Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials
Abstract
1. Introduction
2. Methodology
3. Results
3.1. Literature Search Outcome
3.2. Study Characteristics
3.3. Outcomes
3.3.1. Neutrophil Chemotaxis
3.3.2. Phagocytosis and Oxidative Burst
3.3.3. Neutrophil Enzyme Activities
3.3.4. Neutrophil Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.A. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Introne, W.; Boissy, R.E.; Gahl, W.A. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol. Genet. Metab. 1999, 68, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Spiller, F.; Cunha, F.Q. Neutrophil paralysis in sepsis. Shock 2010, 34 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. Vitamin C in pneumonia and sepsis. In Vissers; Chen, M.Q., Ed.; Oxidative Stress and Disease; Boca Raton Taylor and Francis Group: Boca Raton, FL, USA, 2019; in press. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Goldschmidt, M.C. Reduced bactericidal activity in neutrophils from scorbutic animals and the effect of ascorbic acid on these target bacteria in vivo and in vitro. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1214S–1220S. [Google Scholar] [CrossRef] [PubMed]
- Shilotri, P.G. Glycolytic, hexose monophosphate shunt and bactericidal activities of leukocytes in ascorbic acid deficient guinea pigs. J. Nutr. 1977, 107, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Shilotri, P.G. Phagocytosis and leukocyte enzymes in ascorbic acid deficient guinea pigs. J. Nutr. 1977, 107, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Wilkie, R.P. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. J. Leukoc. Biol. 2007, 81, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef]
- Washko, P.W.; Wang, Y.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar]
- Hume, R.; Weyers, E. Changes in leucocyte ascorbic acid during the common cold. Scott. Med. J. 1973, 18, 3–7. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Butterworth, D.E.; Warren, B.J.; Davis, J.M.; Fagoaga, O.R.; Nehlsen-Cannarella, S.L. Vitamin C supplementation does not alter the immune response to 2.5 hours of running. Int. J. Sport Nutr. 1997, 7, 173–184. [Google Scholar] [CrossRef]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar]
- Peters, E.M.; Bateman, E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S. Afr. Med. J. 1983, 64, 582–584. [Google Scholar]
- Carr, A.C.; Vissers, M.C. Synthetic or food-derived vitamin C—Are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Anderson, R.; Hay, I.; van Wyk, H.A.; Theron, A. Ascorbic acid in bronchial asthma. S. Afr. Med. J. 1983, 63, 649–652. [Google Scholar] [PubMed]
- Charlton, A.J.; Harvey, B.A.; Hatch, D.J.; Soothill, J.F. Neutrophil mobility during anaesthesia in children. A trial of ascorbate premedication. Acta Anaesthesiol. Scand. 1987, 31, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Maderazo, E.G.; Woronick, C.L.; Hickingbotham, N.; Jacobs, L.; Bhagavan, H.N. A randomized trial of replacement antioxidant vitamin therapy for neutrophil locomotory dysfunction in blunt trauma. J. Trauma 1991, 31, 1142–1150. [Google Scholar] [CrossRef]
