Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection Criteria
- Study design: RCTs or meta-analysis of RCTs. Meta-analyses were reviewed for cross-referencing. When treatment allocation was not truly random, such as assigning a treatment intervention based on day of admission or month of service (pseudo-randomized trials), these trials were excluded.
- Population: Adult patients undergoing open heart surgery (with or without cardiopulmonary bypass)
- Intervention: Any form of perioperative vitamin C administration, defined by at least 1 day before, until 7 days after cardiac surgery. Studies with co-administration of other pharmacologic substances including pharmaconutrients and antioxidants were excluded, except for trials using the clinical standard treatment “ß-blockers” in both groups (see below).
- Outcomes included in our meta-analysis: Incidence of organ dysfunction, adverse events, intensive care unit (ICU), and hospital length of stay (LOS), hospital discharge location, and mortality
2.3. Selection of Studies and Data Extraction
2.4. Assessment of Risk of Bias in Included Studies
2.5. Measures of Treatment Effect
2.6. Assessment of Statistical Heterogeneity
- The I2 value was high (exceeding 30%); and either:
- There was inconsistency between trials in the direction or magnitude of effects (judged visually), or there was a low p value (< 0.10) in the Chi2 test for heterogeneity; or
- The estimate of between-study heterogeneity (Tau2) was above zero.
3. Results
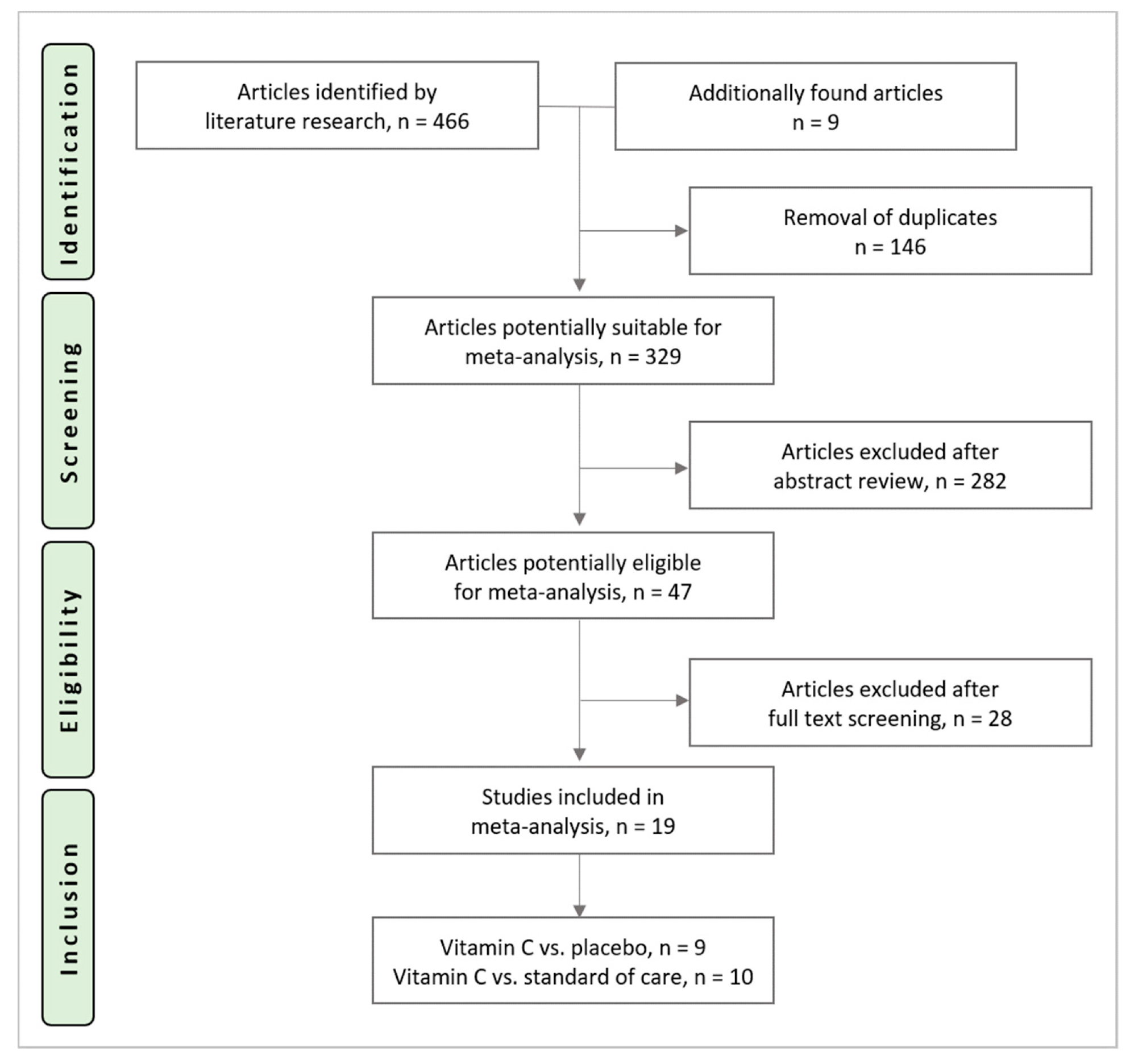
3.1. Study Selection Process
3.2. Characteristics of Included Studies
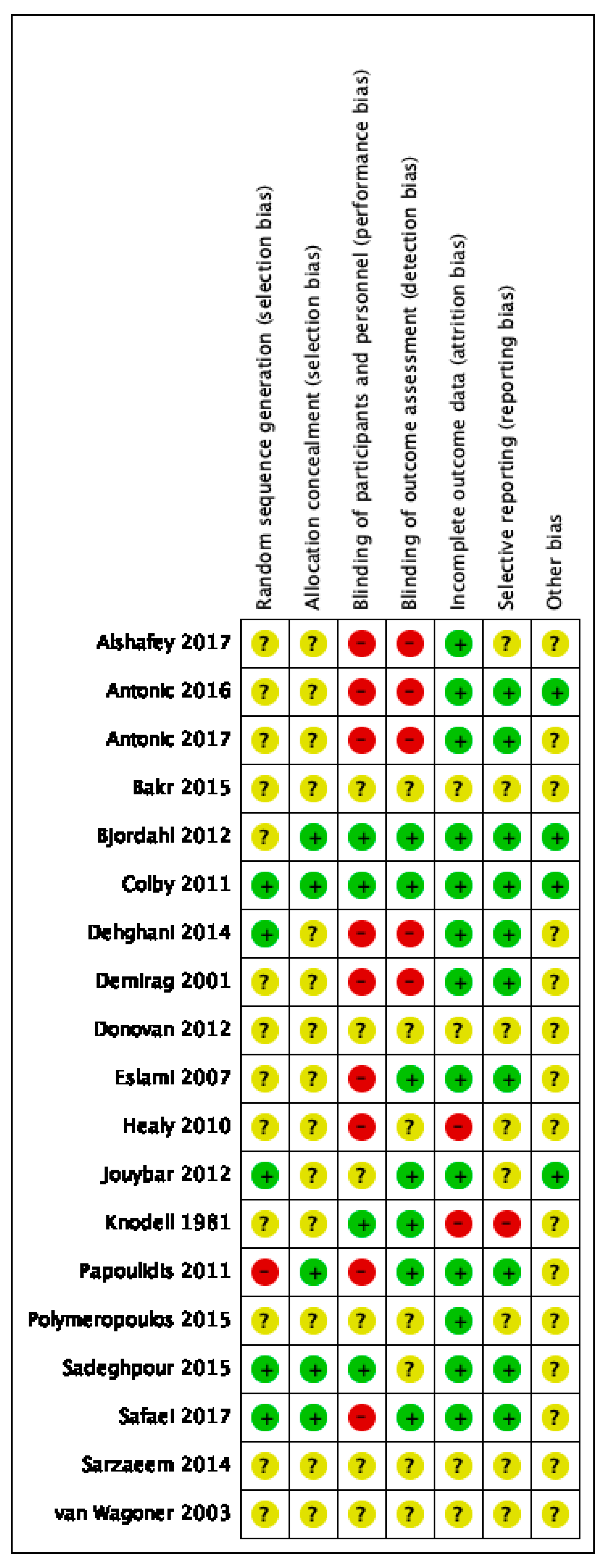
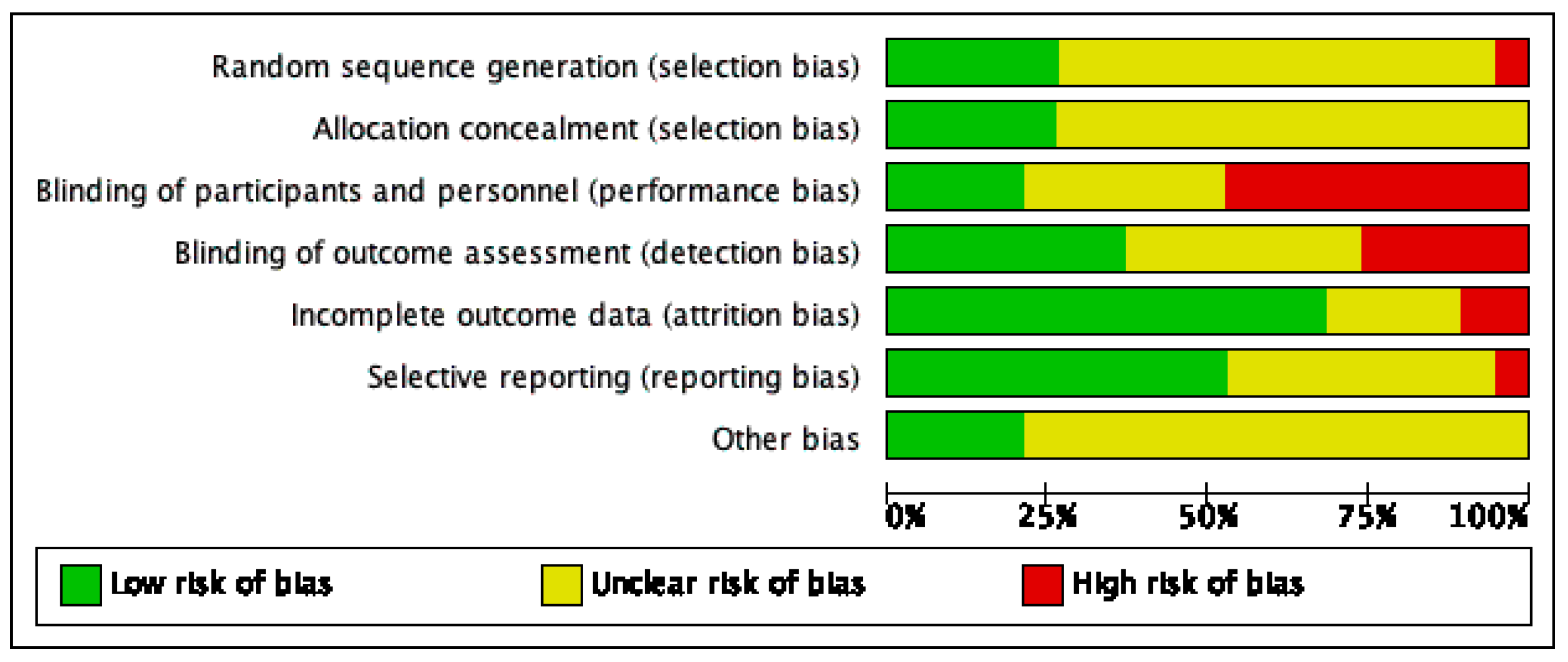
3.3. Risk of Bias Assessment
4. Organ Function
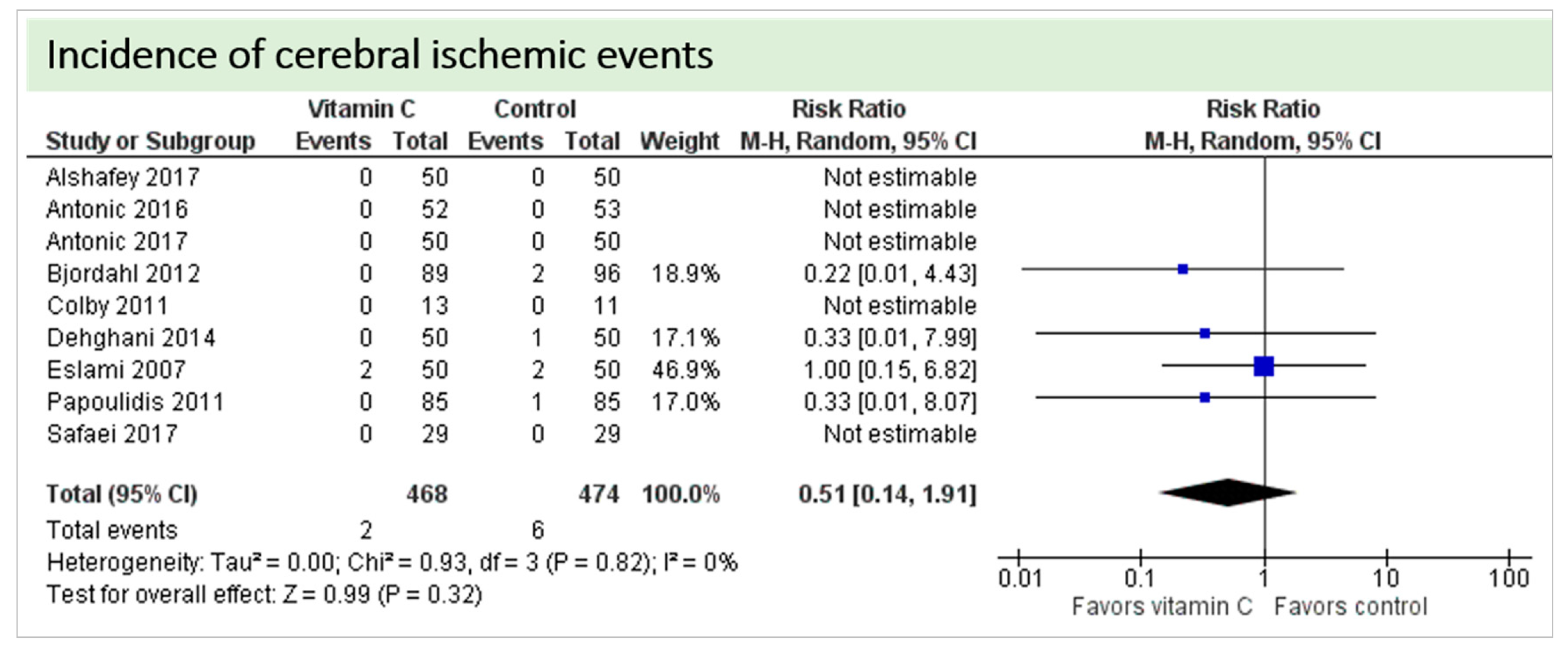
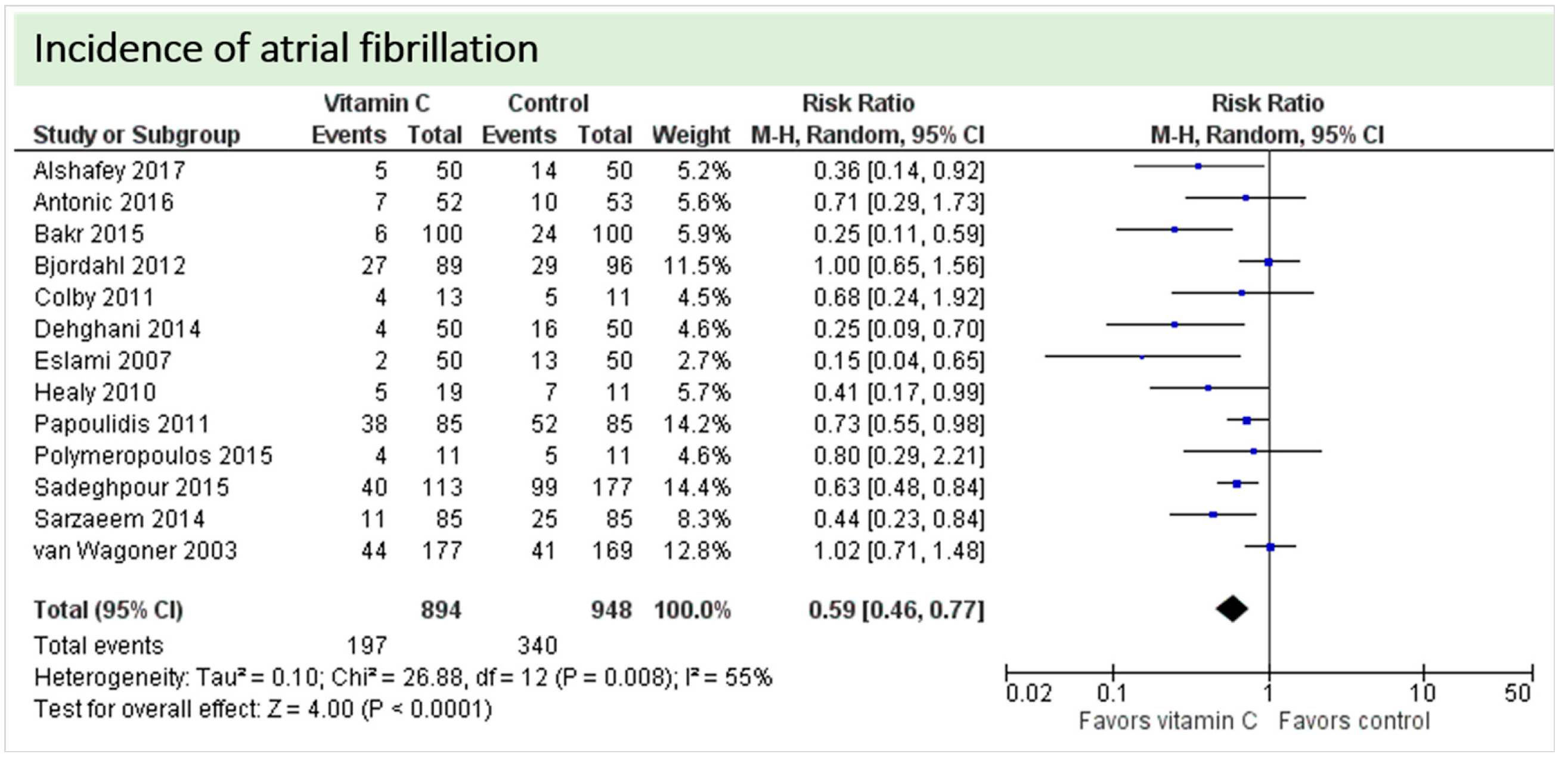
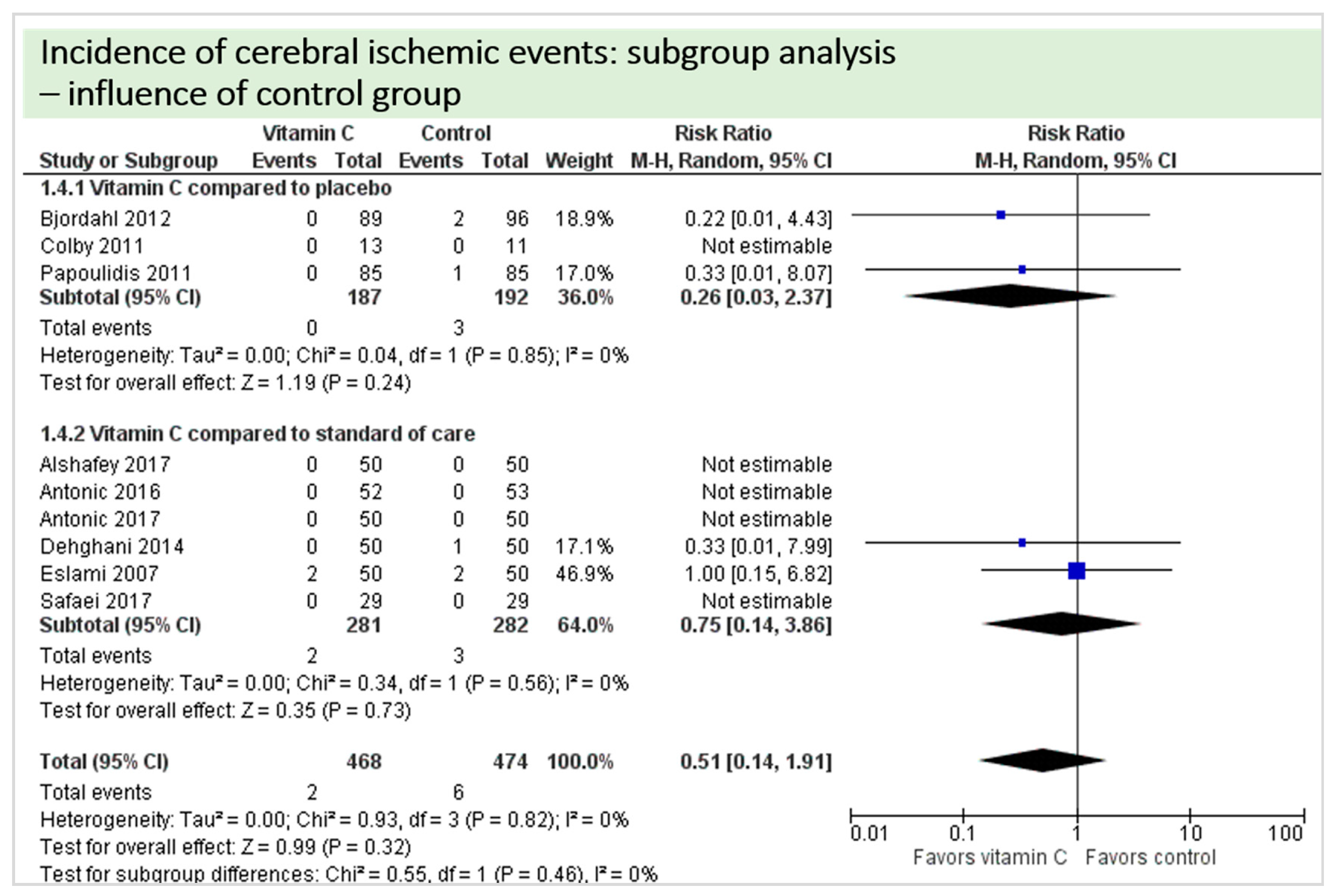
4.1. Neurologic Function
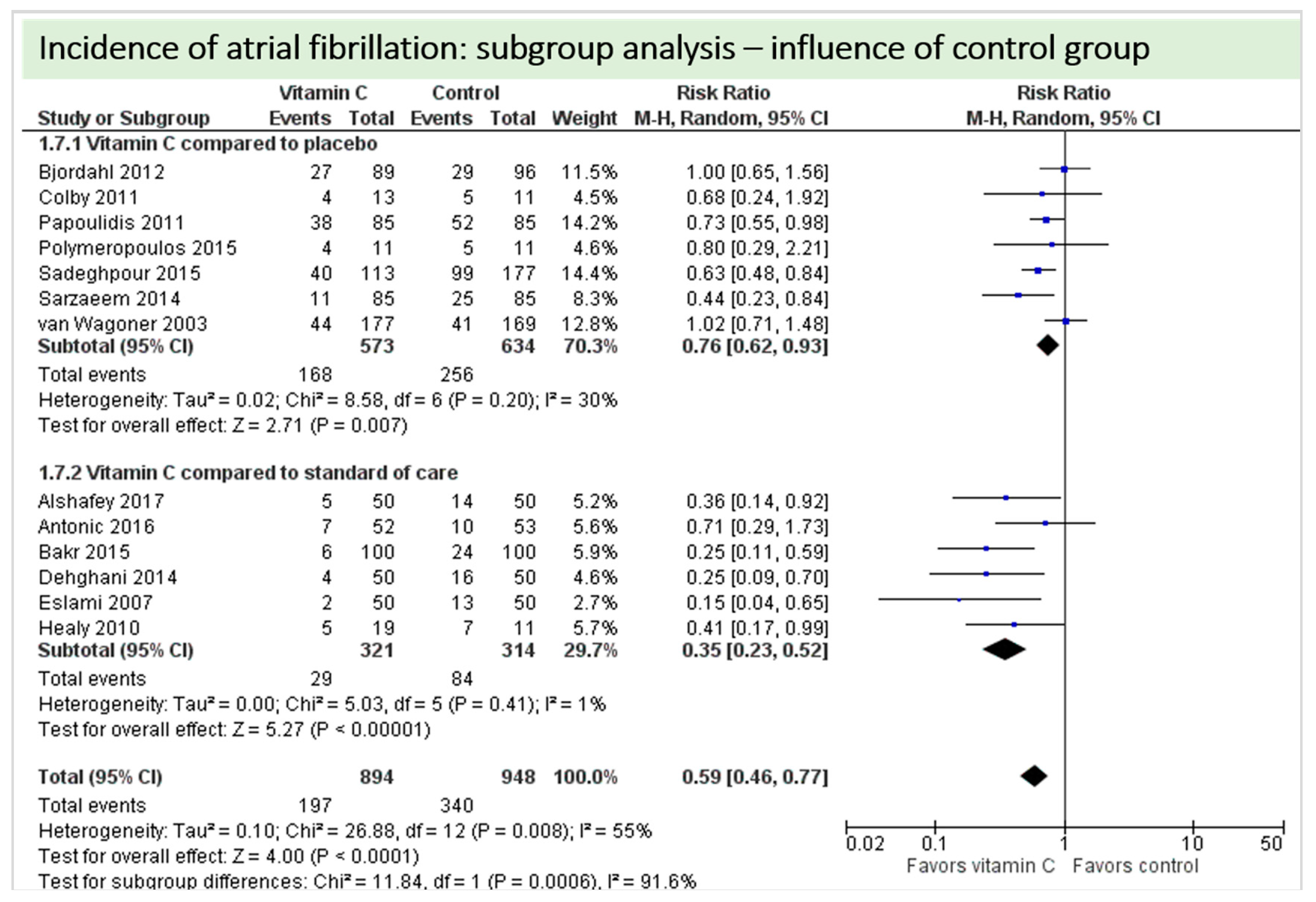
4.2. Cardiac Function
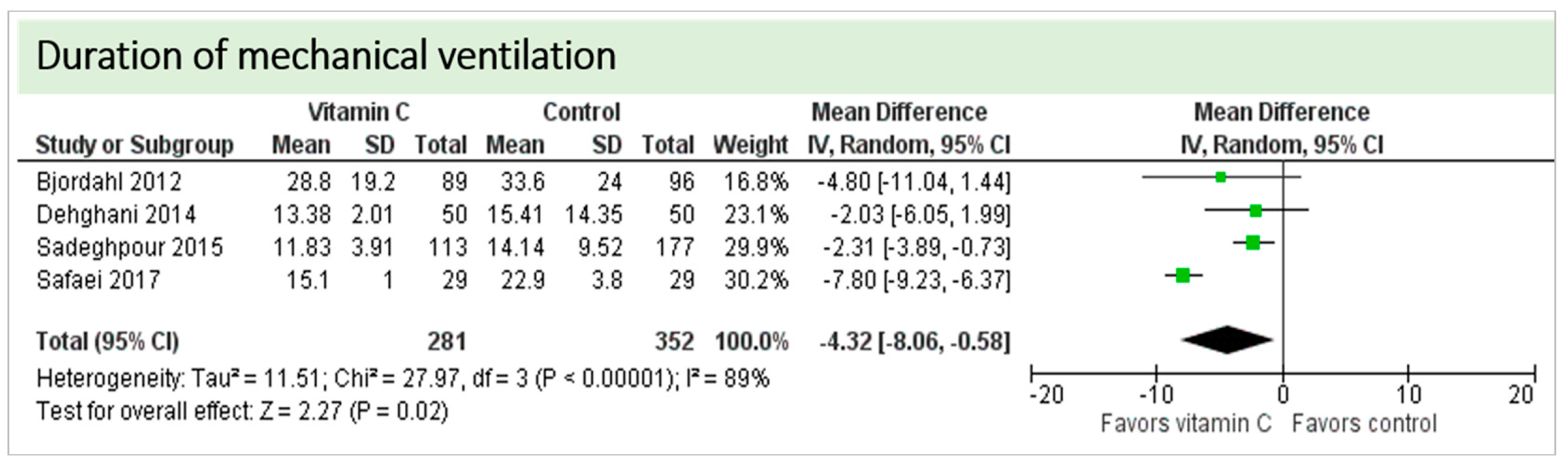
4.3. Pulmonary Function
4.4. Renal Function
4.5. Adverse Events
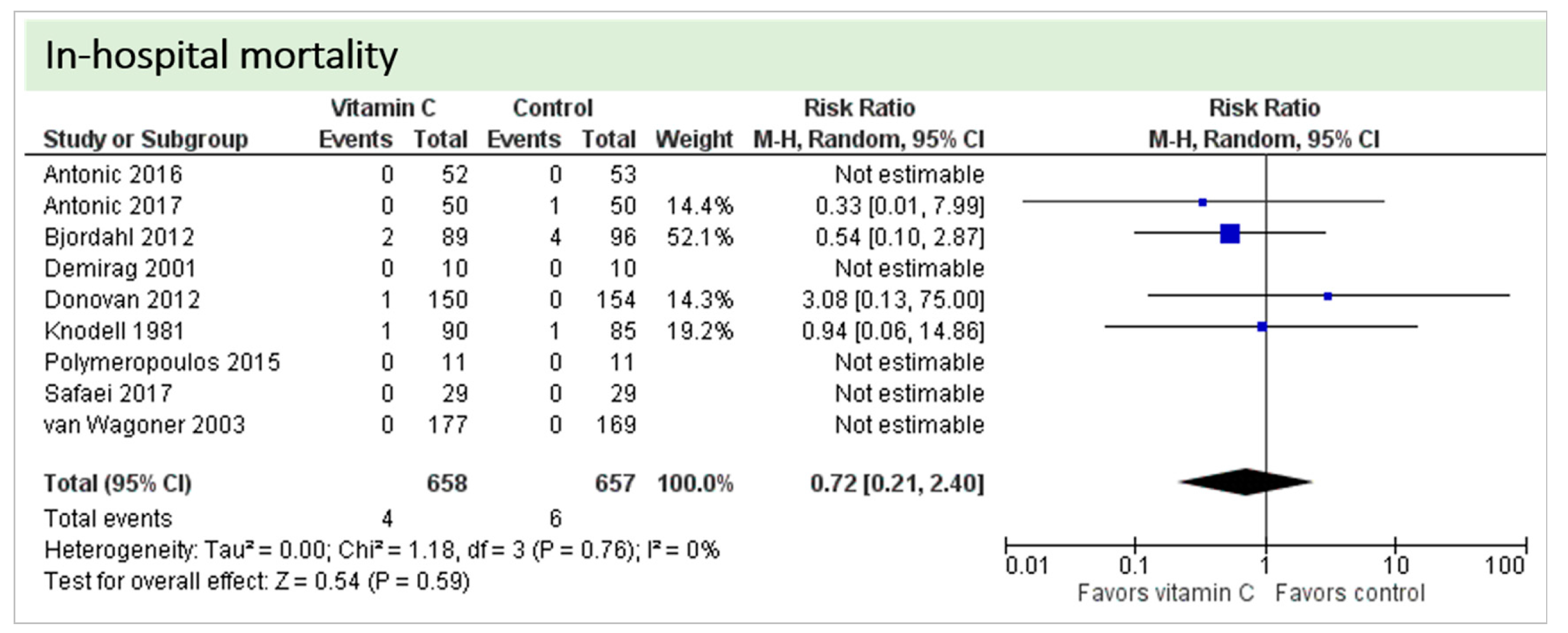
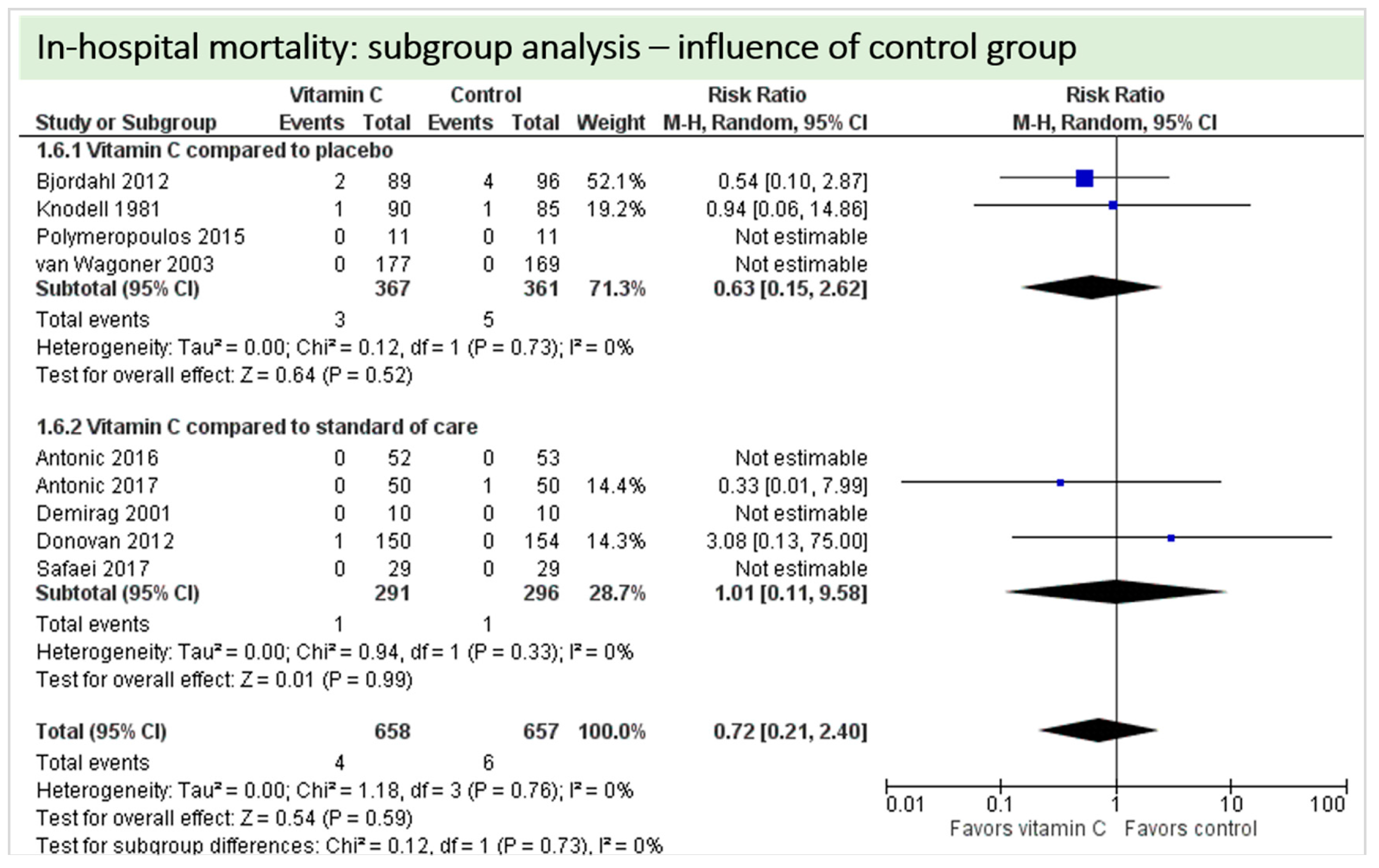
4.6. In-Hospital Mortality
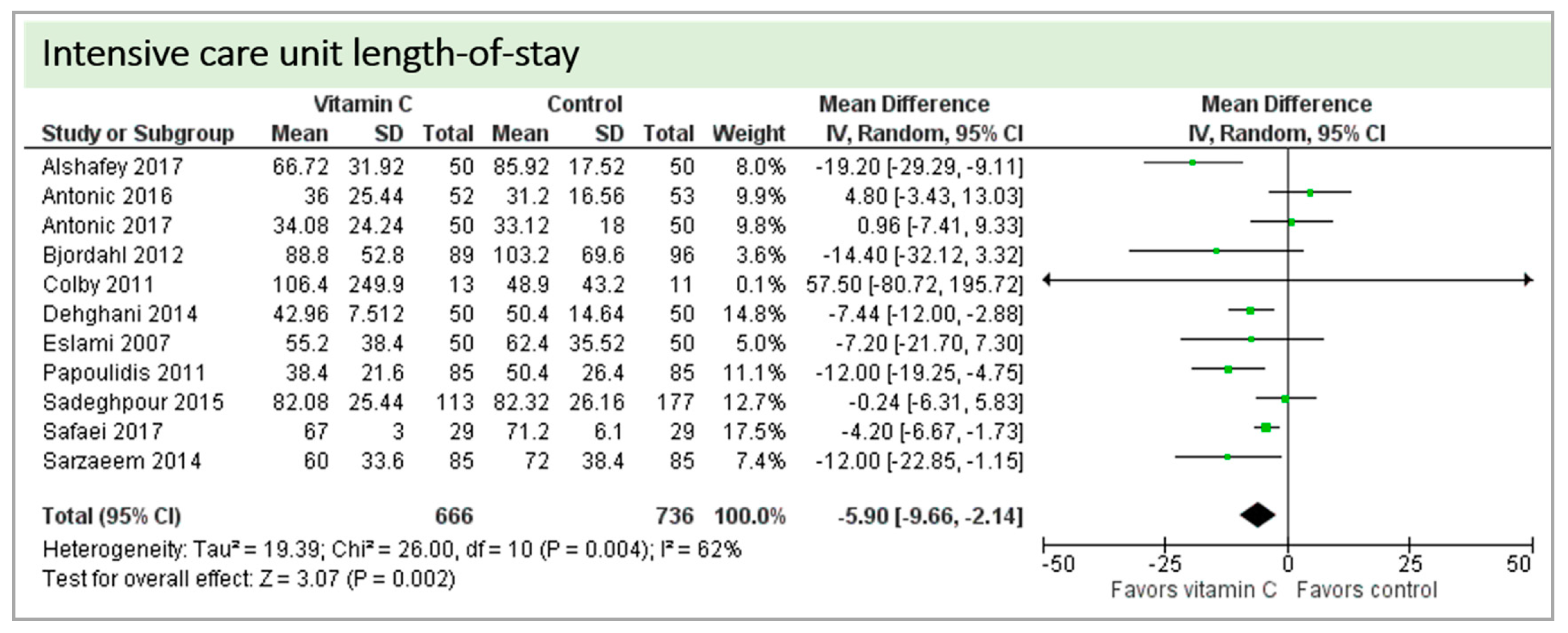
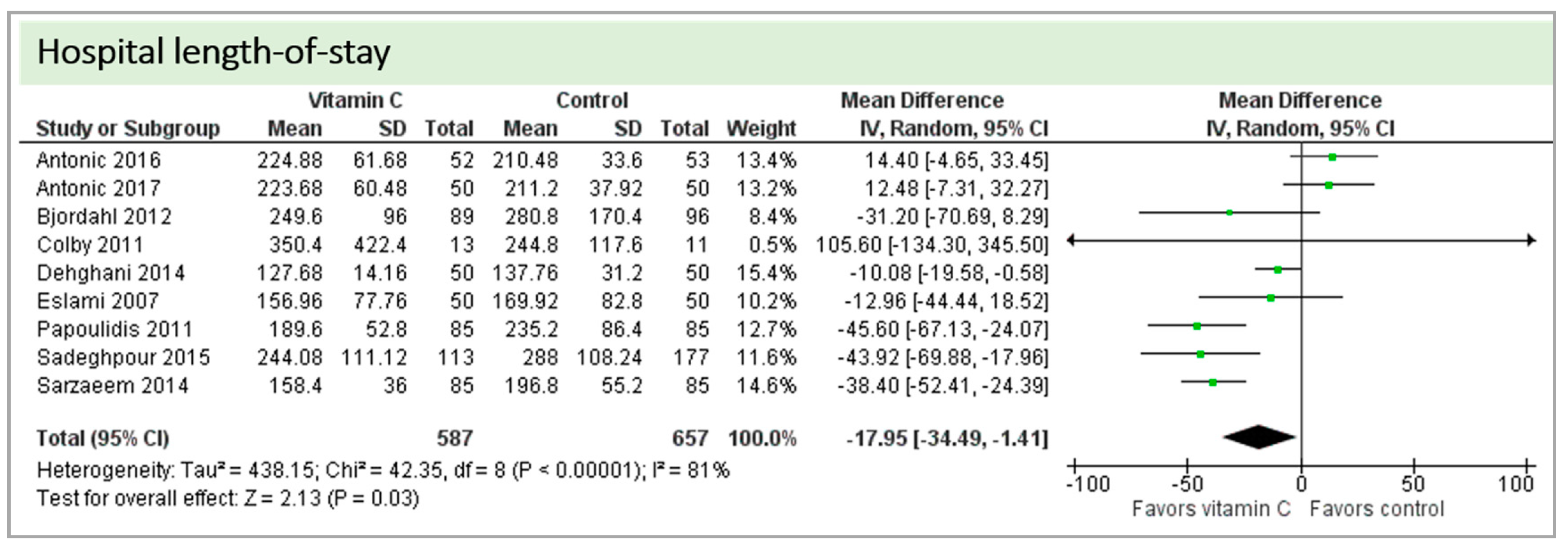
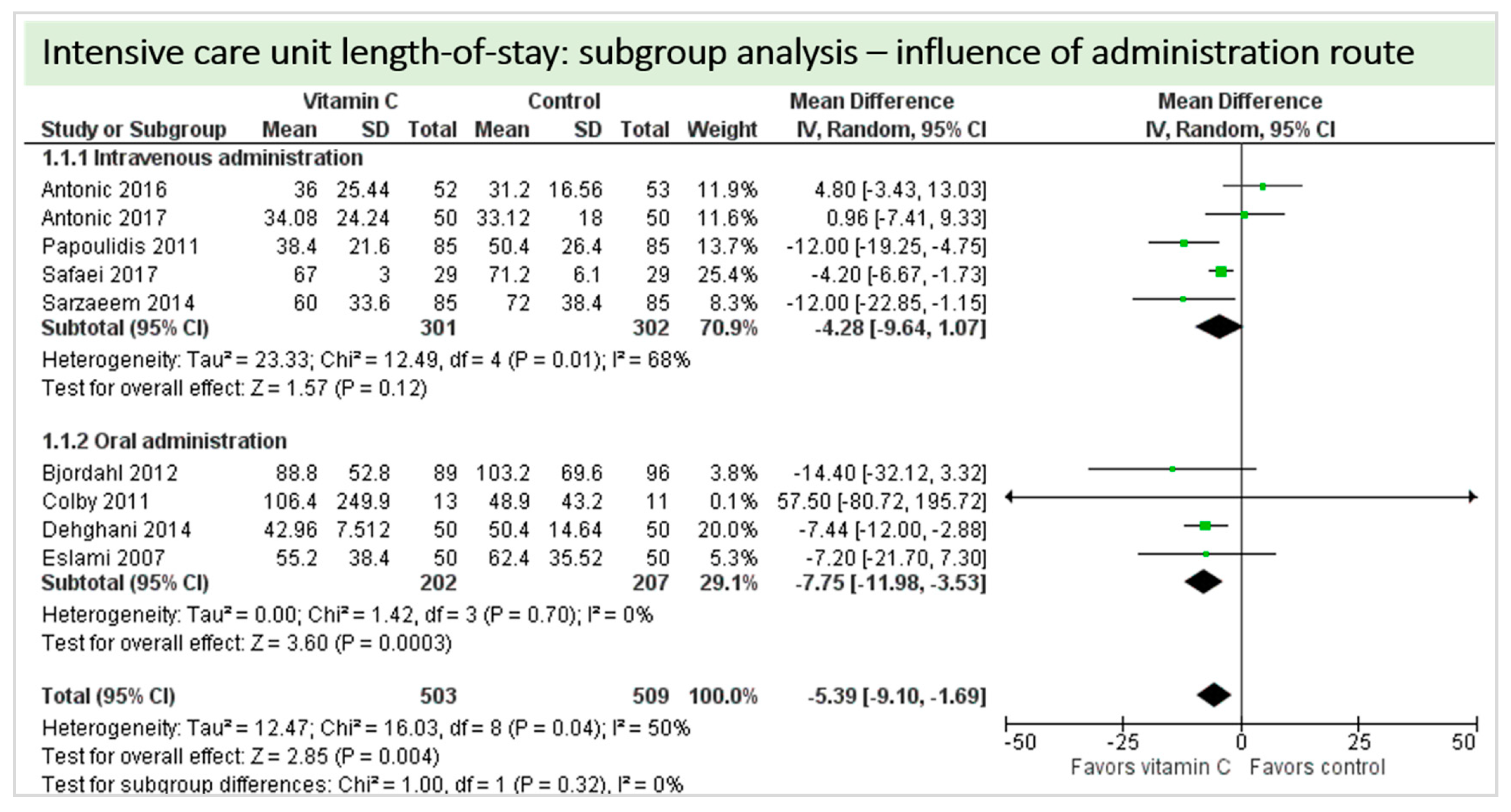
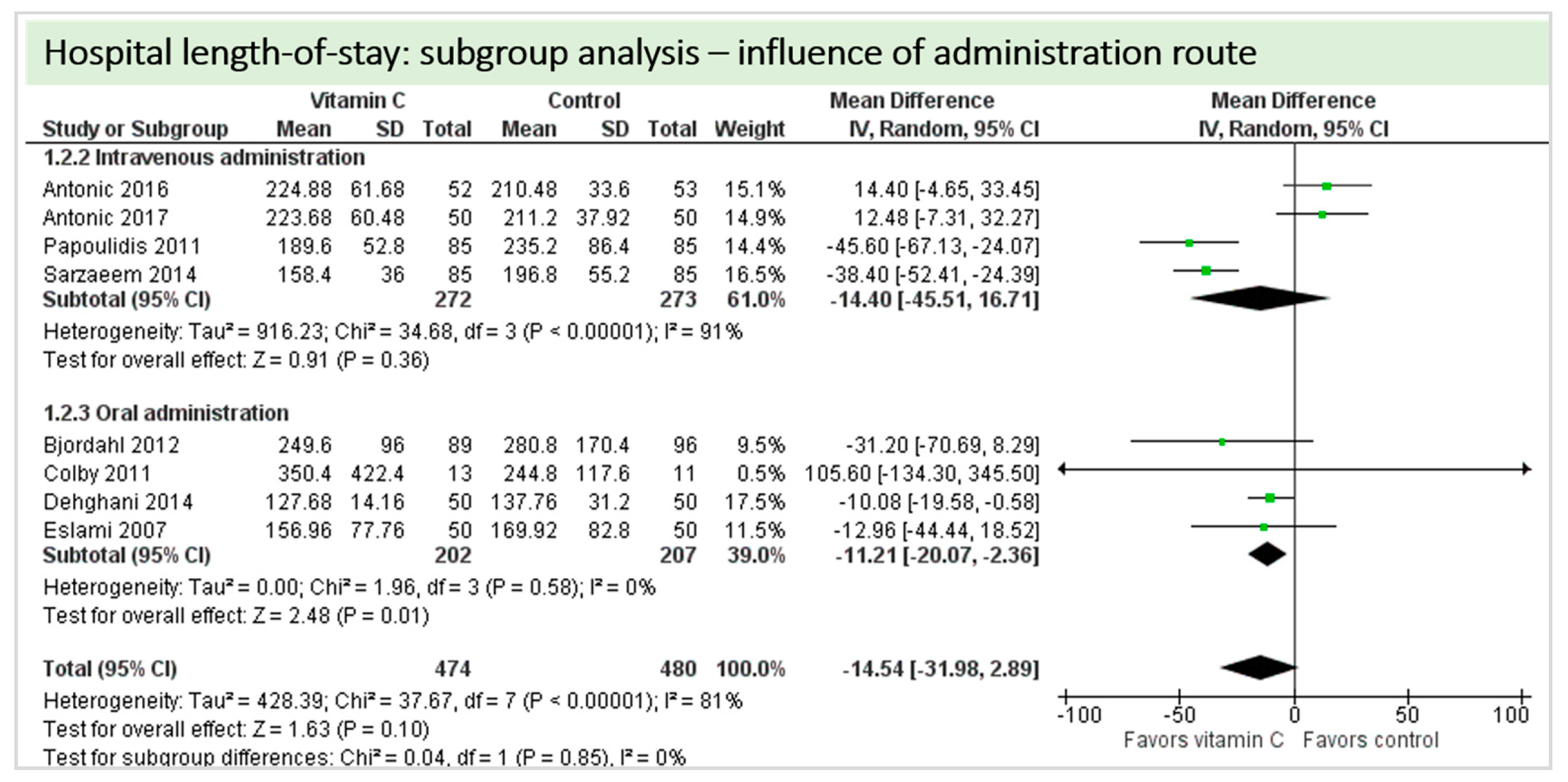
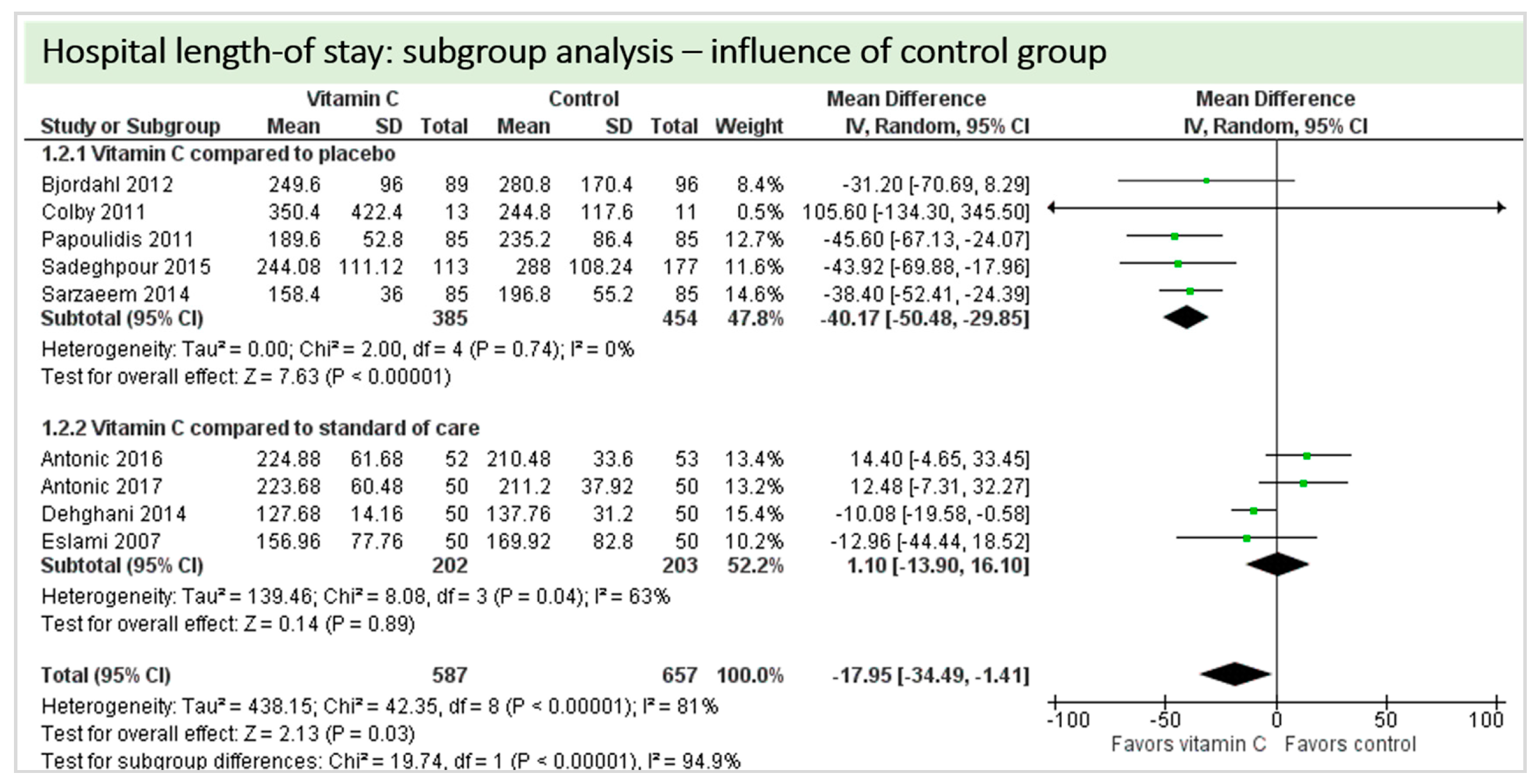
4.7. Length of Stay
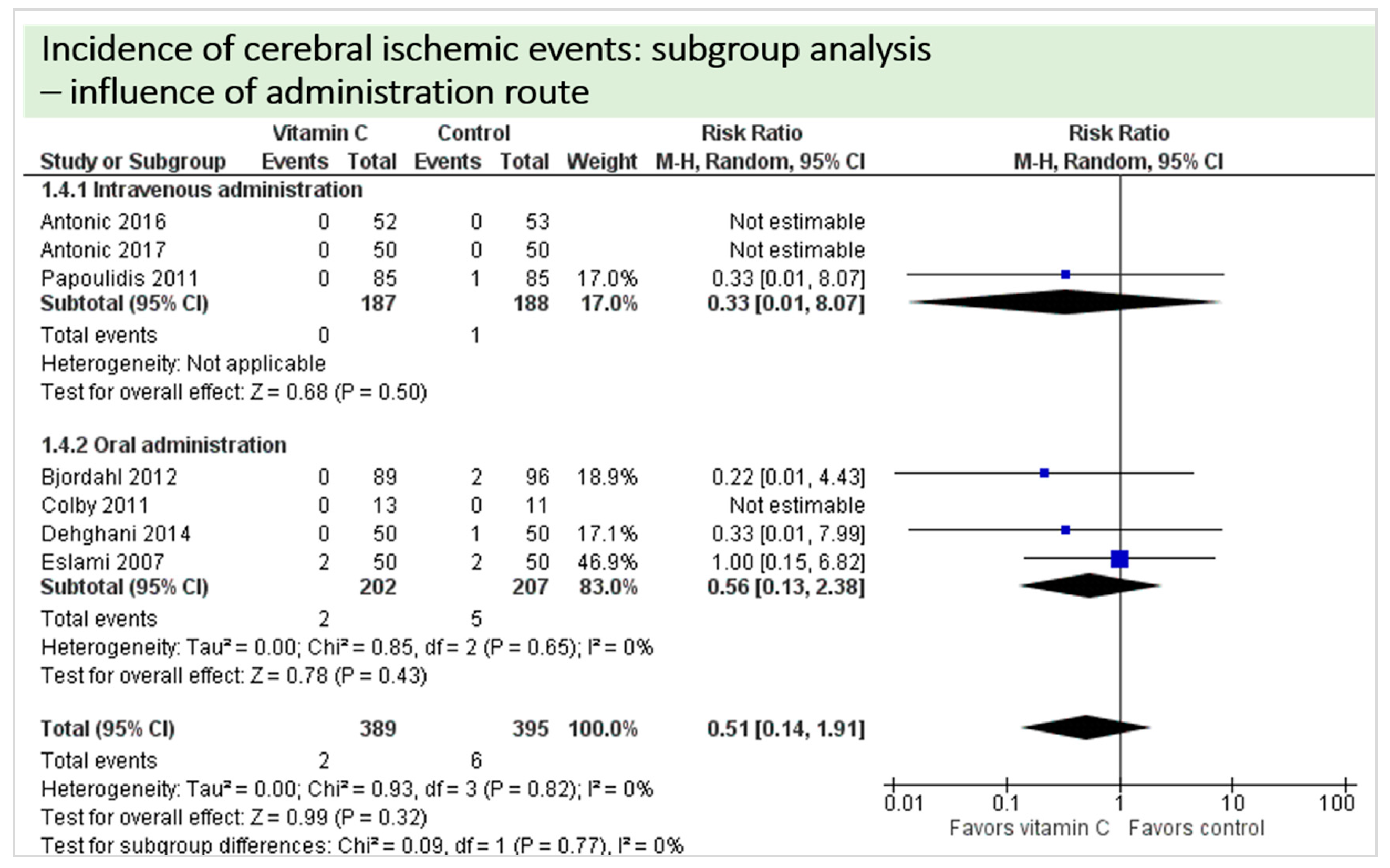
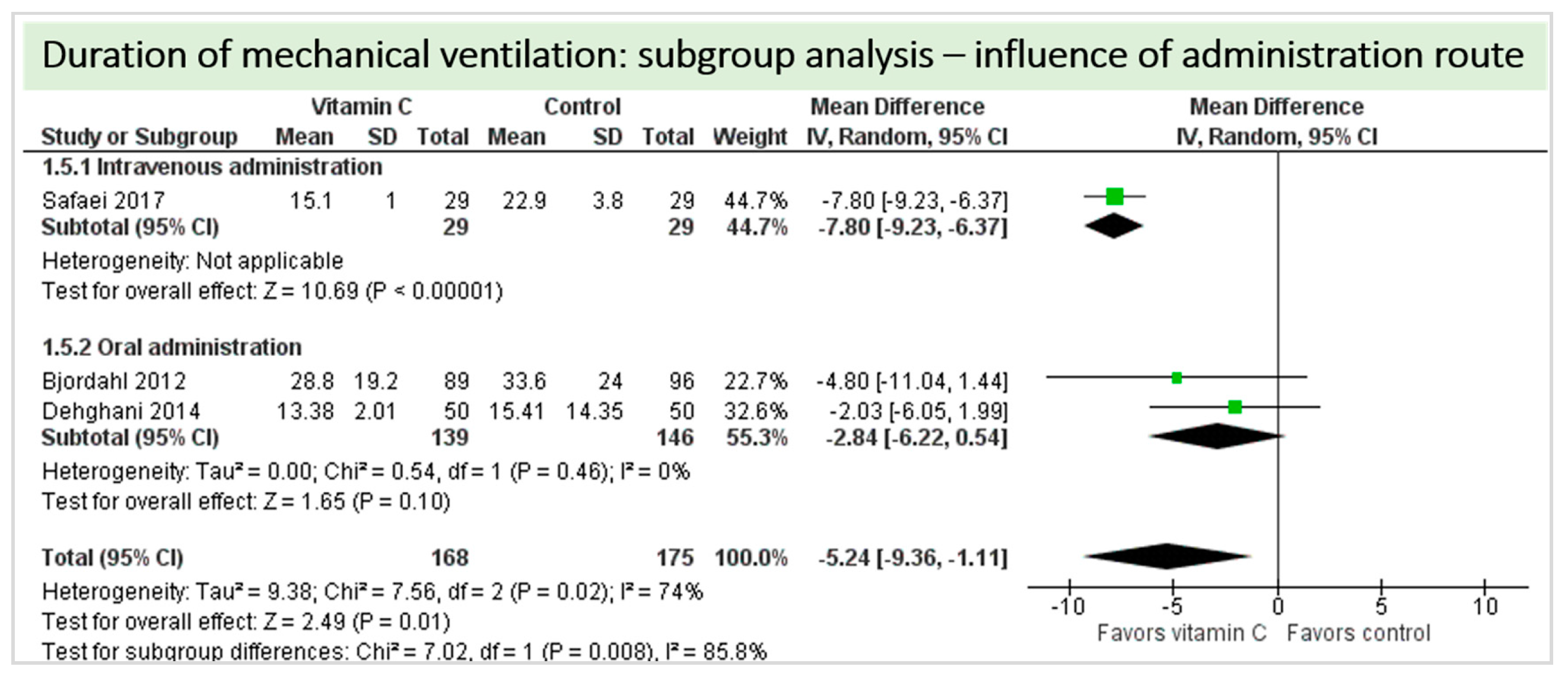
