.S.; Sung, M.K. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors 2006, 27, 245–265. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.; Im, J.A.; Lee, H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model. BMC Complementary Altern. Med. 2015, 15, 185. [Google Scholar] [CrossRef]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Islam, M.R.; Zaman, A.; Jahan, I.; Chakravorty, R.; Chakraborty, S. In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer’s disease. J. Young Pharm. 2013, 5, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Unsal, C.; Aktas, C.; Erboga, M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol. Ind. Health 2016, 32, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.H.; Cheng, C.; Sun, J.M. Quercetin protects mouse brain against lead-induced neurotoxicity. J. Agric. Food Chem. 2013, 61, 7630–7635. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Hayakawa, M.; Itoh, M.; Ohta, K.; Li, S.; Ueda, M.; Wang, M.X.; Nishida, E.; Islam, S.; Suzuki, C.; Ohzawa, K. Quercetin reduces eIF2α phosphorylation by GADD34 induction. Neurobiol. Aging 2015, 36, 2509–2518. [Google Scholar] [CrossRef]
- Qin, X.Y.; Cheng, Y.; Yu, L.C. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci. Lett. 2012, 513, 170–173. [Google Scholar] [CrossRef]
- Mukai, R.; Shirai, Y.; Saito, N.; Fukuda, I.; Nishiumi, S.; Yoshida, K.I.; Ashida, H. Suppression mechanisms of flavonoids on aryl hydrocarbon receptor-mediated signal transduction. Arch. Biochem. Biophys. 2010, 501, 134–141. [Google Scholar] [CrossRef]
- Ferreira, N.; Saraiva, M.J.; Almeida, M.R. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011, 585, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.T.; Jung, C.H.; Lee, S.-R.; Bae, J.-H.; Baek, W.-K.; Suh, M.-H.; Park, J.; Park, C.-W.; Suh, S.-I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol(−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Choi, J.W.; Choi, J.M.; Maeda, T.; Fujii, H.; Yokozawa, T.; Cho, E.J. Protective role of oligonol from oxidative stress-induced inflammation in C6 glial cell. Nutr. Res. Pract. 2015, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).