Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Ethical Considerations
2.3. Intervention
2.4. Outcome Assessment and Clinical Monitoring
2.5. Sample Size Calculations
2.6. Statistical Analysis
3. Results
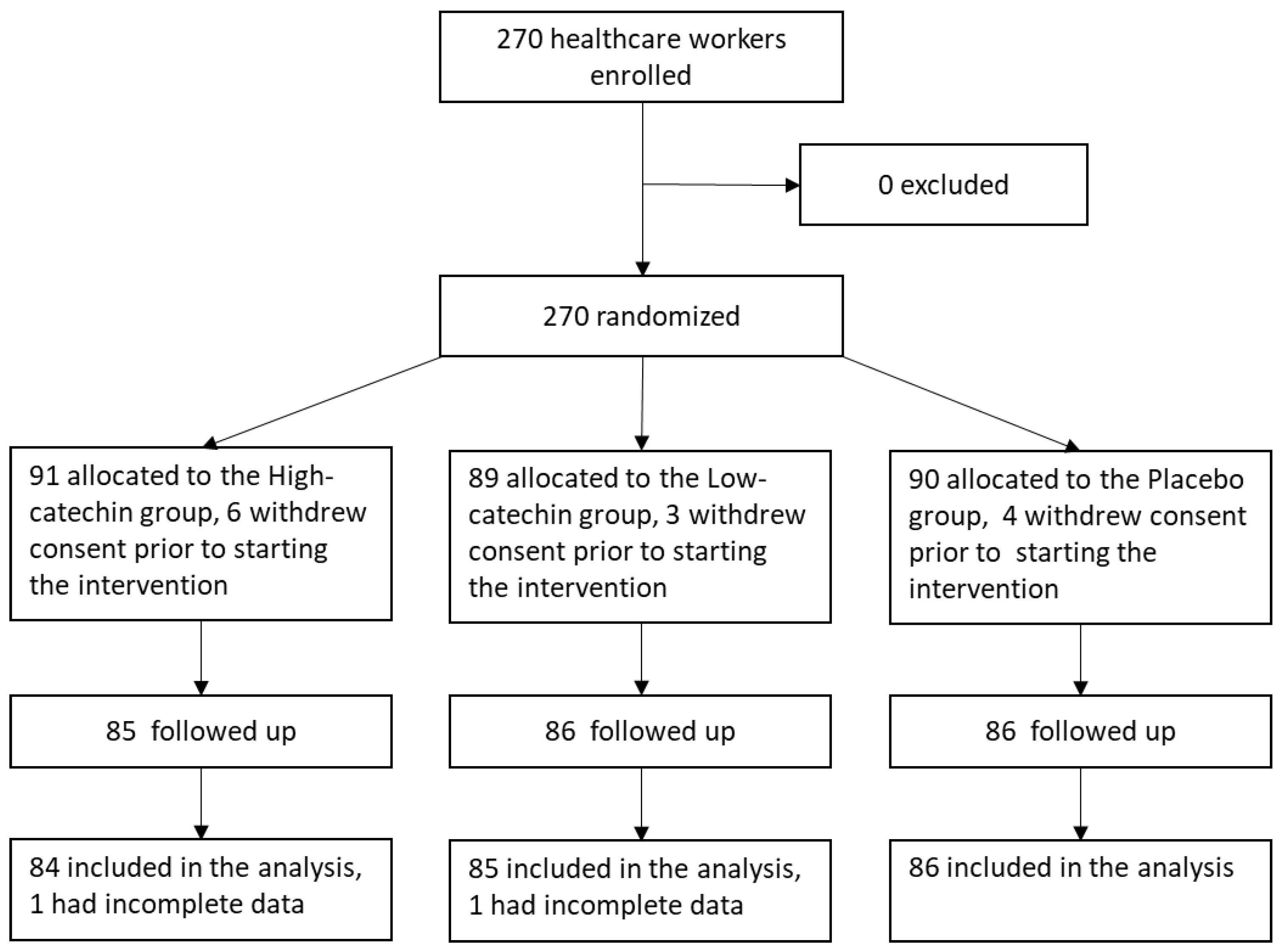
3.1. Subjects
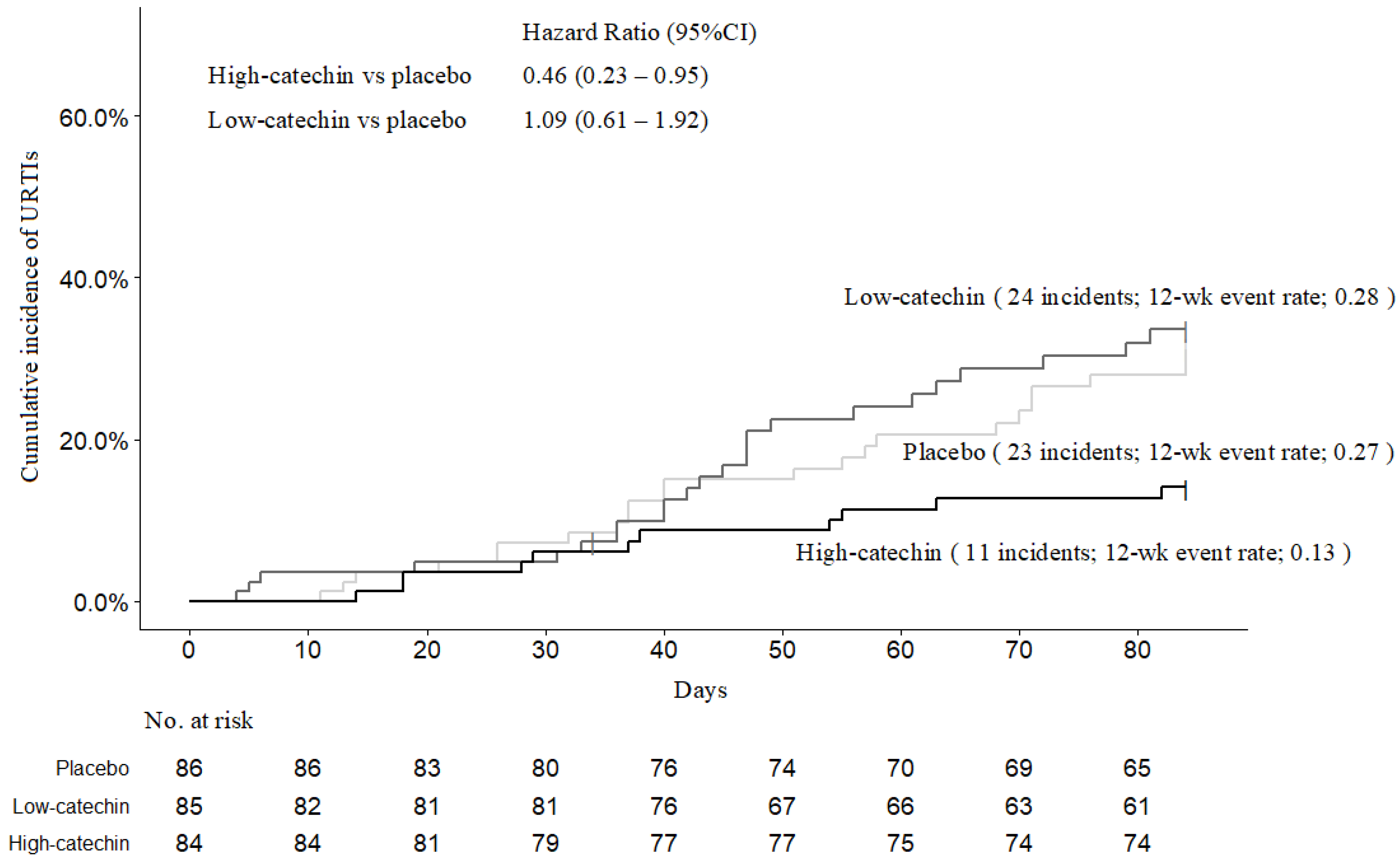
3.2. Incidence of Acute Upper Respiratory Tract Infections and Influenza-Like Illness
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef]
- Kassel, J.C.; King, D.; Spurling, G.K. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2010, 3, CD006821. [Google Scholar] [CrossRef]
- Chalumeau, M.; Duijvestijn, Y.C. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho-pulmonary disease. Cochrane Database Syst. Rev. 2013, 5, CD003124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruuskanen, O.; Arola, M.; Heikkinen, T.; Zeigler, T. Viruses in acute otitis media: Increasing evidence for clinical significance. Pediatr. Infect. Dis. J. 1991, 10, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Pinchichero, M.E. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 2004, 23, 824–828. [Google Scholar] [CrossRef]
- Tan, T.; Little, P.; Stokes, T.; Guideline Development Group. Antibiotic prescribing for self limiting respiratory tract infections in primary care: Summary of NICE guidance. BMJ 2008, 337, a437. [Google Scholar] [CrossRef]
- Kenealy, T.; Arroll, B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst. Rev. 2013, 6, CD000247. [Google Scholar] [CrossRef]
- Goins, W.P.; Talbot, H.K.; Talbot, T.R. Health care-acquired viral respiratory diseases. Infect. Dis. Clin. N. Am. 2011, 25, 227–244. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.L.; Ohde, S.; Takahashi, O.; Tokuda, Y.; Omata, F.; Fukui, T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: A randomized controlled trial. Am. J. Infect. Control 2009, 37, 417–419. [Google Scholar] [CrossRef]