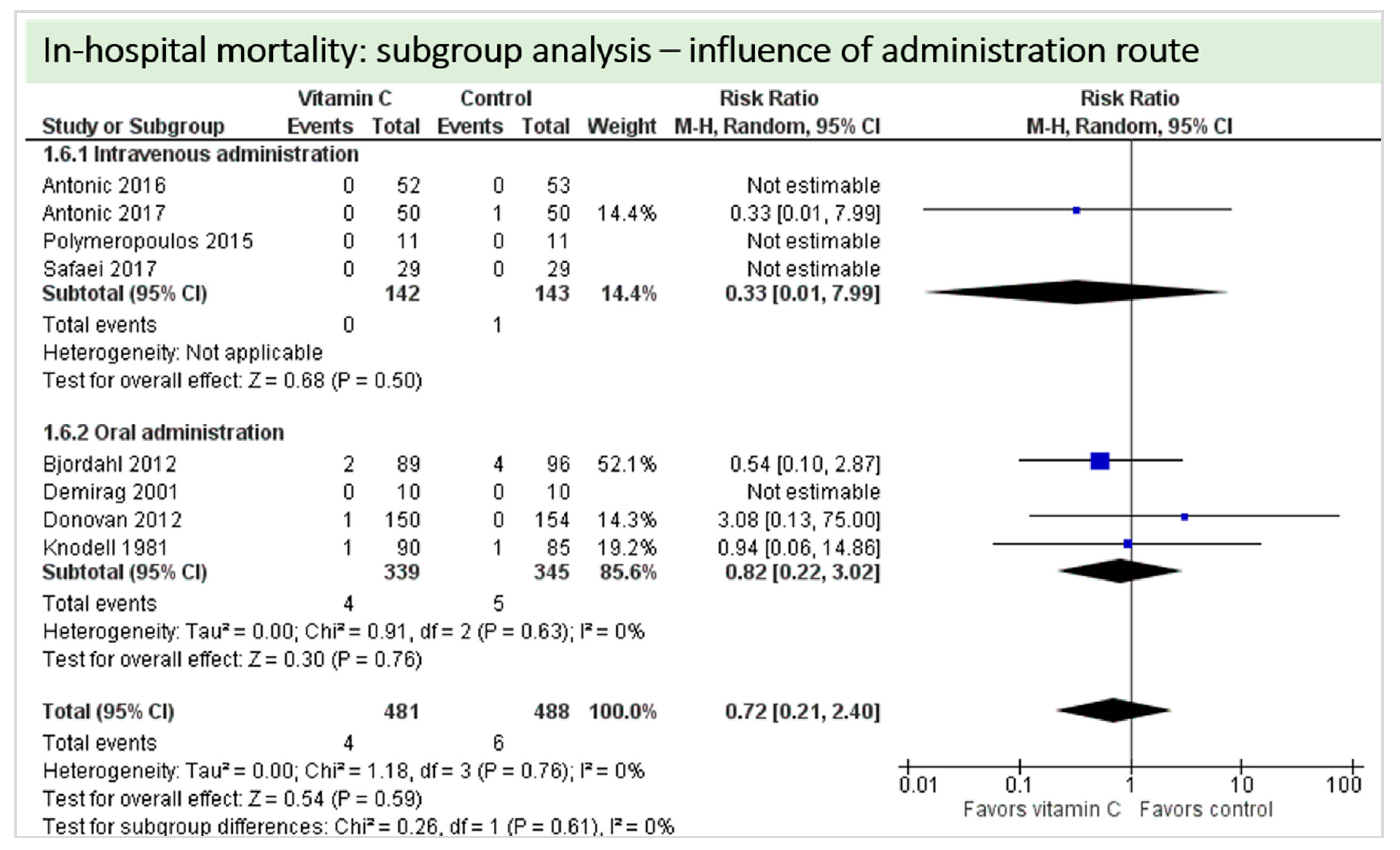
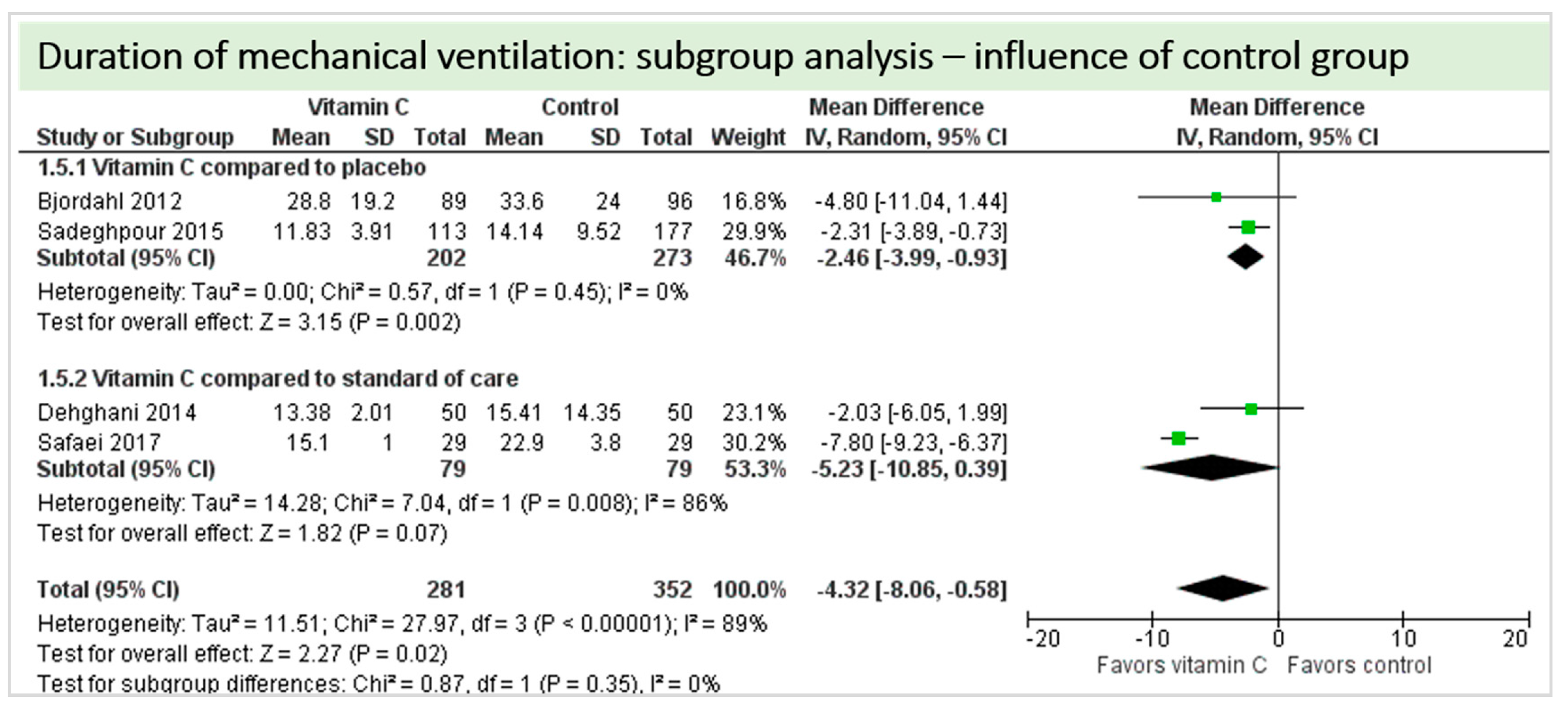
4.8. Subgroup Analysis Influence of Administration Route: Intravenous Administration versus Oral Administration of Vitamin C
4.9. Subgroup Analysis Influence of Control Group: “Vitamin C versus Placebo” versus “Vitamin C versus Standard of Care”
4.10. Sensitivity Analysis
5. Discussion
5.1. Quality of the Evidence
5.2. Potential Biases in the Review Process
5.3. Agreements and Disagreements with Other Reviews
5.4. Implications for Practice
5.5. Implications for Research
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CABG | Coronary Artery Bypass Graft |
| CPB | Cardiopulmonary bypass |
| CI | Confidence interval |
| GSE | Grape seed extract |
| ICU | Intensive care unit |
| LOS | Length of stay |
| n.a. | Not available |
| p.o. | Per os/orally |
| Postop | After surgery |
| Preop | Before surgery |
| RCT | Randomized controlled trial |
| Vit C | Vitamin C |
Appendix A. Manuscripts Excluded from this Analysis after Full Text Screening
| Author and Year | Rationale for Exclusion | |
| 1 | Ali-Hasan Al Saegh 2016 [41] | Systematic review and meta-analysis |
| 2 | Ali-Hassan-Sayegh 2014 [39] | Systematic review and meta-analysis |
