Population Approaches Targeting Metabolic Syndrome Focusing on Japanese Trials
Abstract
:1. Introduction
2. Obesity and Obesity Disease
3. Visceral Fat and Subcutaneous Fat
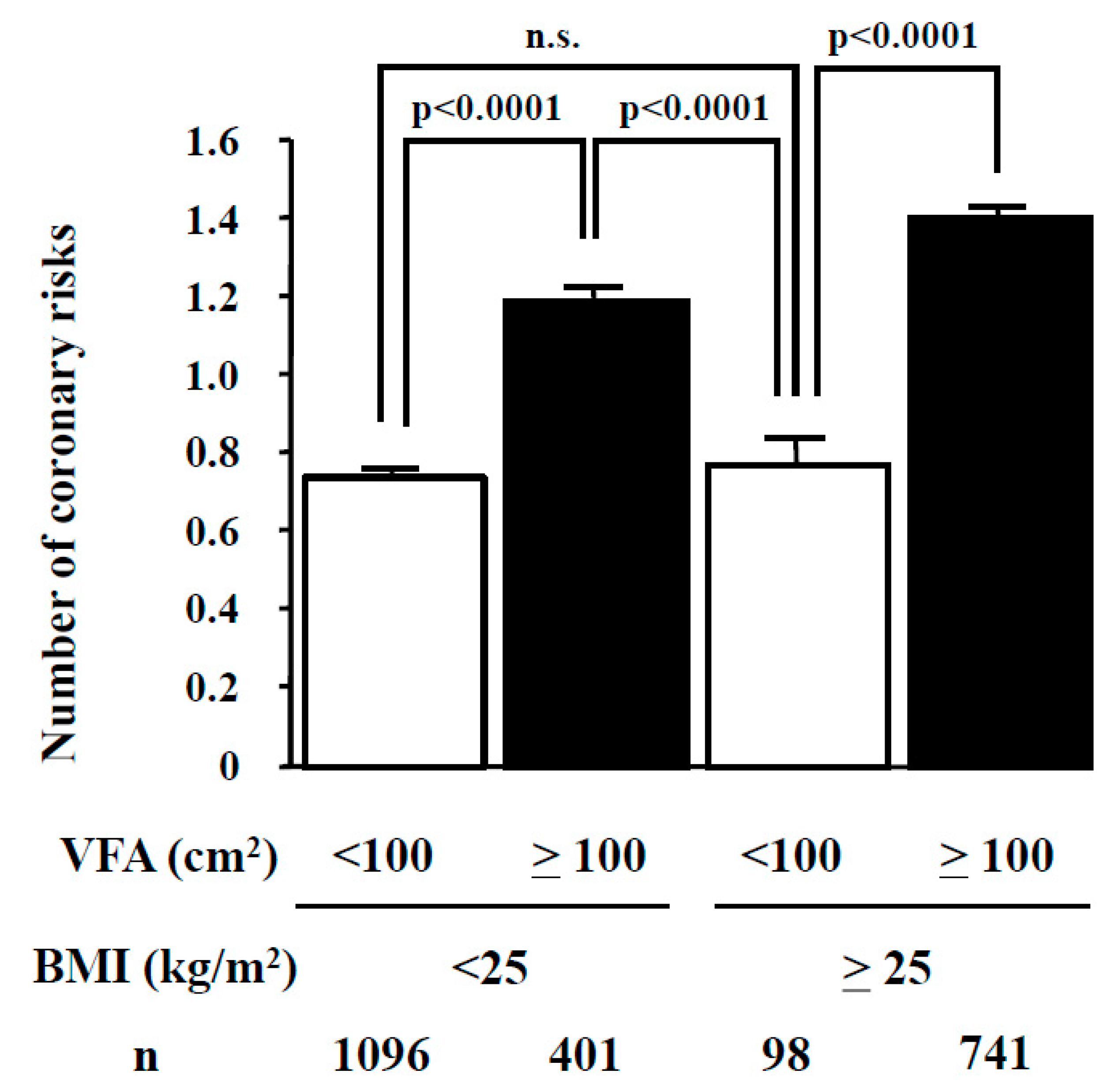
4. BMI and Visceral Fat Area
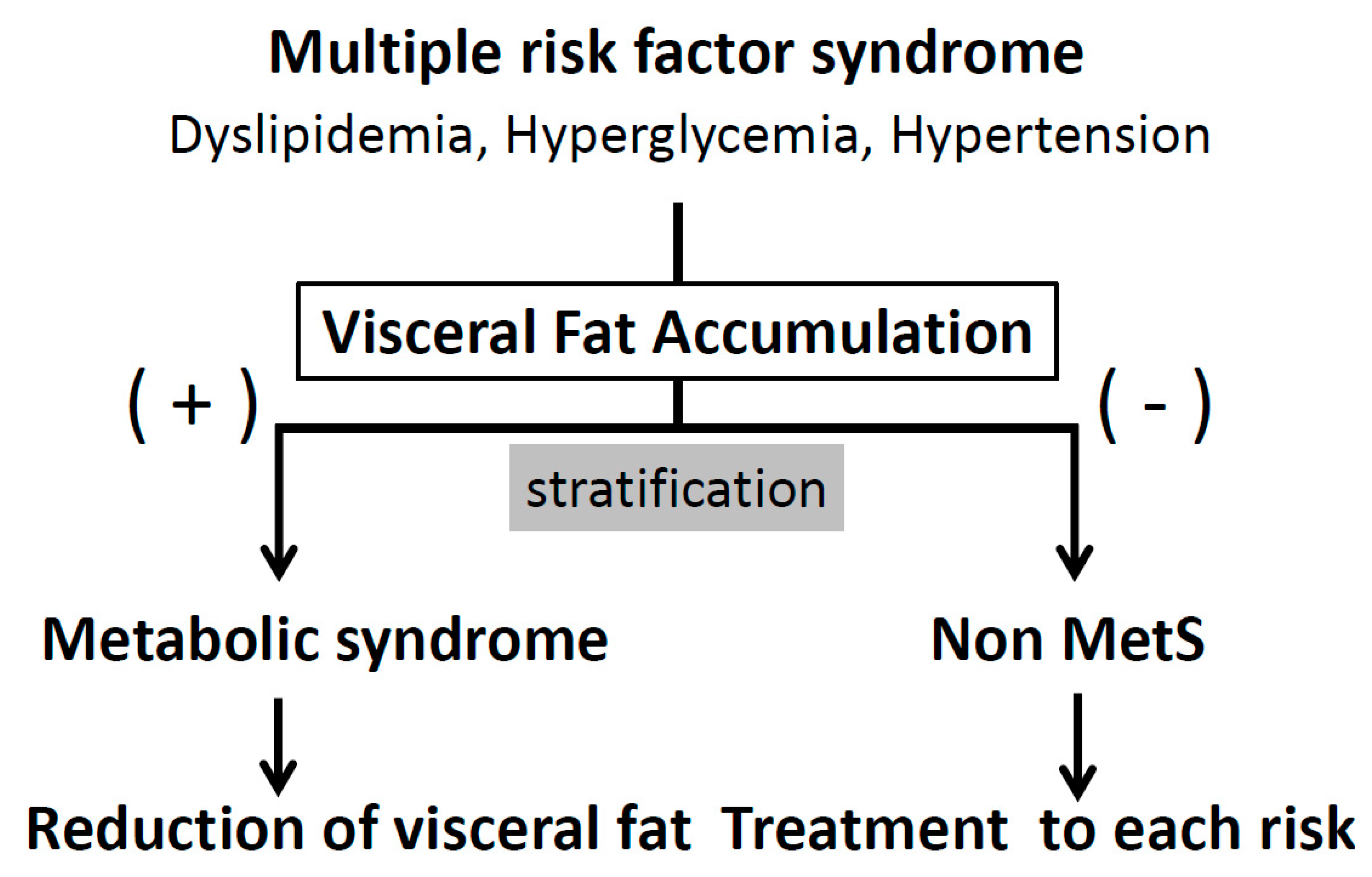
5. Clinical Significance of Metabolic Syndrome in Atherosclerotic Cardiovascular Diseases
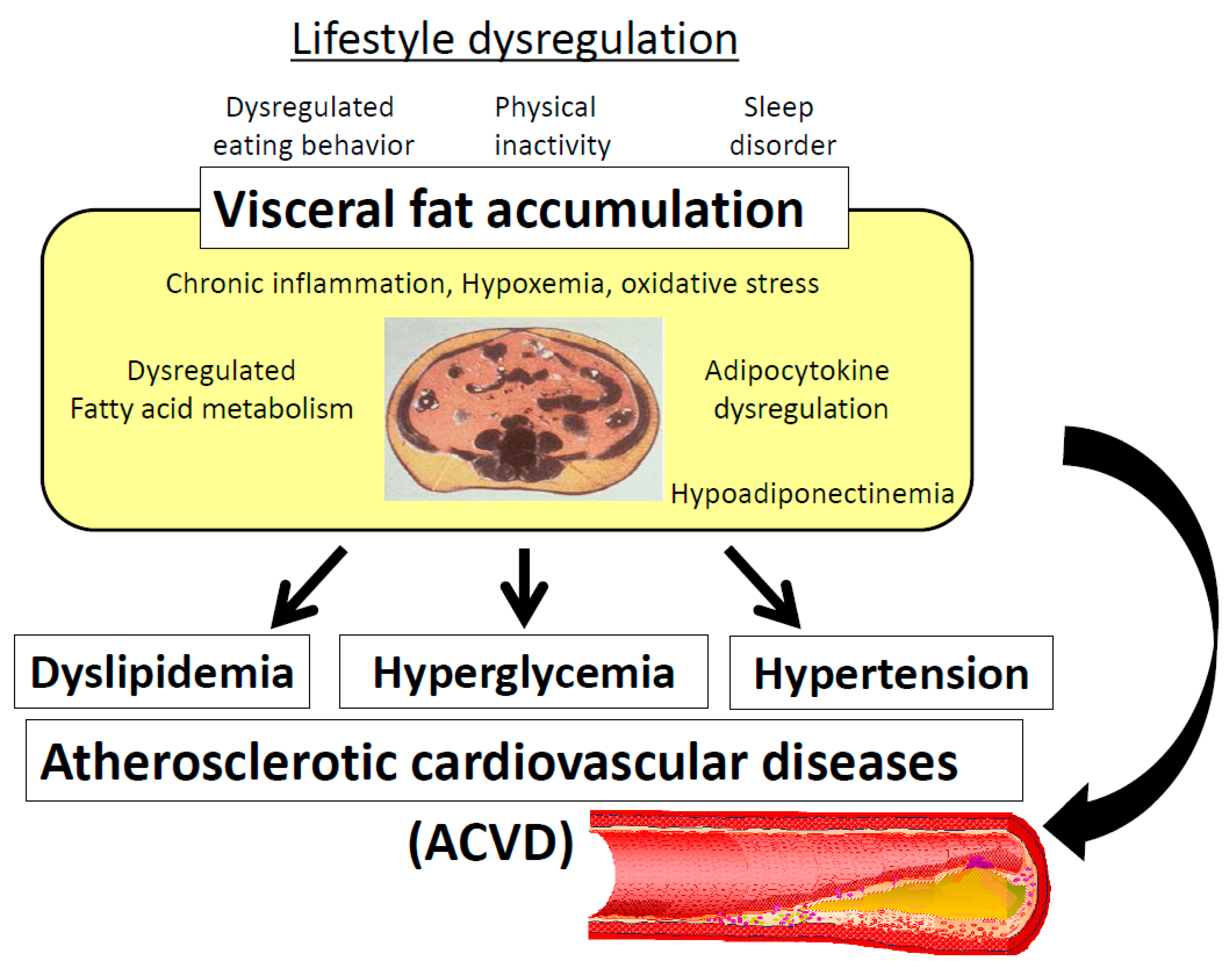
6. Pathogenesis of Visceral Fat Accumulation
7. Population Approaches Targeting the Metabolic Syndrome
8. Strategies Addressing Lifestyle Behavior and Policies Targeting the Environment (Diet, Physical Activity, Sleep, and Mental Health)
9. Screening and Intervention Program Against the Metabolic Syndrome
9.1. Community or Organization-Based Prevention
9.2. Healthcare Program by the Public System