- Lin, J.-K.; Lin, C.-L.; Liang, Y.-C.; Lin-Shiau, S.-Y.; Juan, I.-M. Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu-erh, and black teas. J. Agric. Food Chem. 1998, 46, 3635–3642. [Google Scholar] [CrossRef]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z.; Weinreich, B.; Otto, F.; Gruner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.; Brighenti, F.; Crozier, A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.K.; Wei, T.T.; Chiu, Y.F.; Tung, C.P.; Chuang, J.Y.; Hung, S.K.; Li, C.; Liu, S.T. Inhibition of Epstein-Barr virus lytic cycle by (-)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003, 301, 1062–1068. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Downard, K.M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jang, Y.H.; Kim, Y.S.; Kim, J.; Seong, B.L. Evaluation of green tea extract as a safe personal hygiene against viral infections. J. Biol. Eng. 2018, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Kaihatsu, K.; Yamabe, M.; Ebara, Y. Antiviral Mechanism of Action of Epigallocatechin-3-O-gallate and Its Fatty Acid Esters. Molecules 2018, 23, 2475. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.A.; Nantz, M.P.; Bukowski, J.F.; Percival, S.S. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma, delta T cell function: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Nutr. 2007, 26, 445–452. [Google Scholar] [CrossRef]
- Park, M.; Yamada, H.; Matsushita, K.; Kaji, S.; Goto, T.; Okada, Y.; Kosuge, K.; Kitagawa, T. Green tea consumption is inversely associated with the incidence of influenza infection among schoolchildren in a tea plantation area of Japan. J. Nutr. 2011, 141, 1862–1870. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Daimon, T.; Matsuda, K.; Yoshida, M.; Takuma, N.; Hara, Y. A randomized controlled study on the effects of gargling with tea catechin extracts on the prevention of influenza infection in healthy adults. Jpn. J. Clin. Pharmacol. Ther. 2007, 38, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Takuma, N.; Daimon, T.; Hara, Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: A prospective clinical study. J. Altern. Complement. Med. 2006, 12, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Furushima, D.; Ide, K.; Yamada, H.K. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules 2018, 23, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, K.; Yamada, H.; Matsushita, K.; Ito, M.; Nojiri, K.; Toyoizumi, K.; Matsumoto, K.; Sameshima, Y. Effects of green tea gargling on the prevention of influenza infection in high school students: A randomized controlled study. PLoS ONE 2014, 9, e96373. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef]
- Boivin, G.; Hardy, I.; Tellier, G.; Maziade, J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin. Infect. Dis. 2000, 31, 1166–1169. [Google Scholar] [CrossRef]
- Saijo, R.; Takeda, Y. HPLC analysis of catechins in various kinds of green teas produced in Japan and abroad. Nippon Shokuhin Kagaku Kogaku Kaishi. 1999, 46, 138–147. [Google Scholar] [CrossRef]
- Chan, K.H.; Hardy, I.; Tellier, G.; Maziade, J. Comparative analytical sensitivities of six rapid influenza A antigen detection test kits for detection of influenza A subtypes H1N1, H3N2 and H5N1. J. Clin. Virol. 2007, 38, 169–171. [Google Scholar] [CrossRef]
- National Institute of Infectious Diseases, Japan. Available online: https://www.niid.go.jp/niid/en/ (accessed on 25 November 2019).