| 3 | Baines 2002 [42] | Review |
| 4 | Baker 2016 [11] | Meta-analysis |
| 5 | Carnes 2001 [43] | No randomization and retrospective control group |
| 6 | Das 2016 [44] | Use of inappropriate medication in control group (antacid instead of placebo) |
| 7 | Dingchao 1994 [45] | No randomization, “divided into two groups” |
| 8 | Ebade 2014 [46] | No randomization, “divided into three equal groups” |
| 9 | Hemilae 2017 [13] | Systematic review and meta-analysis |
| 10 | Hill 2018 [4] | Review |
| 11 | Hu 2017 [14] | Meta-analysis |
| 12 | Kumar 2013 [47] | Meta-analysis |
| 13 | Li 1990 [48] | Randomization strategy not mentioned in abstract. Full text not accessible, attempts to contact author unsuccessful. |
| 14 | Liu 2010 [49] | Letter to the editor |
| 15 | Moludi 2016 [50] | Paper and abstract within the university’s journal, abstract identical to included publiction by Sadeghpour et al. [19] |
| 16 | Oktar 2001 [51] | No randomization |
| 17 | Oudemans-van Straten [7] | Review |
| 18 | Polymeropoulos 2016 [12] | Meta-analysis |
| 19 | Rasoli 2011 [52] | Review |
| 20 | Rodrigo 2008 [53] | Review |
| 21 | Rodrigo 2009 [54] | Review |
| 22 | Rebrova [55] | No randomization |