- Davison, G.; Gleeson, M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 465–479. [Google Scholar] [CrossRef]
- Davison, G.; Gleeson, M. The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur. J. Appl. Physiol. 2006, 97, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.J.; May, M.A.; Martin, K.J. The effect of vitamin E and vitamin C supplementation on LDL oxidizability and neutrophil respiratory burst in young smokers. J. Am. Coll. Nutr. 2000, 19, 361–369. [Google Scholar] [CrossRef]
- Krause, R.; Patruta, S.; Daxbock, F.; Fladerer, P.; Biegelmayer, C.; Wenisch, C. Effect of vitamin C on neutrophil function after high-intensity exercise. Eur. J. Clin. Investig. 2001, 31, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Tauler, P.; Aguilo, A.; Cases, N.; Tur, J.A.; Pons, A. Hypoxia/reoxygenation and vitamin C intake influence NO synthesis and antioxidant defenses of neutrophils. Free Radic. Biol. Med. 2004, 37, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Ferron-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.M.; Bueno, P.; Ferrón, J.A. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 2009, 153, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Herbaczynska-Cedro, K.; Ko-W, B.; Cedro, K.; Wasek, W.; Panczenko-Kresowska, B.; Wartanowicz, M. Supplementation with vitamins C and E suppresses leukocyte oxygen free radical production in patients with myocardial infarction. Eur. Heart J. 1995, 16, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.J.; Bouic, P.J.; Myburgh, K.H. Antioxidant supplementation enhances neutrophil oxidative burst in trained runners following prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.C.; Skinner, M.A.; Wolber, F.M.; Booth, C.L.; Loh, J.M.; Wohlers, M.; Stevenson, L.M.; Kruger, M.C. Consumption of gold kiwifruit reduces severity and duration of selected upper respiratory tract infection symptoms and increases plasma vitamin C concentration in healthy older adults. Br. J. Nutr. 2012, 108, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Sha, W. Ingestion of micronutrient fortified breakfast cereal has no influence on immune function in healthy children: A randomized controlled trial. Nutr. J. 2011, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Wolvers, D.A.; van Herpen-Broekmans, W.M.; Logman, M.H.; van der Wielen, R.P.; Albers, R. Effect of a mixture of micronutrients, but not of bovine colostrum concentrate, on immune function parameters in healthy volunteers: A randomized placebo-controlled study. Nutr. J. 2006, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Tauler, P.; Aguilo, A.; Fuentespina, E.; Tur, J.A.; Pons, A. Diet supplementation with vitamin, E.; vitamin C and beta-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflügers Arch. 2002, 443, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; Demuth, D.R.; Scott, D.A. Tobacco use increases susceptibility to bacterial infection. Tob. Induc. Dis. 2008, 4, 12. [Google Scholar] [CrossRef]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef]