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3. 2018. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 10 August 2019).
- Tamura, M.; Saito, H.; Kikuchi, K.; Ishigami, T.; Toyama, Y.; Takami, M.; Ochiai, K. Antimicrobial activity of gel-entrapped catechins toward oral microorganisms. Biol. Pharm. Bull. 2011, 34, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Azziz Baumgartner, E.; Dao, C.N.; Nasreen, S.; Bhuiyan, M.U.; Mah-E-Muneer, S.; Al Mamun, A.; Sharker, M.A.; Zaman, R.U.; Cheng, P.Y.; Klimov, A.I.; et al. Seasonality, timing, and climate drivers of influenza activity worldwide. J. Infect. Dis. 2012, 206, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Althouse, B.M.; Flasche, S.; Minh, L.N.; Thiem, V.D.; Hashizume, M.; Ariyoshi, K.; Anh, D.D.; Rodgers, G.L.; Klugman, K.P.; Hu, H.; et al. Seasonality of respiratory viruses causing hospitalizations for acute respiratory infections in children in Nha Trang, Vietnam. Int. J. Infect. Dis. 2018, 75, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Rambhatla, S.B.; Lai, A.G.; McKeating, J.A. Interplay between circadian clock and viral infection. J. Mol. Med. 2017, 95, 1283–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.S.; Stangherlin, A.; Nagy, A.D.; Nicoll, M.P.; Efstathiou, S.; O’Neill, J.S.; Reddy, A.B. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Natl. Acad. Sci. 2016, 113, 10085–10090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Kinouchi Shimizu, N.; Hakamata, W.; Unno, K.; Asai, T.; Oku, N. Preventive effect of green tea catechins on experimental tumor metastasis in senescence-accelerated mice. Biol. Pharm. Bull. 2010, 33, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.L.; Chen, T.S.; Liou, S.Y.; Hsieh, C.C. Immunomodulatory effects of EGCG fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol. Immunotoxicol. 2014, 36, 364–370. [Google Scholar] [CrossRef]
- Pae, M.; Ren, Z.; Meydani, M.; Shang, F.; Smith, D.; Meydani, S.N.; Wu, D. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J. Nutr. Biochem. 2012, 23, 526–531. [Google Scholar] [CrossRef]
- Pogacnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kim, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F. Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Alvarez, P.; Alvarado, C.L.; Mathieu, F.; Jiménez, L.; De la Fuente, M. Diet supplementation for 5 weeks with polyphenol-rich cereals improves several functions and the redox state of mouse leucocytes. Eur. J. Nutr. 2006, 45, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Sharma, A.; Kumari, A.; Kulurkar, P.M.; Raj, R.; Gulati, A.; Padwad, Y.S. Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology 2017, 18, 367–382. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green tea catechin metabolites exert immunoregulatory effects on CD4(+) T cell and natural killer cell activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
| Contents | Catechin Beverage | Placebo Beverage |
|---|---|---|
| Catechins (mg) | 57 | 0 |
| Epigallocatechin gallate (mg) | 20 | 0 |
| Epigallocatechin (mg) | 18 | 0 |
| Epicatechin gallate (mg) | 6 | 0 |
| Epicatechin (mg) | 5 | 0 |
| Gallocatechin (mg) | 4 | 0 |
| Gallocatechin gallate (mg) | 2 | 0 |
| Catechin gallate (mg) | 1 | 0 |
| Catechin (mg) | 1 | 0 |
| Caffeine (mg) | 10 | 10 |
