Magnesium: Are We Consuming Enough?
Abstract
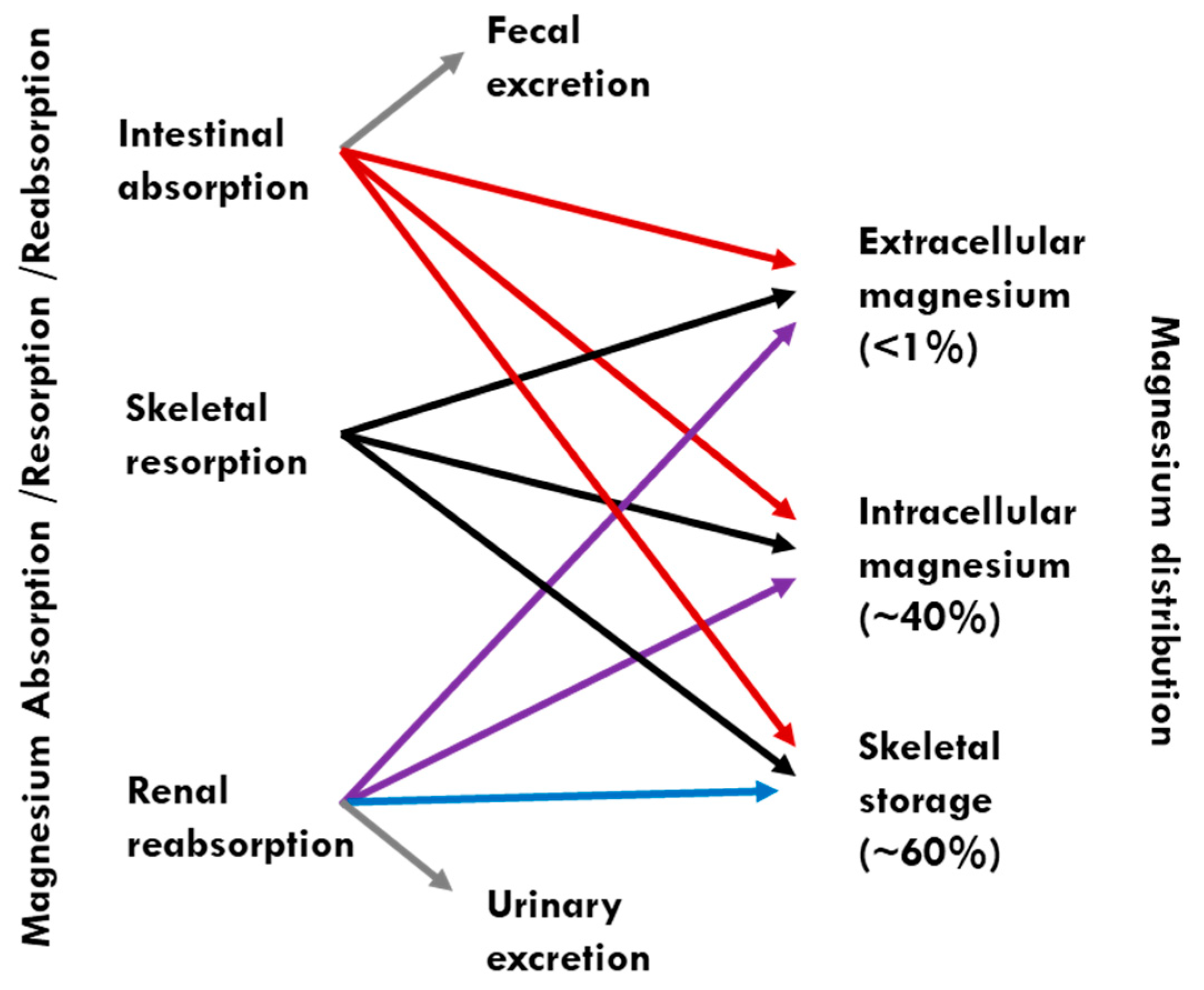
1. Introduction
- ▪
- Does serum magnesium level reflect total body magnesium status?
- ▪
- Normal level of serum magnesium does not rule out moderate to severe magnesium deficiency.
- ▪
- Normal level of serum magnesium does not rule out moderate to severe magnesium deficiency.