9.3. Behavioral Approach Including Motivational Interviewing
10. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACVD | atherosclerotic cardiovascular disease |
| BMI | body mass index |
| Mets | metabolic syndrome |
| SFA | subcutaneous fat area |
| VFA | visceral fat area |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Metabolic syndrome--definition and diagnostic criteria in Japan. J. Atheroscler. Thromb. 2005, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Fujioka, S.; Matsuzawa, Y.; Tokunaga, K.; Tarui, S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987, 36, 54–59. [Google Scholar] [CrossRef]
- Hiuge-Shimizu, A.; Kishida, K.; Funahashi, T.; Ishizaka, Y.; Oka, R.; Okada, M.; Suzuki, S.; Takaya, N.; Nakagawa, T.; Fukui, T.; et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (The VACATION-J study). Ann. Med. 2012, 44, 82–92. [Google Scholar] [CrossRef]
- Okauchi, Y.; Nishizawa, H.; Funahashi, T.; Ogawa, T.; Noguchi, M.; Ryo, M.; Kihara, S.; Iwahashi, H.; Yamagata, K.; Nakamura, T.; et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care 2007, 30, 2392–2394. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; O’Donovan, G.; Stensel, D.; Stamatakis, E. Normal-weight central obesity and risk for mortality. Ann. Intern. Med. 2017, 166, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X.; Blackburn, G.; Brancati, F.L.; Bray, G.A.; Bright, R.; Clark, J.M.; Curtis, J.M.; Espeland, M.A.; Foreyt, J.P.; Graves, K.; et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2007, 30, 1374–1383. [Google Scholar] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP)Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421.