- Dietrich, M.; Block, G.; Norkus, E.P.; Hudes, M.; Traber, M.G.; Cross, C.E.; Packer, L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am. J. Clin. Nutr. 2003, 77, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Loft, S.; Nielsen, J.B.; Poulsen, H.E. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. Am. J. Clin. Nutr. 1997, 65, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: Associations with glycaemic control, obesity, and smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar]
- Kinnula, V.L.; Soini, Y.; Kvist-Makela, K.; Savolainen, E.R.; Koistinen, P. Antioxidant defense mechanisms in human neutrophils. Antioxid. Redox Signal. 2002, 4, 27–34. [Google Scholar] [CrossRef]
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006, 40, 689–697. [Google Scholar] [CrossRef]
- Schectman, G.; Byrd, J.C.; Hoffmann, R. Ascorbic acid requirements for smokers: Analysis of a population survey. Am. J. Clin. Nutr. 1991, 53, 1466–1470. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef]
- Hoffman-Goetz, L.; Pedersen, B.K. Exercise and the immune system: A model of the stress response? Immunol. Today 1994, 15, 382–387. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. Duration of intravenous vitamin C therapy is a critical consideration. Crit. Care Resusc. 2019, 21, 220–221. [Google Scholar] [PubMed]
- Lykkesfeldt, J.; Poulsen, H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 2010, 103, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 1, CD000980. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013, Cd005532. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Zhao, W.; Wang, J.; Wang, H.; Zhao, Y.; Tseng, Y.; Bu, H. Extra dose of vitamin C based on a daily supplementation shortens the common cold: A meta-analysis of 9 randomized controlled trials. BioMed Res. Int. 2018, 2018, 1837634. [Google Scholar] [CrossRef] [PubMed]
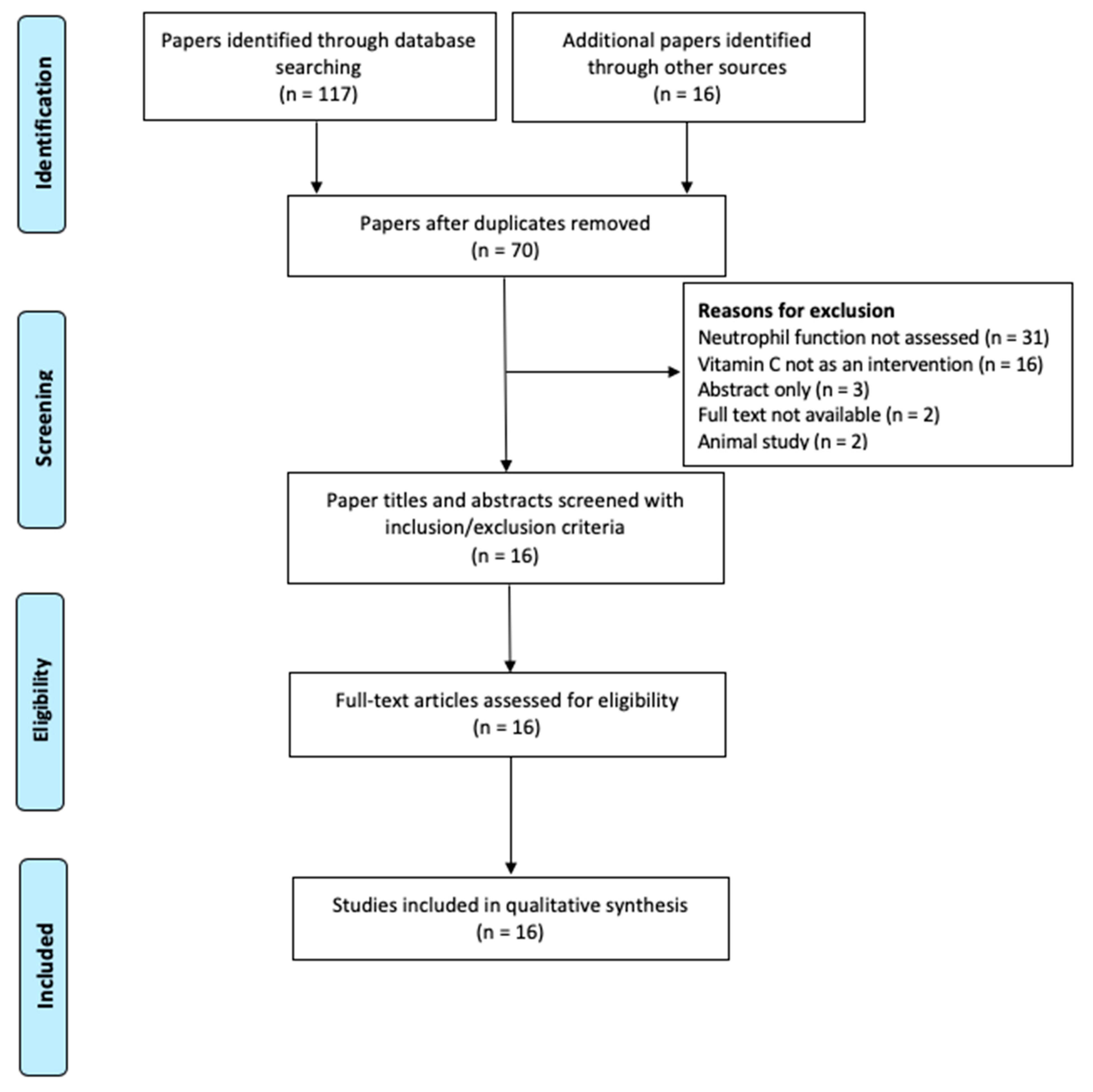
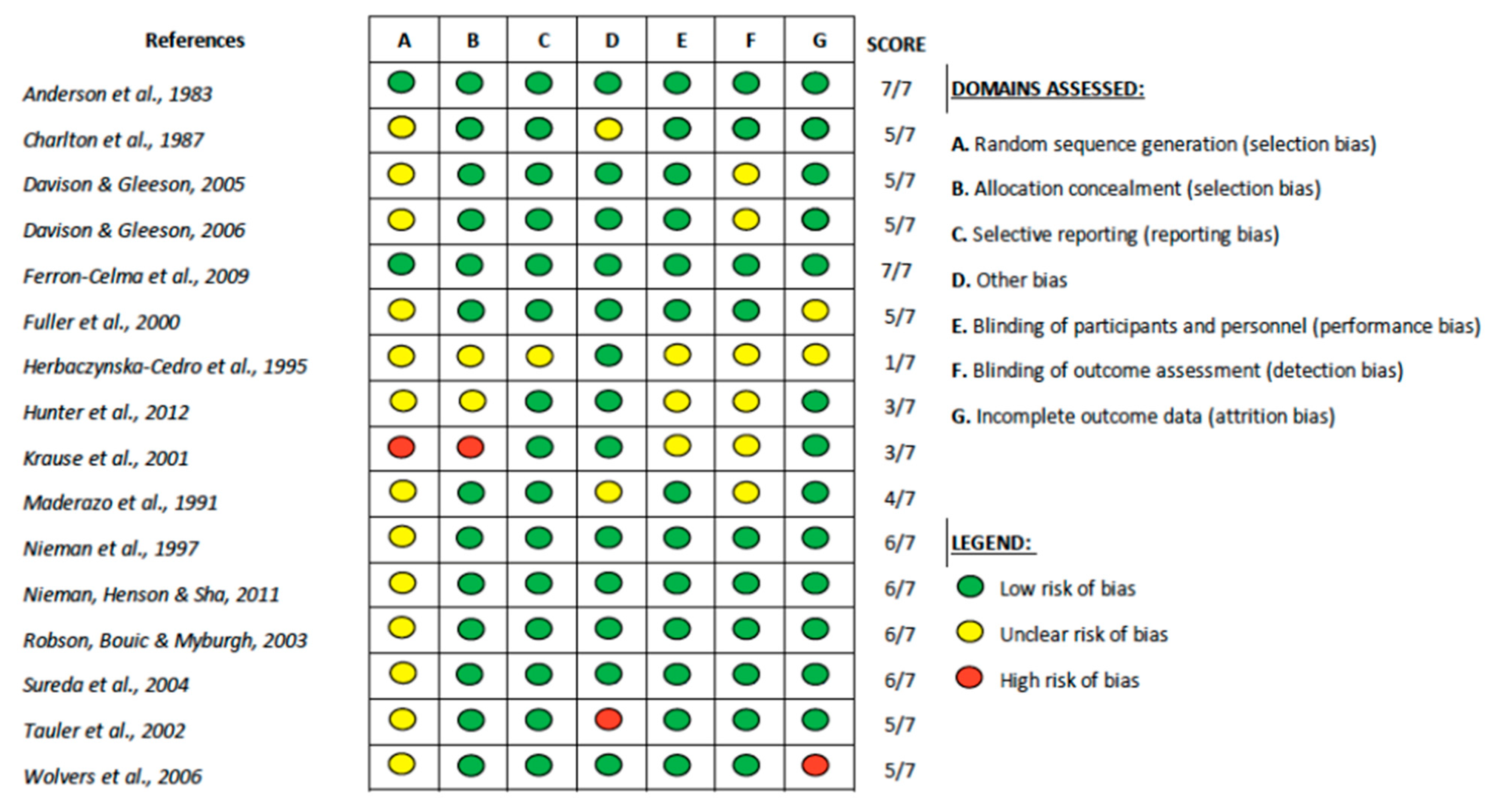
| Inclusion | Exclusion |
|---|---|
| Randomized Controlled Trial Peer-reviewed publication Human Subjects Full-text access English papers Any of the following neutrophil functions as primary or secondary outcomes: Migration/chemotaxis/motility Phagocytosis Oxidative burst Enzyme activity Apoptosis/clearance Necrosis/necrotic cell death | Participants with existing neutrophilic dysfunction disorders Review articles |
| Reference | Title of Study | Location | Trial Setting | Time Frame of Study | Neutrophil Function Assessed |
|---|---|---|---|---|---|
| Anderson et al. (1983) [25] | Ascorbic acid in bronchial asthma | Africa | Hospital Paediatric Respiratory Clinic | 6 months | Chemotaxis, phagocytosis (isolated cells) |
| Charlton et al. (1987) [26] | Neutrophil mobility during anaesthesia in children. A trial for ascorbate premedication. | United Kingdom | Surgical Hospital | 1 day | Chemotaxis (isolated cells) |
| Maderazo et al. (1991) [27] | A randomized trial of replacement antioxidant vitamin therapy for neutrophil locomotory dysfunction in blunt trauma | United States | Hospital | 1 week | Chemotaxis (isolated cells) |
