Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Radiographic Analyses of the Femur
2.3. Microcomputed Tomography (μCT) Analysis of the Distal Femur
2.4. Weight of Caecal Content, pH, and β-Glucosidase Activity
2.5. Biochemical Marker of Bone Resorption
2.6. DNA Extraction from Faeces
2.7. Polymerase Chain Reaction (PCR) Conditions and Terminal Restriction Fragment Length Polymorphism (T-RFLP) Analyses
2.8. Analyses of Clone Libraries
2.9. RNA Extraction and Quantitative Real-Time PCR of Large-Intestine Tissue or the Bone Marrow of the Tibia
2.10. Statistical Analyses
3. Results
3.1. Body Weight and Tissue Weight
3.2. Weight of Caecal Content, pH, and β-Glucosidase Activity
3.3. Biochemical Marker of Bone Resorption
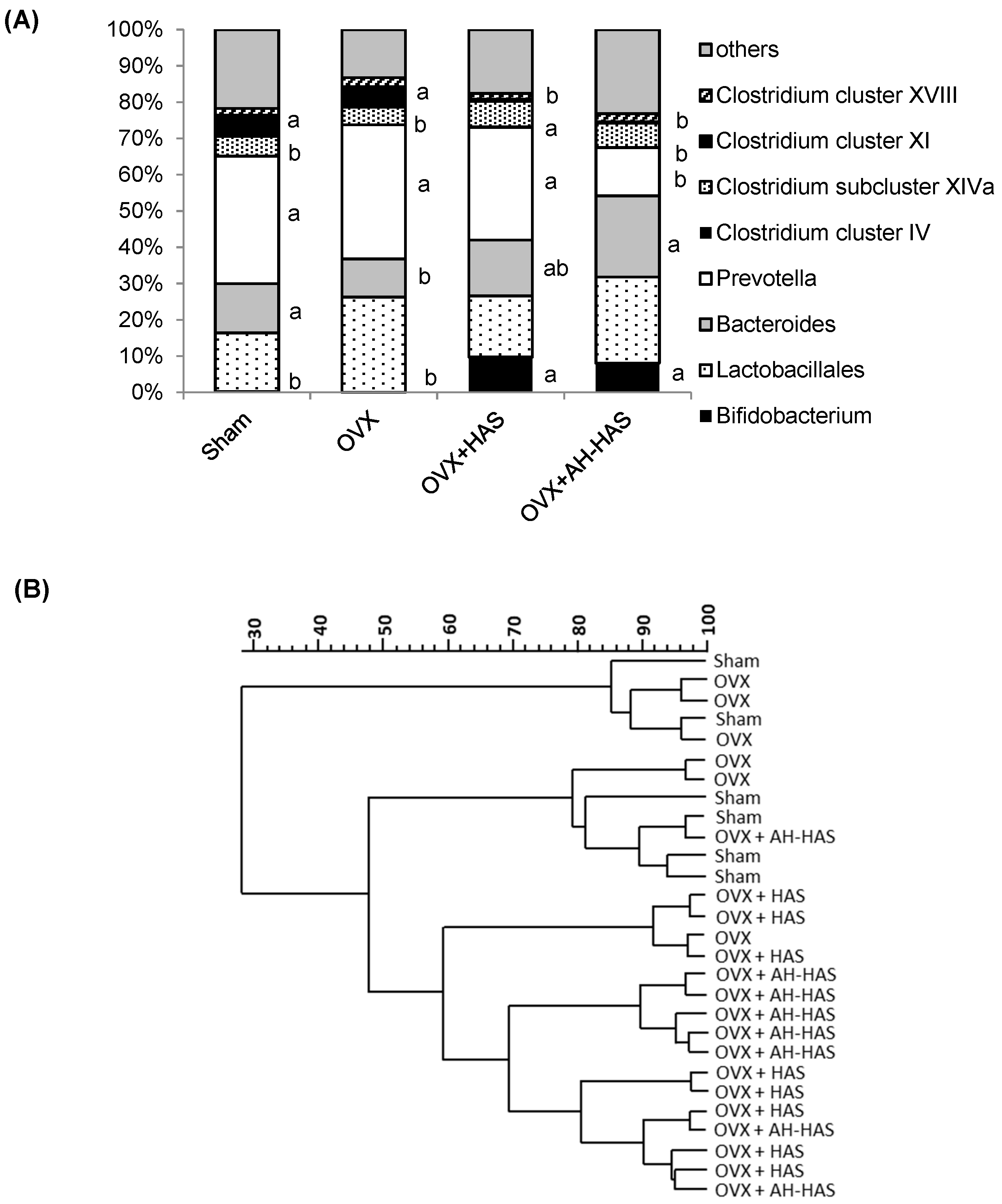
3.4. Analyses of Faecal Microbiota
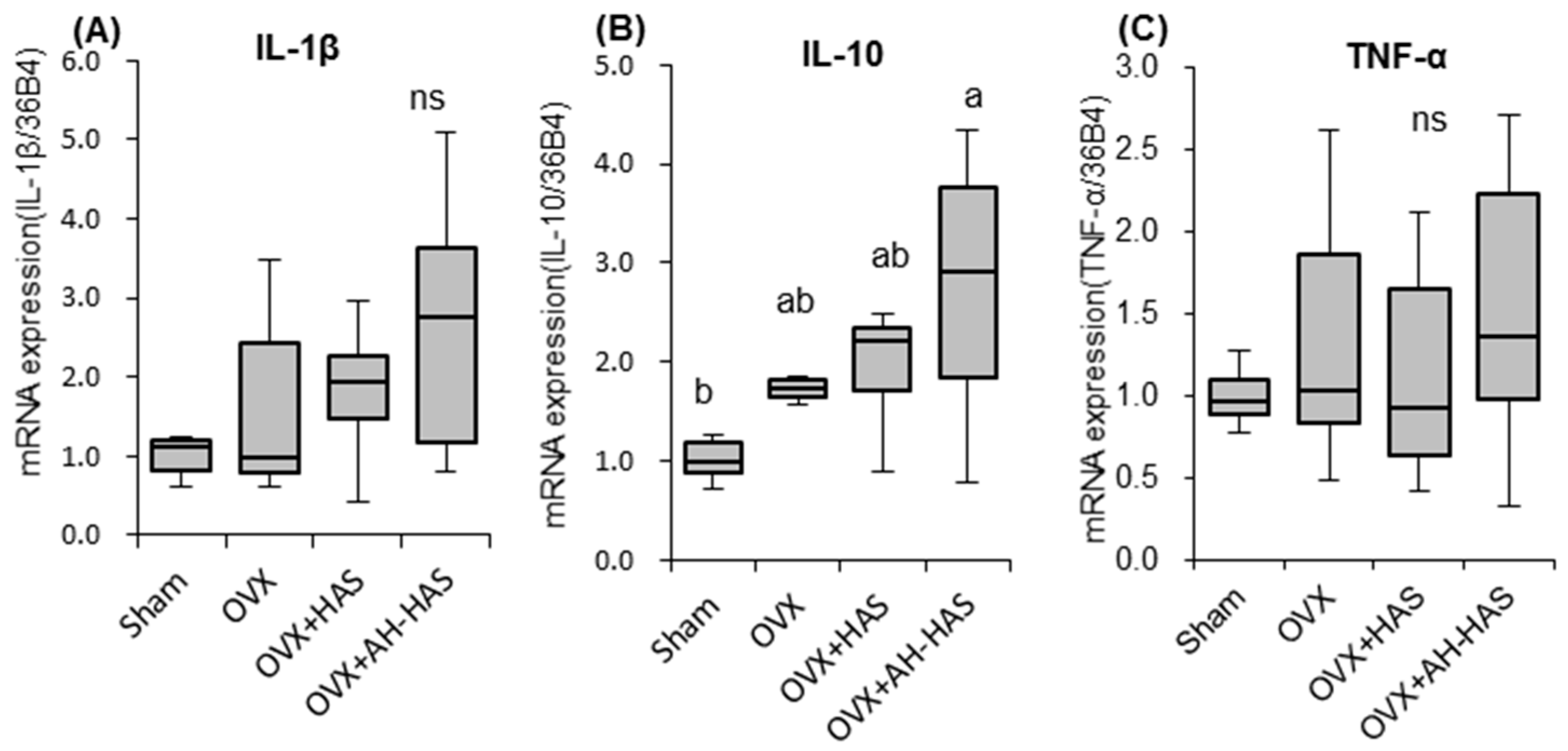
3.5. Expression of Genes Associated with Inflammation or Tight-Junction Proteins in the Colon
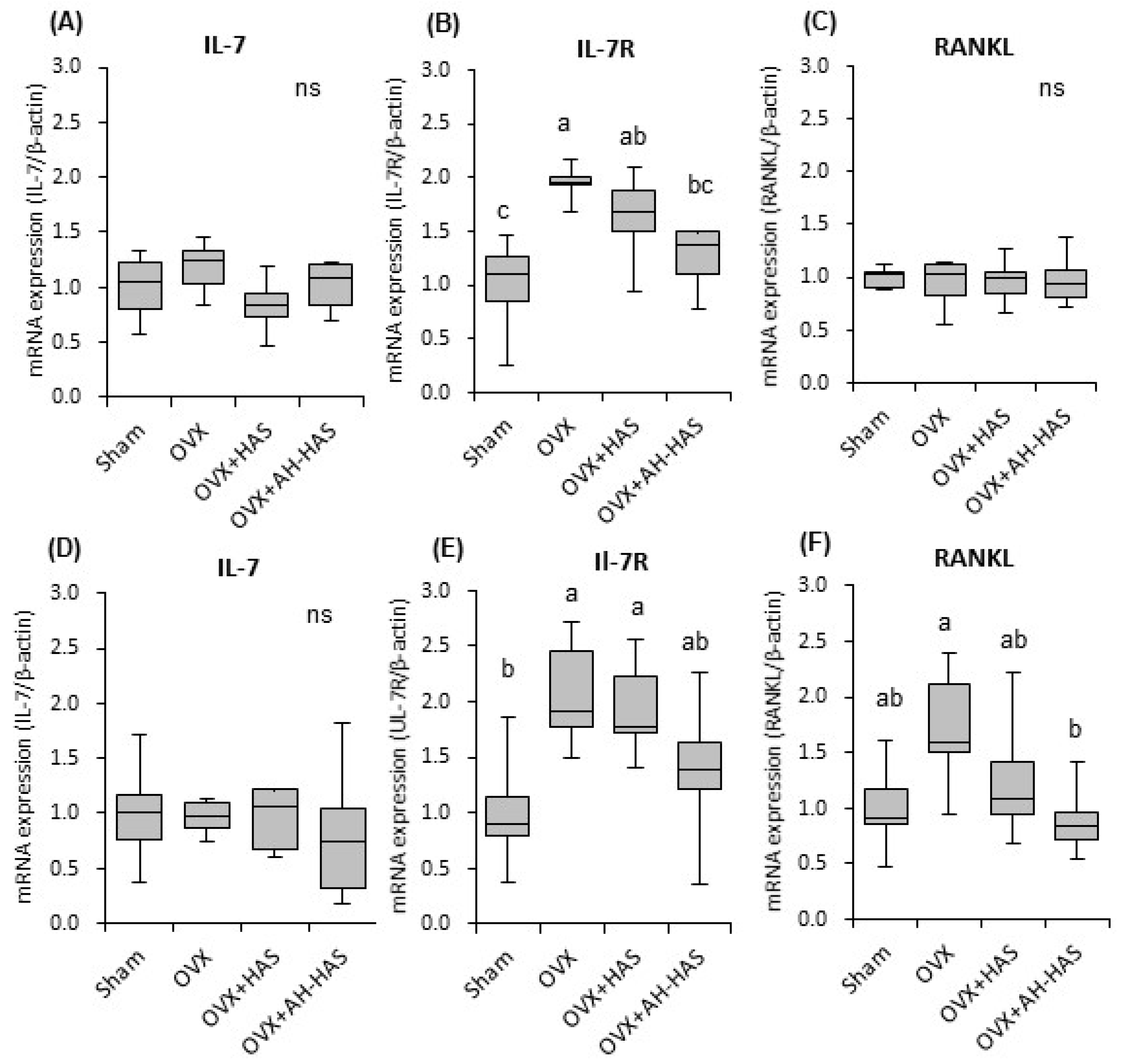
3.6. Expression of Inflammation-Related Genes in Bone Marrow
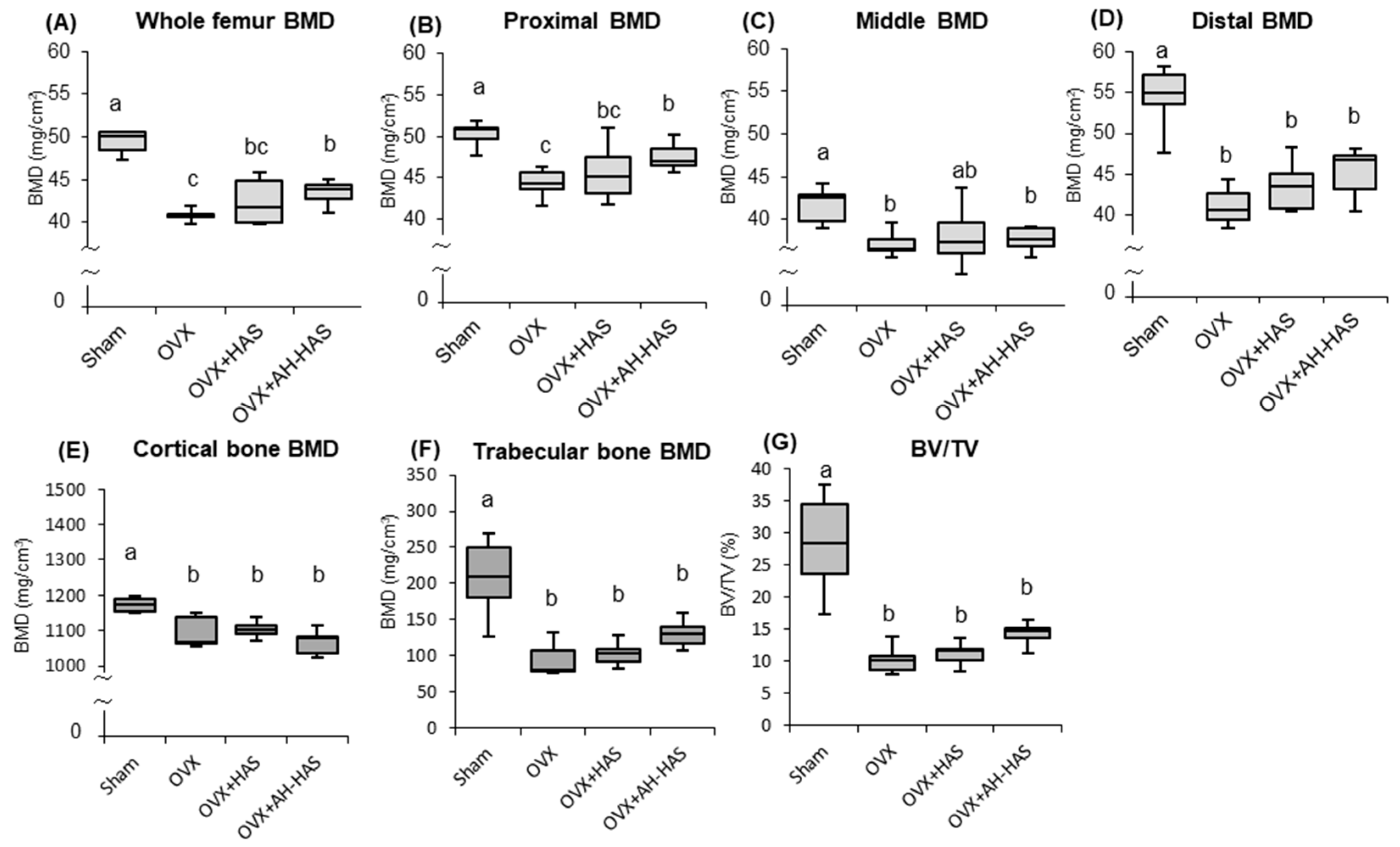
3.7. BMD of the Femur