- Iso, H. A Japanese health success story: Trends in cardiovascular diseases, their risk factors, and the contribution of public health and personalized approaches. EPMA J. 2011, 2, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Mizuno, K.; Matsuzawa, Y.; Matsubara, K. Analysis of an expression profile of genes in the human adipose tissue. Gene 1997, 190, 227–235. [Google Scholar] [CrossRef]
- Nyamdorj, R.; Pitkäniemi, J.; Tuomilehto, J.; Hammar, N.; Stehouwer, C.D.; Lam, T.H.; Ramachandran, A.; Janus, E.D.; Mohan, V.; Söderberg, S.; et al. Ethnic comparison of the association of undiagnosed diabetes with obesity. Int. J. Obes. 2010, 34, 332–339. [Google Scholar] [CrossRef]
- Kadowaki, T.; Sekikawa, A.; Murata, K.; Maegawa, H.; Takamiya, T.; Okamura, T.; El-Saed, A.; Miyamatsu, N.; Edmundowicz, D.; Kita, Y.; et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int. J. Obes. (Lond) 2006, 30, 1163–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, E913. [Google Scholar] [CrossRef] [PubMed]
- Näslund, U.; Ng, N.; Lundgren, A.; Fhärm, E.; Grönlund, C.; Johansson, H.; Lindahl, B.; Lindahl, B.; Lindvall, K.; Nilsson, S.K.; et al. VIPVIZA trial group. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): A pragmatic, open-label, randomised controlled trial. Lancet 2019, 393, 133–142. [Google Scholar]
- Neumark-Sztainer, D.; Wall, M.; Larson, N.I.; Eisenberg, M.E.; Loth, K. Dieting and disordered eating behaviors from adolescence to young adulthood: Findings from a 10-year longitudinal study. J. Am. Diet. Assoc. 2011, 111, 1004–1011. [Google Scholar] [CrossRef]
- Shah, R.; Kennedy, S.; Clark, M.D.; Bauer, S.C.; Schwartz, A. Primary care-based interventions to promote positive parenting behaviors: A meta-analysis. Pediatrics 2016, 137, e20153393. [Google Scholar] [CrossRef]
- Ewart-Pierce, E.; Mejía Ruiz, M.J.; Gittelsohn, J. “Whole-of-Community” Obesity Prevention: A review of challenges and opportunities in multilevel, multicomponent interventions. Curr. Obes. Rep. 2016, 5, 361–374. [Google Scholar] [CrossRef]
- Cornelsen, L.; Green, R.; Dangour, A.; Smith, R. Why fat taxes won’t make us thin. J. Public Health (Oxford) 2015, 37, 18–23. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R.M. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Giles-Corti, B.; Vernez-Moudon, A.; Reis, R.; Turrell, G.; Dannenberg, A.L.; Badland, H.; Foster, S.; Lowe, M.; Sallis, J.F.; Stevenson, M.; et al. City planning and population health: A global challenge. Lancet 2016, 388, 2912–2924. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Clarke, J.; Janssen, I. Is the frequency of weekly moderate-to-vigorous physical activity associated with the metabolic syndrome in Canadian adults? Appl. Physiol. Nutr. Metab. 2013, 38, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Pract. Res. Endcrinol. Metab. 2010, 24, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, S.; Hirata, A.; Nishizawa, H.; Nagao, H.; Kimura, T.; Fujishima, Y.; Yamaoka, M.; Kozawa, J.; Imagawa, A.; Funahashi, T.; et al. Characteristics of sleep-wake cycle and sleep duration in Japanese type 2 diabetes patients with visceral fat accumulation. J. Diabetes Invest. 2018, 9, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, E.S.; O’Connor, E.; Whitlock, E.P.; Patnode, C.D.; Kapka, T. Effectiveness of primary care-relevant treatments for obesity in adults: A systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.; Wyke, S.; Gray, C.M.; Anderson, A.S.; Brady, A.; Bunn, C.; Donnan, P.T.; Fenwick, E.; Grieve, E.; Leishman, J.; et al. A gender-sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): A pragmatic randomised controlled trial. Lancet 2014, 383, 1211–1221. [Google Scholar] [CrossRef]