| 23 | Sisto 1995 [56] | Inappropriate comedication (allopurinol) |
| 24 | Samadikah 2014 [57] | Inappropriate comedication (statin) |
| 25 | Shi 2018 [15] | Meta-analysis |
| 26 | NCT00519337 | Registered trial on clinicaltrials.gov, no results posted or found |
| 28 | NCT01167569 | Registered trial on clinicaltrials.gov. Contact to author unsuccessful, no results posted or found |
Appendix B. Methods for Risk of Bias Assessment
B.1. Selection Bias
- Low risk (any truly random process, e.g., random number table; computer random number generator);
- High risk (any non-random process, e.g., odd or even date of birth; hospital or clinic record number);
- Unclear risk (insufficient information to permit judgement).
B.2. Allocation Concealment
- Low risk (e.g., telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
- High risk (open random allocation; unsealed or non-opaque envelopes; alternation; date of birth);
- Unclear risk (insufficient information to permit judgement).
B.3. Performance Bias
- Low, high or unclear risk of bias for participants;
- Low, high or unclear risk of bias for personnel.
B.4. Detection Bias
- Low risk (no blinding of outcome assessment but the authors judged that the outcome was not likely to be influenced by this);
- High risk (no blinding of outcome assessment and the outcome measurement was likely to have been influenced by this);
- Unclear risk (insufficient information to permit judgement; the study did not address this).
B.5. Attrition Bias
- Low risk (20% or less missing data);
- High risk (more than 20% missing data);
- Unclear risk (insufficient reporting to permit judgement; the study did not address this).
B.6. Reporting Bias
- Low risk (where it was clear that all of the study’s prespecified outcomes as identified in the study protocol (where available) and in the methods section were reported on; that all expected outcomes of interest to the review were reported on);
- High risk (where it was clear that not all of the study’s prespecified outcomes as identified in the study protocol (where available) and in the methods section were reported on; failure to include a key outcome that would have been expected to have been included);
- Unclear risk (insufficient information to permit judgement).
B.7. Other Bias
- Low risk (study appeared to be free of bias);
- High risk (had at least one important risk of bias, for example related to study design);
- Unclear risk (insufficient information to permit judgement).