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Joestl, J.; Lang, N.; Bukaty, A.; Tiefenboeck, T.M.; Platzer, P. Osteoporosis associated vertebral fractures-Health economic implications. PLoS ONE 2017, 12, e0178209. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Bull, D.; Reeves, G.; Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Blanco, R. Osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) in an osteoporotic patient chronically treated with bisphosphonates. Osteoporos. Int. 2017, 28, 1145–1147. [Google Scholar] [CrossRef]
- Li, J.Y.; Tawfeek, H.; Bedi, B.; Yang, X.; Adams, J.; Gao, K.Y.; Zayzafoon, M.; Weitzmann, M.N.; Pacifici, R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc. Natl. Acad. Sci. USA 2011, 108, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Takahashi, K.; Munshi, M.; Williams, D.C.; Roberson, P.K.; Manolagas, S.C. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J. Clin. Invest. 1998, 101, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen regulation of immune cell bone interactions. Ann. N. Y. Acad. Sci. 2006, 1068, 256–274. [Google Scholar] [CrossRef]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef]
- Heyman, M.; Abed, J.; Lebreton, C.; Cerf-Bensussan, N. Intestinal permeability in coeliac disease: Insight into mechanisms and relevance to pathogenesis. Gut 2012, 61, 1355–1364. [Google Scholar] [CrossRef]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Sjogren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Backhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zheng, B.; Lu, X.; Zhuang, W. The in vitro effects of retrograded starch (resistant starch type 3) from lotus seed starch on the proliferation of Bifidobacterium adolescentis. Food Funct. 2013, 4, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Tousen, Y.; Matsumoto, Y.; Matsumoto, C.; Nishide, Y.; Nagahata, Y.; Kobayashi, I.; Ishimi, Y. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice. Br. J. Nutr. 2016, 116, 247–257. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Nagahata, Y.; Kobayashi, I.; Goto, M.; Nakaura, Y.; Inouchi, N. The Formation of Resistant Starch during Acid Hydrolysis of High-amylose Corn Starch. J. Appl. Glycosci. 2013, 60, 123–130. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tousen, Y.; Ono, R.; Kurasawa, S.; Nagahata, Y.; Kobayashi, I.; Ishimi, Y. Effects of Resistant Starch on the Inhibition of Bone Loss and Fat Accumulation in Ovariectomized Mice. J. Jpn. Diet. Fib. Res. 2016, 20, 13–20. (In Japanese) [Google Scholar]