2. Sources of Magnesium
3. Magnesium Deficiency
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Bone-kidney axis in systemic phosphate turnover. Arch. Biochem. Biophys. 2014, 561, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Shi, P.; Mumena, C.H.; Haq, A.; Razzaque, M.S. Dietary phosphorus burden increases cariogenesis independent of vitamin D uptake. J. Steroid Biochem. Mol. Biol. 2017, 167, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Erem, S.; Razzaque, M.S. Dietary phosphate toxicity: An emerging global health concern. Histochem. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Phosphate toxicity: New insights into an old problem. Clin. Sci. 2011, 120, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011, 79, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Fgf23-mediated regulation of systemic phosphate homeostasis: Is klotho an essential player? Am. J. Physiol. Renal Physiol. 2009, 296, F470–F476. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The fgf23-klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The metacyc database of metabolic pathways and enzymes and the biocyc collection of pathway/genome databases. Nucleic Acids Res. 2012, 40, D742–D753. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Noronha, J.L.; Matuschak, G.M. Magnesium in critical illness: Metabolism, assessment, and treatment. Intensive Care Med. 2002, 28, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Houillier, P. Mechanisms and regulation of renal magnesium transport. Annu. Rev. Physiol. 2014, 76, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Weglicki, W.B.; Mak Iu, T.; Chmielinska, J.J.; Tejero-Taldo, M.I.; Komarov, A.M.; Kramer, J.H. The role of magnesium deficiency in cardiovascular and intestinal inflammation. Magnes. Res. 2010, 23, S199–S206. [Google Scholar] [PubMed]
- De Rouffignac, C.; Quamme, G. Renal magnesium handling and its hormonal control. Physiol. Rev. 1994, 74, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Wolf, F.I. A guided tour of presentations at the xiv international magnesium symposium. Magnes. Res. 2016, 29, 55–59. [Google Scholar] [PubMed]
- Nielsen, F.H. Guidance for the determination of status indicators and dietary requirements for magnesium. Magnes. Res. 2016, 29, 154–160. [Google Scholar] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The case for an evidence-based reference interval for serum magnesium: The time has come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Nes, M.; Ronneberg, R.; Midtvedt, K.; Falch, D.; Kjekshus, J. Magnesium status in healthy free-living elderly norwegians. J. Am. Coll. Nutr. 1994, 13, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Midtvedt, K.; Dolva, L.O.; Norseth, J.; Kjekshus, J. The magnesium loading test: Reference values in healthy subjects. Scand. J. Clin. Lab. Investig. 1994, 54, 23–31. [Google Scholar] [CrossRef]
- Ozono, R.; Oshima, T.; Matsuura, H.; Higashi, Y.; Ishida, T.; Watanabe, M.; Yoshimura, M.; Hiraga, H.; Ono, N.; Kajiyama, G. Systemic magnesium deficiency disclosed by magnesium loading test in patients with essential hypertension. Hypertens. Res. 1995, 18, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S. Evolving concepts in epithelial magnesium transport. Curr. Opin. Nephrol. Hypertens. 2001, 10, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Magnesium intake and risk of type 2 diabetes: A meta-analysis. J. Intern. Med. 2007, 262, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Wang, K.C.; Hayward, S. Serum magnesium concentrations in the canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients 2017, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated serum magnesium associated with sglt2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, D.; Heimbeck, I.; Herpich, C.; Naue, N.; Höfler, J.; Timmer, W.; Michalke, B. Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr. 2017, 3, 7. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, S.M.; Rouhi-Boroujeni, H.; Kheiri, M.; Mobasheri, M.; Shirani, M.; Ahrani, S.; Karami, J.; Hafshejani, Z.K. Effect of topical application of the cream containing magnesium 2% on treatment of diaper dermatitis and diaper rash in children a clinical trial study. J. Clin. Diagn. Res. 2016, 10, WC04–WC06. [Google Scholar] [CrossRef] [PubMed]
- Hirschfelder, A.D.; Haury, V.G. Clinical manifestations of high and low plasma magnesium: Dangers of epsom salt purgation in nephritis. J. Am. Med. Assoc. 1934, 102, 1138–1141. [Google Scholar] [CrossRef]