- Kohro, T.; Furui, Y.; Mitsutake, N.; Fujii, R.; Morita, H.; Oku, S.; Ohe, K.; Nagai, R. The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int. Heart J. 2008, 49, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.A.; Redaelli, M.; Lauterbach, K.W. Disease management and health care reforms in Germany - does more competition lead to less solidarity? Health Policy 2007, 80, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tsushita, K.; Hosler, A.S.; Miura, K.; Ito, Y.; Fukuda, T.; Kitamura, A.; Tatara, K. Rationale and descriptive analysis of specific health guidance: The nationwide lifestyle intervention program targeting metabolic syndrome in Japan. J. Atheroscler. Thromb. 2018, 25, 308–322. [Google Scholar] [CrossRef]
- Gregg, E.W.; Ali, M.K.; Moore, B.A.; Pavkov, M.; Devlin, H.M.; Garfield, S.; Mangione, C.M. The importance of natural experiments in diabetes prevention and control and the need for better health policy research. Prev. Chronic Dis. 2013, 10, E14. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Kojima, S.; Sairenchi, T.; Kinuta, M.; Yamakawa, M.; Nishizawa, H.; Takahara, M.; Imano, H.; Kitamura, A.; Yoshida, T.; et al. Study Profile: Japan Trial in High-risk Individuals to Accelerate their Referral to Physicians (J-HARP)—A Nurse-led, Community-based Prevention Program of Lifestyle-related Disease. J. Epidemiol. in press.
- Kelley, C.P.; Sbrocco, G.; Sbrocco, T. Behavioral modification for the management of obesity. Prim. Care 2016, 43, 159–175. [Google Scholar] [CrossRef]
- Ryo, M.; Nakamura, T.; Funahashi, T.; Noguchi, M.; Kishida, K.; Okauchi, Y.; Nishizawa, H.; Ogawa, T.; Kojima, S.; Ohira, T.; et al. Health education “Hokenshido” program reduced metabolic syndrome in the Amagasaki visceral fat study. Three-year follow-up study of 3,174 Japanese employees. Intern. Med. 2011, 50, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F. A series of studies examining internet treatment of obesity to inform Internet interventions for substance use and misuse. Subst. Use Misuse 2011, 46, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Hirata, A.; Nishizawa, H.; Nagao, H.; Kashine, S.; Kimura, T.; Inoue, K.; Fujishima, Y.; Yamaoka, M.; Kozawa, J.; et al. Systemic arteriosclerosis and eating behavior in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2015, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I. Visceral adiposity as a target for the metabolic syndrome. Ann. Med. 2012, 44, 233–241. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishizawa, H.; Shimomura, I. Population Approaches Targeting Metabolic Syndrome Focusing on Japanese Trials. Nutrients 2019, 11, 1430. https://doi.org/10.3390/nu11061430
Nishizawa H, Shimomura I. Population Approaches Targeting Metabolic Syndrome Focusing on Japanese Trials. Nutrients. 2019; 11(6):1430. https://doi.org/10.3390/nu11061430
Chicago/Turabian StyleNishizawa, Hitoshi, and Iichiro Shimomura. 2019. "Population Approaches Targeting Metabolic Syndrome Focusing on Japanese Trials" Nutrients 11, no. 6: 1430. https://doi.org/10.3390/nu11061430