| Xanthan gum (mg) | 100 | 100 |
| Variable | High-Catechin Group (n = 84) | Low-Catechin Group (n = 85) | Placebo Group (n = 86) | p-Value 3 |
|---|---|---|---|---|
| Sex 1 | ||||
| Male | 17 (20.2) | 27 (31.8) | 15 (17.4) | n/a |
| Female | 67 (79.8) | 58 (68.2) | 71 (82.6) | 0.06 |
| Age (years) 2 | 43.1 (1.37) | 43.1 (1.30) | 43.1 (1.27) | 0.98 |
| BMI (kg/m2) 2 | 22.9 (0.45) | 23.0 (0.46) | 22.6 (0.39) | 0.75 |
| Non-smoker 1 | 64 (76.2) | 70 (82.4) | 70 (81.4) | 0.56 |
| Full-time employee 1 | 64 (76.2) | 70 (82.4) | 67 (77.9) | 0.60 |
| Daily preventive behavior 1 | ||||
| Hand washing 1 | 80 (95.2) | 84 (98.8) | 85 (98.8) | 0.29 |
| Hand antisepsis 1 | 54 (64.3) | 55 (64.7) | 51 (59.3) | 0.72 |
| Gargling 1 | 50 (59.5) | 47 (55.3) | 49 (57.0) | 0.86 |
| Flu vaccination 1 | 83 (98.8) | 79 (92.9) | 82 (95.3) | 0.18 |
| Green tea drinking habit 1,4 | 62 (73.8) | 60 (70.6) | 64 (74.4) | 0.83 |
| Daily use of public transportation 1 | 19 (22.6) | 16 (18.8) | 18 (11.9) | 0.83 |
| Variable | Low-Catechin Group (n = 24) | High-Catechin Group (n = 11) | Placebo Group (n = 23) | ||
|---|---|---|---|---|---|
| Score | p-Value + | Score | p-Value ++ | Score | |
| Nasopharyngeal symptoms 1 | 35.5 (16.2–54.8) | 0.04 | 17.2 (4.6–29.7) | 0.02 | 81.0 (40.6–121.4) |
| Hypo-pharyngeal symptoms 1 | 33.9 (18.0–49.9) | 0.08 | 33.5 (16.8–50.3) | 0.18 | 62.9 (35.6–90.2) |
| Systemic symptoms 1 | 40.8 (23.5–58.0) | 0.79 | 41.8 (8.2–75.5) | 0.89 | 49.4 (23.2–75.6) |
| Variable | High-Catechin Group (n = 84) | Low-Catechin Group (n = 85) | Placebo Group (n = 86) |
|---|---|---|---|
| Total number of adverse events | 24 | 14 | 17 |
| Blood clot | 0 | 0 | 1 |
| Body pain | 6 | 0 | 2 |
| Broken hand bone | 1 | 0 | 0 |
| Cervical lymphadenitis | 0 | 0 | 1 |
| Dizziness | 1 | 1 | 0 |
| Eczema | 0 | 1 | 0 |
| Gastrointestinal complaint | 12 | 6 | 7 |
| Headache | 1 | 0 | 1 |
| Hearing impairment | 1 | 0 | 0 |
| Hemorrhoids | 0 | 1 | 0 |
| High blood pressure | 0 | 1 | 0 |
| Insomnia | 1 | 0 | 0 |
| Joint pain | 0 | 1 | 0 |
| Nettle rash | 0 | 0 | 1 |
| Nosebleed | 0 | 0 | 1 |
| Periodontitis | 0 | 1 | 1 |
| Polyuria | 1 | 0 | 0 |
| Sores mouth | 0 | 1 | 2 |
| Sprain | 0 | 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furushima, D.; Nishimura, T.; Takuma, N.; Iketani, R.; Mizuno, T.; Matsui, Y.; Yamaguchi, T.; Nakashima, Y.; Yamamoto, S.; Hibi, M.; et al. Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial. Nutrients 2020, 12, 4. https://doi.org/10.3390/nu12010004
Furushima D, Nishimura T, Takuma N, Iketani R, Mizuno T, Matsui Y, Yamaguchi T, Nakashima Y, Yamamoto S, Hibi M, et al. Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial. Nutrients. 2020; 12(1):4. https://doi.org/10.3390/nu12010004
Chicago/Turabian StyleFurushima, Daisuke, Takuma Nishimura, Norikata Takuma, Ryo Iketani, Tomohito Mizuno, Yuji Matsui, Tohru Yamaguchi, Yu Nakashima, Shinji Yamamoto, Masanobu Hibi, and et al. 2020. "Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial" Nutrients 12, no. 1: 4. https://doi.org/10.3390/nu12010004
APA StyleFurushima, D., Nishimura, T., Takuma, N., Iketani, R., Mizuno, T., Matsui, Y., Yamaguchi, T., Nakashima, Y., Yamamoto, S., Hibi, M., & Yamada, H. (2020). Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial. Nutrients, 12(1), 4. https://doi.org/10.3390/nu12010004