- Risco, F.; Traba, M.L. Possible involvement of a magnesium dependent mitochondrial alkaline phosphatase in the regulation of the 25-hydroxyvitamin D3-1 alpha-and 25-hydroxyvitamin D3-24r-hydroxylases in llc-pk1 cells. Magnes. Res. 1994, 7, 169–178. [Google Scholar] [PubMed]
- Risco, F.; Traba, M.L. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes. Res. 1992, 5, 5–14. [Google Scholar] [PubMed]
- Brown, R.B.; Haq, A.; Stanford, C.F.; Razzaque, M.S. Vitamin D, phosphate, and vasculotoxicity. Can. J. Physiol. Pharmacol. 2015, 93, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Lanske, B.; Razzaque, M.S. Vitamin D and aging: Old concepts and new insights. J. Nutr. Biochem. 2007, 18, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Medalle, R.; Waterhouse, C.; Hahn, T.J. Vitamin D resistance in magnesium deficiency. Am. J. Clin. Nutr. 1976, 29, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Ozsoylu, S.; Hanioglu, N. Serum magnesium levels in children with vitamin D deficiency rickets. Turk. J. Pediatr. 1977, 19, 89–96. [Google Scholar] [PubMed]
- Anast, C.S. Magnesium studies in relation to vitamin D-resistant rickets. Pediatrics 1967, 40, 425–435. [Google Scholar] [PubMed]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, vitamin D status and mortality: Results from us national health and nutrition examination survey (nhanes) 2001 to 2006 and nhanes iii. BMC Med. 2013, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J. Steroid Biochem. Mol. Biol. 2018, 180, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; LaCroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium intake, bone mineral density, and fractures: Results from the women’s health initiative observational study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Deyneli, O.; Yavuz, D.; Gozu, H.; Mutlu, N.; Kaygusuz, I.; Akalin, S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol. Trace Elem. Res. 2010, 133, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Park, T.J. Magnesium metabolism. Electrolyte Blood Press 2008, 6, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Joao Matias, P.; Azevedo, A.; Laranjinha, I.; Navarro, D.; Mendes, M.; Ferreira, C.; Amaral, T.; Jorge, C.; Aires, I.; Gil, C.; et al. Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif. 2014, 38, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Cook, N.R.; Albert, C.M.; Buring, J.E.; Liu, S. Dietary magnesium intake and risk of cardiovascular disease among women. Am. J. Cardiol. 2005, 96, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Yan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Yeh, T.H.; Huang, Y.C.; Chen, P.Y. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Phys. 2016, 19, E97–E112. [Google Scholar]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Serebryansky, E.P.; Evsyukov, O.Y.; Spasov, A.A.; Skalny, A.V. Low magnesium diet alters distribution of macroelements and trace elements in tissues and organs of female rats. J. Trace Elem. Med. Biol. 2017, 39, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Castaneda, J.R.; Pendon-Ruiz de Mier, M.V.; Rodriguez, M.; Rodriguez-Ortiz, M.E. Magnesium replacement to protect cardiovascular and kidney damage? Lack of prospective clinical trials. Int. J. Mol. Sci. 2018, 19, 664. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.S.; Severo, J.S.; de Alencar, G.R.R.; de Oliveira, A.R.S.; Cruz, K.J.C.; Marreiro, D.D.N.; Freitas, B.; de Carvalho, C.M.R.; Martins, M.; Frota, K.M.G. Effect of magnesium supplementation on insulin resistance in humans: A systematic review. Nutrition 2017, 38, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Investigating the influence of vitamin D replacement therapy on magnesium status [response to letter]. J. Am. Osteopath. Assoc. 2018, 118, 773–774. [Google Scholar] [CrossRef] [PubMed]
| ▪ Total serum magnesium |
| ▪ Oral magnesium loading test |
| ▪ Intravenous magnesium loading test |
| ▪ RBC magnesium content |
| ▪ Hair magnesium content |
| ▪ Muscle magnesium content (biopsy) |
| ▪ Bone magnesium content |
| ▪ 24-h urinary magnesium |
| ▪ The ratio of ionized to total magnesium |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. https://doi.org/10.3390/nu10121863
Razzaque MS. Magnesium: Are We Consuming Enough? Nutrients. 2018; 10(12):1863. https://doi.org/10.3390/nu10121863
Chicago/Turabian StyleRazzaque, Mohammed S. 2018. "Magnesium: Are We Consuming Enough?" Nutrients 10, no. 12: 1863. https://doi.org/10.3390/nu10121863
APA StyleRazzaque, M. S. (2018). Magnesium: Are We Consuming Enough? Nutrients, 10(12), 1863. https://doi.org/10.3390/nu10121863