- Nagashima, K.; Mochizuki, J.; Hisada, T.; Suzuki, S.; Shimomura, K. Phylogenetic Analysis of 16S Ribosomal RNA Gene Sequences from Human Fecal Microbiota and Improved Utility of Terminal Restriction Fragment Length Polymorphism Profiling. Biosci. Microflora 2006, 25, 99–107. [Google Scholar] [CrossRef]
- Nagashima, K.; Hisada, T.; Sato, M.; Mochizuki, J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol. 2003, 69, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, D.; Cai, W.; Geng, S.; Chen, L.; Han, T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin. Nutr. 2009, 28, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; McKinnon, R.; Young, G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; DiGuardo, M.; Viladomiu, M.; de Horna, A.; Sanchez, S.; Einerhand, A.W.; Sanders, L.; Hontecillas, R. Soluble fibers and resistant starch ameliorate disease activity in interleukin-10-deficient mice with inflammatory bowel disease. J. Nutr. 2011, 141, 1318–1325. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liu, S.M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef] [PubMed]
- Clowes, J.A.; Riggs, B.L.; Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 2005, 208, 207–227. [Google Scholar] [CrossRef]
- Younes, H.; Coudray, C.; Bellanger, J.; Demigné, C.; Rayssiguier, Y.; Rémésy, C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br. J. Nutr. 2001, 86, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.W.; Levrat-Verny, M.A.; Coudray, C.; Besson, C.; Krespine, V.; Messager, A.; Demigné, C.; Rémésy, C. Class2 Resistant starches lower plasma and liver lipids and improve mineral retention in rats. J. Nutr. 2001, 131, 1283–1289. [Google Scholar] [CrossRef]
- Zafar, T.A.; Martin, B.; Weaver, C.M. Resistant Starches (RS2 and RS3) have Variable Effects on Bone Mineral Status in Rats. Open Nutr. J. 2009, 3, 17–22. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Cenci, S.; Rifas, L.; Brown, C.; Pacifici, R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood 2000, 96, 1873–1878. [Google Scholar]
| Sham | OVX | OVX + HAS | OVX + AH-HAS | |
|---|---|---|---|---|
| Body weight | ||||
| Initial body weight (g) | 28.1 ± 0.3 | 29.2 ± 0.4 | 28.1 ± 0.4 | 27.7 ± 0.4 |
| Final body weight (g) | 30.5 ± 1.5 | 33.7 ± 0.4 | 33.7 ± 0.8 | 32.5 ± 0.5 |
| Total food intake (g) | 173.7 ± 3.0 | 173.0 ± 2.7 | 173.4 ± 2.3 | 173.9 ± 2.3 |
| Uterine weight (g) | 77.6 ± 12.4 a | 13.8 ± 1.0 b | 15.0 ± 1.2 b | 17.0 ± 1.4 b |