| Davison and Gleeson (2005) [28] | Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. | United Kingdom | Laboratory | 3 weeks | Oxidative burst (whole blood) |
| Davison and Gleeson (2006) [29] | The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. | United Kingdom | Laboratory | 2 weeks | Oxidative burst (whole blood) |
| Fuller et al. (2000) [30] | The effect of vitamin E and vitamin C supplementation on LDL oxidizability and neutrophil respiratory burst in young smokers | United States (North Carolina) | Community | 8 weeks | Oxidative burst (isolated cells) |
| Krause et al. (2001) [31] | Effect of vitamin C on neutrophil function after high-intensity exercise | Austria | Outdoor Biathlon | 1 week | Phagocytosis; oxidative burst (isolated cells and whole blood) |
| Nieman et al. (1997) [19] | Vitamin C supplementation does not alter the immune response to 2.5 h of running | United States (North Carolina) | Human Performance Laboratory | 8 days | Phagocytosis; oxidative burst (whole blood) |
| Sureda et al. (2004) [32] | Hypoxia/reoxygenation and vitamin C intake influence NO synthesis and antioxidant defences of neutrophils. | Spain | Not specified | 1 week | Enzyme activity (isolated cells) |
| Ferron-Celma et al. (2009) [33] | Effects of vitamin C administration on neutrophil apoptosis in patients after abdominal surgery. | Spain | Digestive Surgery Department | 6 days | Apoptosis (isolated cells) |
| Reference | Title of Study | Country | Trial Setting | Time Frame of Study | Neutrophil Function Assessed |
|---|---|---|---|---|---|
| Herbaczynska-Cedro et al. (1995) [34] | Supplementation with vitamins C and E suppresses leukocyte oxygen free radical production in patients with myocardial infarction | Poland | Hospital | 2 weeks | Oxidative burst (isolated cells) |
| Robson et al. (2003) [35] | Antioxidant supplementation enhances neutrophil oxidative burst in trained runners following prolonged exercise. | South Africa | Laboratory | 7 weeks | Oxidative burst (whole blood) |
| Hunter et al. (2012) [36] | Consumption of gold kiwifruit reduces severity and duration of selected upper respiratory tract infection symptoms and increases plasma vitamin C concentration in healthy older adults | New Zealand | Community | 20 weeks | Phagocytosis (whole blood) |
| Nieman et al. (2011) [37] | Ingestion of micronutrient fortified breakfast cereal has no influence on immune function in healthy children: A randomized controlled trial | United States (North Carolina) | Community | 8 weeks | Phagocytosis; oxidative burst (whole blood) |
| Wolvers et al. (2006) [38] | Effect of a mixture of micronutrients, but not of bovine colostrum concentrate, on immune function parameters in healthy volunteers: a randomized placebo-controlled study. | The Netherlands | Unilever Food and Health Research Institute | 12 weeks | Phagocytosis; oxidative burst (whole blood) |
| Tauler et al. (2002) [39] | Diet supplementation with vitamin E, vitamin C and B-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. | Spain | Not specified | 12 weeks | Enzyme activity (isolated cells) |