Appendix C. Risk of Bias Assessment
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | Funding for trial: no funding Notable conflicts of interest of authors: all authors declare no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Low risk | “The pharmacy department maintained the randomization list andassigned participants to the […] arms of the study in a blinded fashion.” |
| Blinding of participants and personnel (performance bias) | Low risk | “Participants, clinicians, and evaluators were blinded to the treatment assignments and the blind was not broken until after data analyses were complete.” |
| Blinding of outcome assessment (detection bias) | Low risk | “[…] evaluators were blinded to the treatment assignments and the blind was not broken until after data analyses were complete” |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | Funding for trial: not reported Notable conflicts of interest of authors: all authors report no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Eligible patients were randomized using a computer-generated sequence with a 1:1 allocation and a random block size of 10.” |
| Allocation concealment (selection bias) | Low risk | “Eligible patients were randomized using a computer-generated sequence with a 1:1 allocation and a random block size of 10.” |
| Blinding of participants and personnel (performance bias) | Low risk | “Study patients, cardiothoracic surgeons, caregivers, and investigators, including those responsible for data collection, were blinded to the treatment allocation.” |
| Blinding of outcome assessment (detection bias) | Low risk | “Study patients, cardiothoracic surgeons, caregivers, and investigators, including those responsible for data collection, were blinded to the treatment allocation.” |
| Incomplete outcome data (attrition bias) | Low risk | All data reported (one patient excluded from analysis as the patient did not receive the study drug) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | Funding for trial: Gustavus and Luise Pfeiffer Research Foundation, the sponsor played no role in the design, execution, analysis or submission of the trial and its results Notable conflicts of interest of authors: all authors report no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomized into two groups in a 1:1 ratio using random-number table.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: all authors report no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | “Echocardiography […] was performed before surgery by a single investigator in a blinded fashion.” “All of the Holter recordings were examined by a single investigator who had been blinded to patients’ group assignments.” |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: This study was supported in part by a research grant from Tehran University of Medical Sciences Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | High risk | Interim analysis of only 60 patients reported as abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly assigned to two groups according to the printed table of random numbers, to either receive […].” “A blinded anesthesiologist who was involved neither in the patients’ allocation and management nor in the design of the study and data processing and analysis, generated the randomization list using a computer program.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | “Moreover, the physician responsible for managing the patients did not participate in the study.” |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding of outcome assessment, however, only outcomes were laboratory measures, lack of blinding has minor impact of evaluation of these endpoints |
| Incomplete outcome data (attrition bias) | Low risk | 10% of patients not treated according to protocol, excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | Funding for trial: This work was supported by Shiraz University of Medical Sciences Notable conflicts of interest of authors: all authors report no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Low risk | “double-blind trial” |
| Blinding of outcome assessment (detection bias) | Low risk | “Clinical and laboratory data sheets on all patients with even minor enzyme elevations were submitted to two independent rereviewers […] for evaluation.” “These reviewers either accepted or rejected patients as cases of posttransfusion hepatitis, and analysis of data was based on their decisions.” |
| Incomplete outcome data (attrition bias) | High risk | “40 patients […] who did not complete the study were distributed equally between the placebo and vitamin C treatment groups. The vast majority of patients who did not complete the study either refused to take the study medication postoperatively (11 patients in each group) or refused to have follow-up blood samples drawn.” |
| Selective reporting (reporting bias) | High risk | All outcomes stated in the methods section are NOT adequately reported or explained in results: Serum aminotransferases (only SGPT, SGOT missingAlkaline phosphatase missing Symptoms of congestive heart failure, one month intervals missing |
| Other bias | Unclear risk | Funding for trial: Hoffmann-LaRoche and the Veterans Research Service Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “The initial random assignment was by flipping a coin but simple randomization led to an imbalance with respect to sample size with a treatment group of 130 patients and control group of 85 patients. In order to have an equal sample size, we reevaluated our randomization protocol and using a random generator, the computer chose 85 out of 130 patients which were initially enrolled in the study group.” |
| Allocation concealment (selection bias) | Low risk | “The initial random assignment was by flipping a coin but simple randomization led to an imbalance with respect to sample size with a treatment group of 130 patients and control group of 85 patients. In order to have an equal sample size, we reevaluated our randomization protocol and using a random generator, the computer chose 85 out of 130 patients which were initially enrolled in the study group.” |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | “Echocardiography was performed before surgery by a single echocardiographer in a blinded fashion.” |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The study population was randomized one day before surgery to two groups (by using www.randomaizer.org). The method of randomization was balanced block with an allocation sequence based on a block size of eight, generated with a computer random number generator.” |
| Allocation concealment (selection bias) | Low risk | “The study population was randomized one day before surgery to two groups (by using www.randomaizer.org). The method of randomization was balanced block with an allocation sequence based on a block size of eight, generated with a computer random number generator.” |
| Blinding of participants and personnel (performance bias) | Low risk | “Both the patients and the hospital staff were blind to the treatment allocation.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: not reported |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned to three groups (n = 29 each) using random allocation software.” |
| Allocation concealment (selection bias) | Low risk | “Patients were randomly assigned to three groups (n = 29 each) using random allocation software.” |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | “All data were collected by an independent research nurse assigned to this research study and were blinded to the groups.” “All clinical data were collected by an independent end‑point assessor team including a cardiologist and a nurse who were assigned to this clinical trial and were blinded to group assignment.” |
| Incomplete outcome data (attrition bias) | Low risk | Less than 20% lost to follow-up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Funding for trial: Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran Notable conflicts of interest of authors: all authors report no conflict of interest |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Other bias | Unclear risk | Insufficient information to form judgement, study reported in Farsi, translation difficult |
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to form judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Funding for trial: not reported Notable conflicts of interest of authors: all authors report no conflict of interest |
References
- Levy, J.H.; A Tanaka, K. Inflammatory response to cardiopulmonary bypass. Ann. Thorac. Surg. 2003, 75, S715–S720. [Google Scholar] [CrossRef]
- Richard, H. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 983–1033. [Google Scholar]
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, N.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the Systemic Inflammatory Response to Adult Cardiopulmonary Bypass: A Critical Review of the Evidence Base. J. Extra-Corporeal Technol. 2014, 46, 197–211. [Google Scholar]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.L.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients—Review and Pragmatic Approach. Nutrients 2018, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; McDonald, B.; Benstoem, C.; Elke, G.; Meybohm, P.; Whitlock, R.; Fremes, S.E.; Fowler, R.; Lamarche, Y.; Jiang, X.; et al. Evaluation of Persistent Organ Dysfunction Plus Death as a Novel Composite Outcome in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Stocker, R.; England, L.; Ames, B.N. Ascorbate: The Most Effective Antioxidant in Human Blood Plasma. Vaccine Des. 1990, 264, 155–163. [Google Scholar] [CrossRef]
- Straaten, H.O.-V.; Man, A.M.E.S.-D.; De Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef]
- Berger, M.M.; Straaten, H.M.O.-V. Vitamin C supplementation in the critically ill patient. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 193–201. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Man, A.M.E.S.-D.; Elbers, P.; Straaten, H.M.O.-V. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 2018, 22, 70. [Google Scholar] [CrossRef]
- Baker, W.L.; Coleman, C.I. Meta-analysis of ascorbic acid for prevention of postoperative atrial fibrillation after cardiac surgery. Am. J. Heal. Pharm. 2016, 73, 2056–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polymeropoulos, E.; Bagos, P.G.; Papadimitriou, M.; Rizos, I.; Patsouris, E.; Toumpoulis, I. Vitamin C for the Prevention of Postoperative Atrial Fibrillation after Cardiac Surgery: A Meta-Analysis. Adv. Pharm. Bull. 2016, 6, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H.; Suonsyrjä, T. Vitamin C for preventing atrial fibrillation in high risk patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, S. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int. J. Surg. 2017, 37, 125. [Google Scholar] [CrossRef]
- Shi, R.; Li, Z.-H.; Chen, D.; Wu, Q.-C.; Zhou, X.-L.; Tie, H. Sole and combined vitamin C supplementation can prevent postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2018, 41, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available online: http://handbook.cochrane.org/ (accessed on 11 November 2012).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2008; pp. 243–296. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.; Altman, U.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Sadeghpour, A.; Alizadehasl, A.; Kyavar, M.; Sadeghi, T.; Moludi, J.; Gholizadeh, F.; Totonchi, Z.; Ghadrdoost, B. Impact of Vitamin C Supplementation on Post-Cardiac Surgery ICU and Hospital Length of Stay. Anesthesiol. Pain Med. 2015, 5, 25337. [Google Scholar] [CrossRef]
- Alshafey, M.K.; Elrakhawy, H.M.; Rezk, M.E.; Moustafa, H.M. Role of ascorbic acid in reduction of the incidence of the atrial fibrillation in patients under B-blocker and undergoing coronary artery bypass graft operation in early post-operative period. J. Egypt. Soc. Cardio-Thoracic Surg. 2017, 25, 198–203. [Google Scholar] [CrossRef]
- Bakr, H.; Elsayegh, T.; El Fatah, M.A. 2. Role of ascorbic acid & statin in reduction of the incidence of the atrial fibrillation in patients under B-blocker and undergoing coronary artery bypass graft operation in early post-operative period. J. Saudi Hear. Assoc. 2015, 27, 299–300. [Google Scholar] [CrossRef]
- Healy, R.M.; Day, D.; van Gorder, C. Ascorbic acid utilization for atrial-fibrillation prophylaxis post coronary-artery-bypass graft and valve replacement surgeries: An interim analysis of a prospective, randomized study. Pharmacotherapy 2010, 30, 445e–446e. [Google Scholar]
- Van Wagoner, D.R.; Palumbo, R.; Li, J.; Carnes, C.A.; Gillinov, A.; McCarthy, P.M.; Chung, M.K. Supplemental vitamin c did not reduce the incidence of atrial arrhythmia following cardiac bypass surgery. Heart Rhythm Soc. 2003. Available online: http://www.mv.helsinki.fi/home/hemila/CAF/vanWagoner2003.pdf (accessed on 4 December 2016).
- Antonic, M.; Lipovec, R.; Gregorcic, F.; Juric, P.; Kosir, G. Perioperative ascorbic acid supplementation does not reduce the incidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J. Cardiol. 2017, 69, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Antonic, M. Effect of Ascorbic Acid on Postoperative Acute Kidney Injury in Coronary Artery Bypass Graft Patients: A Pilot Study. Hear. Surg. Forum 2017, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Bjordahl, P.M.; Helmer, S.; Gosnell, D.J.; Wemmer, G.E.; O’Hara, W.W.; Milfeld, D.J. Perioperative supplementation with ascorbic acid does not prevent atrial fibrillation in coronary artery bypass graft patients. Am. J. Surg. 2012, 204, 862–867. [Google Scholar] [CrossRef]
- Colby, J.A.; Chen, W.T.; Baker, W.L.; Coleman, C.I.; Reinhart, K.; Kluger, J.; White, C.M. Effect of ascorbic acid on inflammatory markers after cardiothoracic surgery. Am. J. Heal. Pharm. 2011, 68, 1632–1639. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Madjidi, N.; Rahmani, A.; Askari, B.; Rezaei, Y. Effect of oral vitamin C on atrial fibrillation development after isolated coronary artery bypass grafting surgery: A prospective randomized clinical trial. Cardiol. J. 2014, 21, 492–499. [Google Scholar] [CrossRef]
- Demirag, K.; Askar, F.Z.; Uyar, M.; Cevik, A.; Ozmen, D.; Mutaf, I.; Bayindir, O. The protective effects of high dose ascorbic acid and diltiazem on myocardial ischaemia-reperfusion injury. Middle East J. Anaesthesiol. 2001, 16, 67–79. [Google Scholar]
- Donovan, P.C.; Kramer, R.S. Prophylaxis to Reduce Postoperative Atrial Fibrillation in Cardiac Surgery, NCT00953212. Available online: https://clinicaltrials.gov/ct2/show/NCT00953212 (accessed on 23 November 2018).
- Eslami, M.; Badkoubeh, R.S.; Mousavi, M.; Radmehr, H.; Salehi, M.; Tavakoli, N.; Avadi, M.R. Oral Ascorbic Acid in Combination with Beta-Blockers Is More Effective than Beta-Blockers Alone in the Prevention of Atrial Fibrillation after Coronary Artery Bypass Grafting. Tex. Hear. Inst. J. 2007, 34, 268–274. [Google Scholar]
- Jouybar, R.; Kabgani, H.; Kamalipour, H.; Shahbazi, S.; Allahyary, E.; Rasouli, M.; Akhlagh, S.H.; Shafa, M.; Ghazinoor, M.; Moeinvaziri, M.T.; et al. The perioperative effect of ascorbic acid on inflammatory response in coronary artery bypass graft surgery; a randomized controlled trial coronary artery bypass graft surgery. Int. Cardiovasc. Res. J. 2012, 6, 13–17. [Google Scholar]
- Knodell, R.G.; Tate, M.A.; Akl, B.F.; Wilson, J.W. Vitamin C prophylaxis for posttransfusion hepatitis: Lack of effect in a controlled trial. Am. J. Clin. Nutr. 1981, 34, 20–23. [Google Scholar] [CrossRef]
- Papoulidis, P.; Ananiadou, O.; Chalvatzoulis, E.; Ampatzidou, F.; Koutsogiannidis, C.; Karaiskos, T.; Madesis, A.; Drossos, G. The role of ascorbic acid in the prevention of atrial fibrillation after elective on-pump myocardial revascularization surgery: A single-center experience—A pilot study. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, E. Vitamin C for Prophylaxis of Post-Operative Atrial Fibrillation in On-Pump Cardiac Surgery Procedures, NCT01107730. Available online: https://clinicaltrials.gov/ct2/show/NCT00953212 (accessed on 23 November 2018).