| Caecum | ||||
| Wet weight of caecal content (g) | 0.214 ± 0.024 bc | 0.178 ± 0.033 c | 0.310 ± 0.030 ab | 0.409 ± 0.037 a |
| Caecal content pH | 7.850 ± 0.056 ab | 8.000 ± 0.037 a | 7.663 ± 0.073 b | 7.413 ± 0.079 c |
| Caecal β-glucosidase activity * | 1.099 ± 0.143 b | 1.341 ± 0.098 b | 1.873 ± 0.130 b | 4.057 ± 0.687 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tousen, Y.; Matsumoto, Y.; Nagahata, Y.; Kobayashi, I.; Inoue, M.; Ishimi, Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients 2019, 11, 297. https://doi.org/10.3390/nu11020297
Tousen Y, Matsumoto Y, Nagahata Y, Kobayashi I, Inoue M, Ishimi Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients. 2019; 11(2):297. https://doi.org/10.3390/nu11020297
Chicago/Turabian StyleTousen, Yuko, Yu Matsumoto, Yuya Nagahata, Isao Kobayashi, Masahiro Inoue, and Yoshiko Ishimi. 2019. "Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation" Nutrients 11, no. 2: 297. https://doi.org/10.3390/nu11020297
APA StyleTousen, Y., Matsumoto, Y., Nagahata, Y., Kobayashi, I., Inoue, M., & Ishimi, Y. (2019). Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients, 11(2), 297. https://doi.org/10.3390/nu11020297