| References | Participant Characteristics | Intervention and Dose Administered | Frequency of Intervention | Route of Administration | ||
|---|---|---|---|---|---|---|
| Number (n) | Mean Age (Years) | Gender (% Women) | ||||
| Anderson et al. (1983) [25] | n = 16 asthmatic children | 9.5 | 25% | Vitamin C (1000 mg/day) with standard anti-asthma chemoprophylaxis (SAC) OR SAC only | Once daily for six months | Intravenous |
| Charlton et al. (1987) [26] | n = 20 surgical patients | 10 | - | Vitamin C (10 mg/kg; mean = 363 mg) OR placebo | One-off | Intravenous |
| Maderazo et al. (1991) [27] | n = 46 trauma patients | 24 | 21% | Vitamin C (200 mg/day) OR vitamin E (50 mg/day) OR both OR placebo | Once daily for one week | Intravenous |
| Davison and Gleeson (2005) [28] | n = 6 healthy athletes | 25 | 0% | Vitamin C (3400 mg) OR carbohydrate OR both OR placebo | One-off for each intervention (crossover study) | Oral |
| Davison and Gleeson (2006) [29] | n = 9 healthy athletes | 26 | 0% | Vitamin C (1000 mg/day) OR placebo | Once daily for two weeks | Oral |
| Fuller et al. (2000) [30] | n = 30 healthy smokers | 20 | 73% | Vitamin C (1000 mg/day) OR vitamin E (400 IU/day) OR both OR placebo | Once daily for eight weeks | Oral |
| Krause et al. (2001) [31] | n = 10 healthy adults | 29 | 0% | Vitamin C (2000 mg/day) OR none | Once daily for one week | Oral |
| Nieman et al. (1997) [19] | n = 12 healthy athletes | 41 | 25% | Vitamin C (1000 mg/day) OR placebo | Once daily for eight days | Oral |
| Sureda et al. (2004) [32] | n = 7 healthy divers | - | 0% | Vitamin C (1000 mg/day) OR placebo | Once daily for one week | Oral |
| Ferron-Celma et al. (2009) [33] | n = 20 surgical patients | 67 | 45% | Vitamin C (450 mg/day) OR placebo | Once daily for six days post-operative | Intravenous |
| References | Participant Characteristics | Intervention and Dose Administered | Frequency of Intervention | Route of Administration | ||
|---|---|---|---|---|---|---|
| Number (n) | Mean Age (Years) | Gender (% Women) | ||||
| Herbaczynska-Cedro et al. (1995) [34] | n = 45 cardiac patients | 59 | 13% | Vitamins C and E (600 mg/day) OR conventional treatment only | Once daily for two weeks | Oral |
| Robson et al. (2003) [35] | n = 12 healthy athletes | 30 | 50% | Multivitamin supplement: vitamin C content 60 mg/day AND antioxidant supplement: vitamin C content 900 mg/day OR placebo | Once daily for one week | Oral |
| Hunter et al. (2012) [36] | n = 32 healthy elderly | 71 | 63% | 2 fresh Gold kiwifruit AND 2 freeze dried Gold kiwifruit (comprising total of ~360 mg vitamin C) OR 2 freeze dried bananas | Once daily for four weeks; crossover (8 weeks washout) | Oral |
| Nieman et al. (2011) [37] | n = 65 healthy children | 10 | 43% | Cereal fortified with micronutrients PLUS: Low: vitamin C content of 0.8 mg/day Medium: vitamin C content of 20 mg/day High: vitamin C content of 100 mg/day | Once daily for two months | Oral |