- Babaei, H.; Safaie, N.; Azarfarin, R.; Jodati, A.; Yaghoubi, A.; Sheikhalizadeh, M.-A. Comparative effect of grape seed extract (Vitis vinifera) and ascorbic acid in oxidative stress induced by on-pump coronary artery bypass surgery. Ann. Card. Anaesth. 2017, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sarzaeem, M.; Shayan, N. Vitamin c in prevention of atrial fibrillation after coronary artery bypass graft: Double blind randomized clinical trial. Tehran Univ. Med. J. 2014, 71, 2014. [Google Scholar]
- Geng, J.; Qian, J.; Si, W.; Cheng, H.; Ji, F.; Shen, Z. The clinical benefits of perioperative antioxidant vitamin therapy in patients undergoing cardiac surgery: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Ali-Hassan-Sayegh, S.; Mirhosseini, S.J.; Rezaeisadrabadi, M.; Dehghan, H.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Popov, A.-F.; Liakopoulos, O.J. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: An updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 646–654. [Google Scholar] [CrossRef]
- Stoppe, C.; Ney, J.; Lomivorotov, V.V.; Efremov, S.M.; Benstoem, C.; Hill, A.; Nesterova, E.; Laaf, E.; Goetzenich, A.; McDonald, B.; et al. Prediction of Prolonged ICU Stay in Cardiac Surgery Patients as a Useful Method to Identify Nutrition Risk in Cardiac Surgery Patients: A Post Hoc Analysis of a Prospective Observational Study. J. Parenter. Enter. Nutr. 2018, 43, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Ali-Hassan-Sayegh, S.; Mirhosseini, S.J.; Tahernejad, M.; Mahdavi, P.; Shahidzadeh, A.; Karimi-Bondarabadi, A.A.; Dehghan, A.-M.; Rahimizadeh, E.; Haddad, F.; Ghodratipour, Z.; et al. Impact of antioxidant supplementations on cardio-renal protection in cardiac surgery: An updated and comprehensive meta-analysis and systematic review. Cardiovasc. Ther. 2016, 34, 360–370. [Google Scholar] [CrossRef]
- Baines, M.; Shenkin, A. Use of antioxidants in surgery: A measure to reduce postoperative complications. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 665–670. [Google Scholar] [CrossRef]
- A Carnes, C.; Chung, M.K.; Nakayama, T.; Nakayama, H.; Baliga, R.S.; Piao, S.; Kanderian, A.; Pavia, S.; Hamlin, R.L.; McCarthy, P.M.; et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ. Res. 2001, 89, e32–e38. [Google Scholar] [CrossRef]
- Das, D.; Sen, C.; Goswami, A. Effect of Vitamin C on adrenal suppression by etomidate induction in patients undergoing cardiac surgery: A randomized controlled trial. Ann. Card. Anaesth. 2016, 19, 410–417. [Google Scholar] [CrossRef]
- Dingchao, H.; ZhiDuan, Q.; Liye, H.; Xiaodong, F. The Protective Effects of High-Dose Ascorbic Acid on Myocardium against Reperfusion Injury During and After Cardiopulmonary Bypass. Thorac. Cardiovasc. Surg. 1994, 42, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Ebade, A.; Taha, W.; Saleh, R.; Fawzy, A. Ascorbic acid versus magnesium for the prevention of atrial fibrillation after coronary artery bypass grafting surgery. Egypt. J. Cardiothorac. Anesthesia 2014, 8, 59. [Google Scholar] [CrossRef]
- Kumar, S.; Benjo, A.M.; Pamidimukala, C.K.; Javed, F.; Garcia, W.; Macedo, F.Y.; Garcia, D.C.; Santana, O.; Nascimeto, F.O.; Pierce, M.; et al. Vitamin c decreases atrial fibrillation in cardiac surgery patients but vitamin e may decrease response: A randomized-controlled trial meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 288. [Google Scholar]
- Li, C.C. Changes of creatine phosphokinase and malondialdehyde in the serum and clinical use of large doses of vitamin C following open heart surgery. Chin. J. Surg 1990, 28, 16–17. [Google Scholar] [PubMed]
- Liu, T.; Li, G. Antioxidant interventions as novel preventive strategies for postoperative atrial fibrillation. Int. J. Cardiol. 2009, 145, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Keshavarz, S.; Pakzad, R.; Sedghi, N.; Sadeghi, T.; Alimoradi, F. Effect of vitamin c supplementation in the prevention of atrial fibrillation. Tehran Univ. Med. J. 2016, 73, 791–797. [Google Scholar]
- Oktar, G.L.; Sinci, V.; Kalaycioğlu, S.; Soncul, H.; Gökgöz, L.; Halit, V.; Ersöz, A. Biochemical and hemodynamic effects of ascorbic acid and alpha-tocopherol in coronary artery surgery. Scand. J. Clin. Lab. Investig. 2001, 61, 621–629. [Google Scholar] [CrossRef]
- Rasoli, S.; Kourliouros, A.; Harling, L.; Athanasiou, T. Does prophylactic therapy with antioxidant vitamins have an effect on atrial fibrillation following cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2011, 13, 82–85. [Google Scholar] [CrossRef]
- Rodrigo, R.; Cereceda, M.; Castillo, R.; Asenjo, R.; Zamorano, J.; Araya, J.; Castillo-Koch, R.; Espinoza, J.; Larraín, E. Prevention of atrial fibrillation following cardiac surgery: Basis for a novel therapeutic strategy based on non-hypoxic myocardial preconditioning. Pharmacol. Ther. 2008, 118, 104–127. [Google Scholar] [CrossRef]
- Rodrigo, R.; Vinay, J.; Castillo, R.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Araya, J.; Castillo-Koch, R.; Espinoza, J.; Larraín, E. Use of vitamins C and E as a prophylactic therapy to prevent postoperative atrial fibrillation. Int. J. Cardiol. 2010, 138, 221–228. [Google Scholar] [CrossRef]
- Rebrova, T.I.; Shipulin, V.M.; Afanas’ev, S.A.; Vorob’Eva, E.V.; Kiĭko, O.G. The experience of the application of ascorbinic acid as antioxidant after coronary artery surgery with use of cardiopulmonary bypass. Kardiologiia 2012, 52, 73–76. [Google Scholar] [PubMed]
- Sisto, T.; Paajanen, H.; Metsä-Ketelä, T.; Harmoinen, A.; Nordback, I.; Tarkka, M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann. Thorac. Surg. 1995, 59, 1519–1523. [Google Scholar] [CrossRef]
- Samadikhah, J.; Golzari, S.E.; Sabermarouf, B.; Karimzadeh, I.; Tizro, P.; Khanli, H.M.; Ghabili, K. Efficacy of Combination Therapy of Statin and Vitamin C in Comparison with Statin in the Prevention of Post-CABG Atrial Fibrillation. Adv. Pharm. Bull. 2013, 4, 97–100. [Google Scholar] [PubMed]


| Author and Year | Patients | Dosage and Timing of Vitamin C in the Intervention Group | Route | Control Group | |
|---|---|---|---|---|---|
| 1 | Alshafey 2017 [20] | 100 scheduled CABG | Preop: 2 g daily for at least 3 days pre-operatively | n.a. | Standard of care |
| 2 | Antonic 2016 [24] | 105 elective CABG with CPB | Preop: 2 × 2 g: 24 and 2 h before surgery Postop: 2 × 1 g/day for 5 days | i.v. | Standard of care |
| 3 | Antonic 2017 [25] | 100 elective CABG with CPB | Preop: 2 × 2 g: 24 and 2 h Postop: 2 × 1 g/day for 5 days | i.v. | Standard of care |
| 4 | Bakr 2015 [21] | 200 CABG | Preop: at least one week, dosage and route not specified | n.a. | Standard of care |
| 5 | Bjordahl 2012 [26] | 185 scheduled CABG | Preop: 1 × 2 g night before surgery Postop: 2 × 1 g/day for 5 days | p.o. | Placebo |
| 6 | Colby 2011 [27] | 24 scheduled CABG and/or valvular surgery | Preop: 1 × 2 g night before Postop: 2 × 0.5 g/day for 4 days | p.o. | Placebo |
| 7 | Dehghani 2014 [28] | 100 elective isolated CABG with CPB | Preop: 1 × 2 g before the surgery Postop: 2 × 0.5 g/day for 5 days | p.o. | Standard of care |
| 8 | Demirag 2001 [29] | 30 elective CABG | Group 1: 2 × 50 mg/kg vitamin C after induction and before declamping | i.v. | Standard of care |
| 9 | Donovan 2012 [30] | 150 | Preop: 2 g the morning before surgery Postop: 2 × 1 g for 5 days | p.o. | Standard of care |
| 10 | Eslami 2007 [31] | 100 elective isolated CABG patients with CPB | Preop: 2 g the night before surgery Postop: 2 × 1 g for 5 days | p.o. | Standard of care |
| 11 | Healy 2010 [22] | 60 CABG and/or valve in interim analysis | n.a. | n.a. | Standard of care |
| 12 | Jouybar 2012 [32] | 40 elective CABG | Preop: 2 × 3 g 12–18 h before surgery and after induction of anesthesia | i.v. | Placebo |
| 13 | Knodell 1981 [33] | 175 elective cardiac surgery | Preop: 4 × 800 mg/day for 2 days Postop: 4 × 800 mg/day for 2 weeks, started as soon as patient could take oral liquids | p.o. | Placebo |
| 14 | Papoulidis 2011 [34] | 170 elective isolated CABG with CPB | Preop: 1 × 2 g 3 h prior to initiation of CPB Postop: 2 × 0.5 mg/day for 5 days | i.v. | Placebo |
| 15 | Polymeropoulos 2015 [35] | 22 cardiac surgery with CPB | Preop: 4 x 500 mg/d for 2 days prior to surgery Postop: 4 x 500 mg/d for 4 days | i.v. | Placebo |
| 16 | Sadeghpour 2015 [19] | 290 elective CABG or valve | Preop: 1 × 2 g immediately before surgery Postop: 1 × 1 g/day for 4 days | Preop: i.v. Postop: p.o. | Placebo |
| 17 | Safaei 2017 [36] | 87 elective isolated CABG with CPB | Group 1: 4 × 100 mg GSE 24 h before operation Group 2: 25 mg/kg vitamin C during surgery | i.v. | Standard of care |
| 18 | Sarzaeem 2014 [37] | 170 CABG | Preop: 2 g the night before surgery Postop: 2 × 500 mg/day for 5 days | i.v. | Placebo |
| 19 | Van Wagoner 2003 [23] | 346 CABG | Preop: 2 g the night before surgery Postop: 2 × 500 mg/day for 5 days | n.a. | Placebo |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, A.; Clasen, K.C.; Wendt, S.; Majoros, Á.G.; Stoppe, C.; Adhikari, N.K.J.; Heyland, D.K.; Benstoem, C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2103. https://doi.org/10.3390/nu11092103
Hill A, Clasen KC, Wendt S, Majoros ÁG, Stoppe C, Adhikari NKJ, Heyland DK, Benstoem C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients. 2019; 11(9):2103. https://doi.org/10.3390/nu11092103
Chicago/Turabian StyleHill, Aileen, Kai C. Clasen, Sebastian Wendt, Ádám G. Majoros, Christian Stoppe, Neill K. J. Adhikari, Daren K. Heyland, and Carina Benstoem. 2019. "Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis" Nutrients 11, no. 9: 2103. https://doi.org/10.3390/nu11092103