| Wolvers et al. (2006) [38] | n = 131 healthy volunteers | 57 | 68% | Micronutrient mix (with vitamin C ~375 mg/day) OR bovine colostrum OR both OR placebo | Once daily for ten weeks | Oral |
| Tauler et al. (2002) [39] | n = 20 healthy athletes | 23 | 0% | Antioxidant cocktail (vitamin E and β-carotene) PLUS vitamin C 500 mg/day (only in last 15 days) OR placebo | Once daily for three months (only last 15 days for vitamin C) | Oral |
| References | Mean Plasma Vitamin C Levels | Diet Control | Smoking Status | |
|---|---|---|---|---|
| Baseline | Post-Intervention | |||
| Vitamin C-Only Studies | ||||
| Anderson et al. (1983) [25] | ~61 µmol/L | ~137 µmol/L | Not controlled | - |
| Charlton et al. (1987) [26] | - | - | Not controlled | - |
| Maderazo et al. (1991) [27] | ~51 µmol/L (~25 µmol/L day 1) | ~54 µmol/L | Nutrition by mouth or feeding tube as needed (parenteral nutrition excluded until end of study) | - |
| Davison and Gleeson (2005) [28] | - | - | 24-hour food diary of diet prior to exercise trials and maintained during trials | - |
| Davison and Gleeson (2006) [29] | - | 92 µmol/L | 24-hour food diary prior to exercise trials and maintained during trials | Non-smokers |
| Fuller et al. (2000) [30] | 39 µmol/L | 62 µmol/L | 3-day food record completed and maintained usual diet | All smokers |
| Krause et al. (2001) [31] | - | - | not controlled | - |
| Nieman et al. (1997) [19] | - | - | 7-day food record, carbohydrate intake ~60% of total energy, moderate vitamin C intake (~100 mg/day); refrained from nutrient supplement use | - |
| Sureda et al. (2004) [32] | 30 µmol/L | 80 µmol/L | 7-day 24-hour recall of dietary intake and maintained during trials (Mediterranean diet) | - |
| Ferron-Celma et al. (2009) [33] | - | - | Not controlled | - |
| Combination Studies | ||||
| Herbaczynska-Cedro et al. (1995) [34] | 38 µmol/L | 77 µmol/L | Not controlled | 58% smokers |
| Robson et al. (2003) [35] | ~70 µmol/L | ~90 µmol/L | Dietary intake recorded a week before exercise trials and maintained | - |
| Hunter et al. (2012) [36] | - | 73 µmol/L | Refrained from consumption of vitamin C supplements, kiwifruit and kiwifruit products | Non-smokers |
| Nieman et al. (2011) [37] | - | - | 3-day food record pre-study, at 1 month and at 2 months | - |
| Wolvers et al. (2006) [38] | ~37 µmol/L | ~70 µmol/L | Maintained Dutch dietary habits | Non-smokers |
| Tauler et al. (2002) [39] | ~57 µmol/L | ~94 µmol/L | Not controlled | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liugan, M.; Carr, A.C. Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients 2019, 11, 2102. https://doi.org/10.3390/nu11092102
Liugan M, Carr AC. Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients. 2019; 11(9):2102. https://doi.org/10.3390/nu11092102
Chicago/Turabian StyleLiugan, Mikee, and Anitra C. Carr. 2019. "Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials" Nutrients 11, no. 9: 2102. https://doi.org/10.3390/nu11092102
APA StyleLiugan, M., & Carr, A. C. (2019). Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients, 11(9), 2102. https://doi.org/10.3390/nu11092102






