Assessment of Potential Toxicity of Hyaluronic Acid-Coated Magnetic Nanoparticles on Maize (Zea mays) at Early Development Stages
Abstract
1. Introduction
2. Results
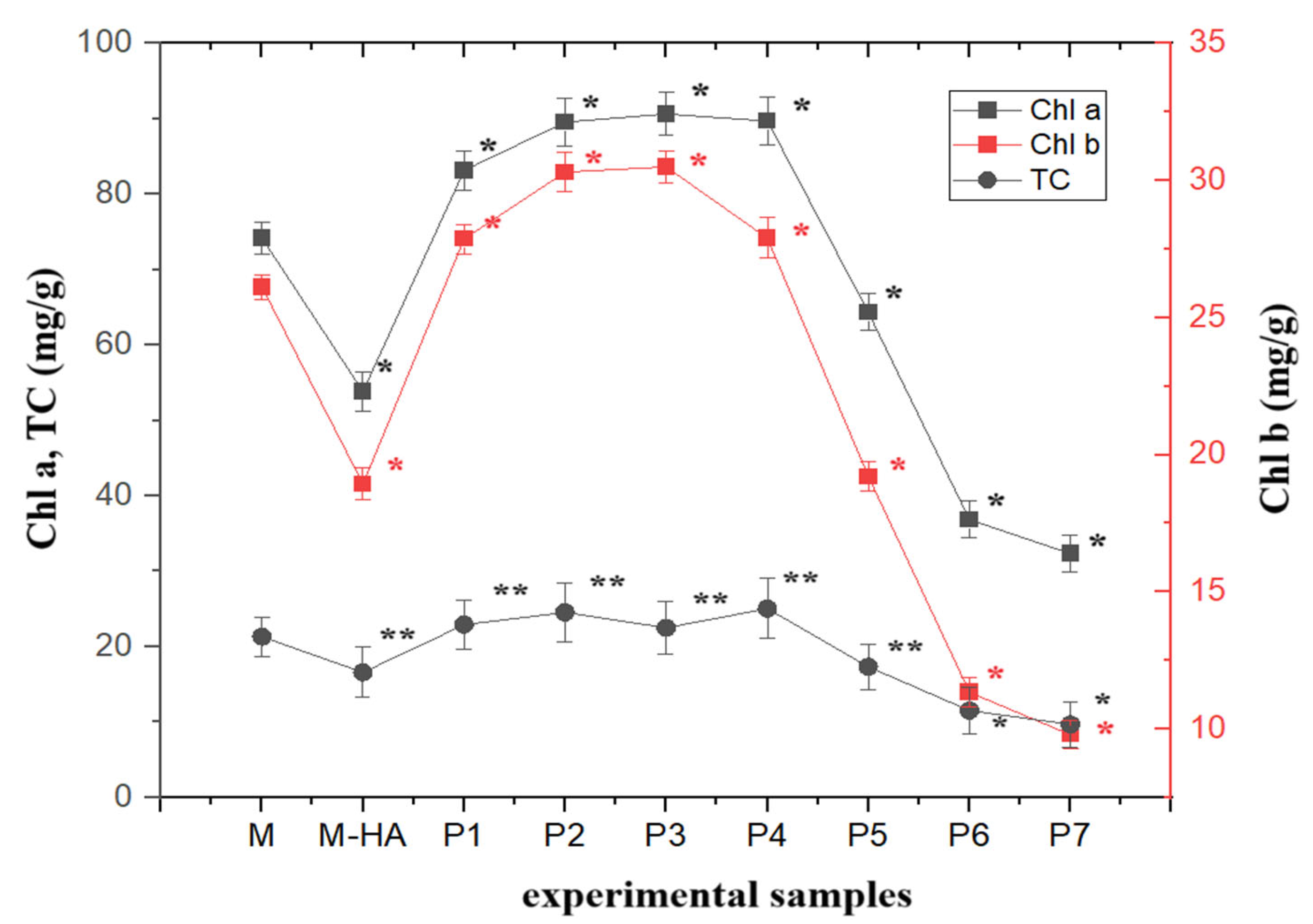
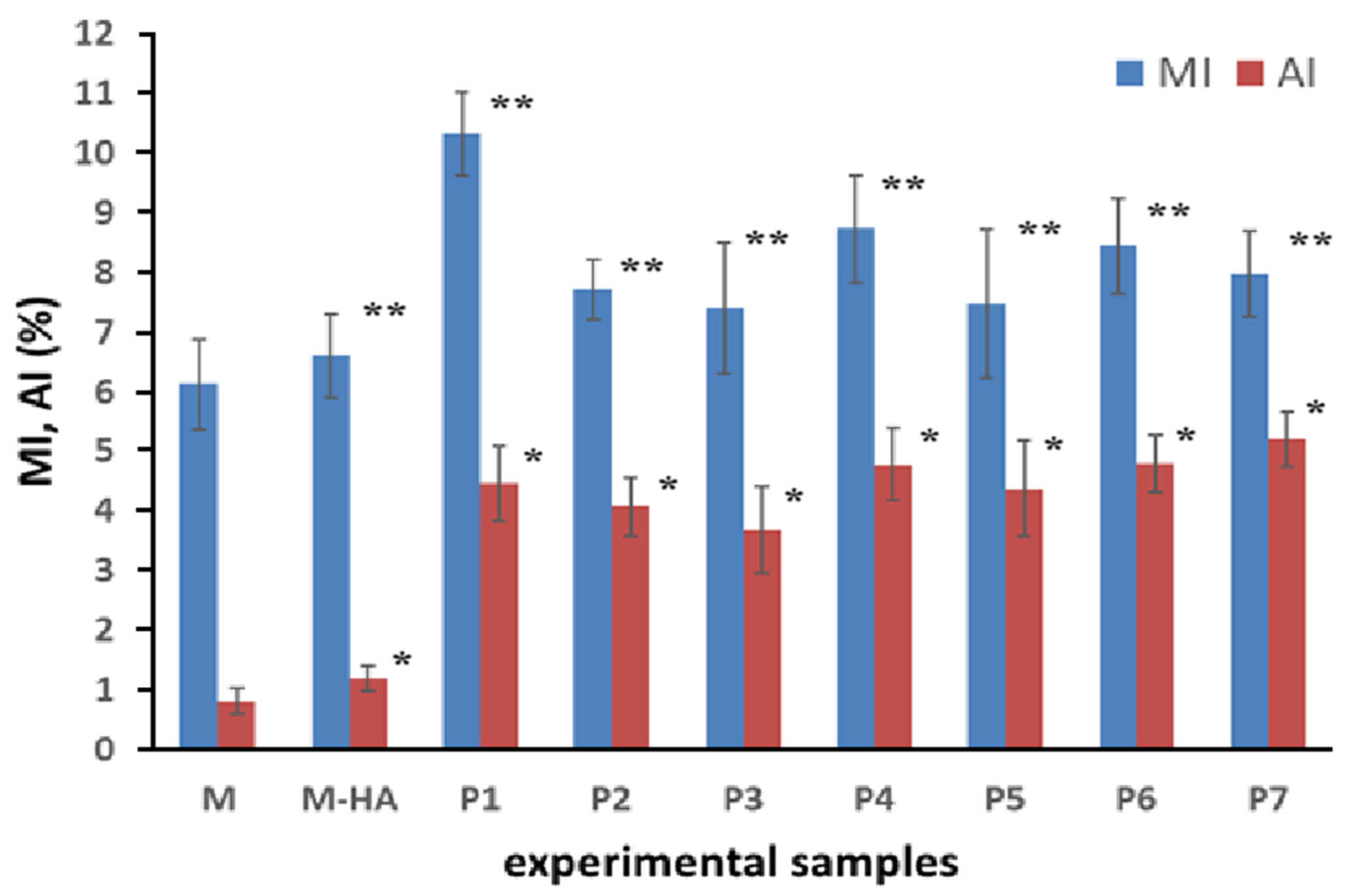
2.1. HA-MNP Phytotoxicity on Maize
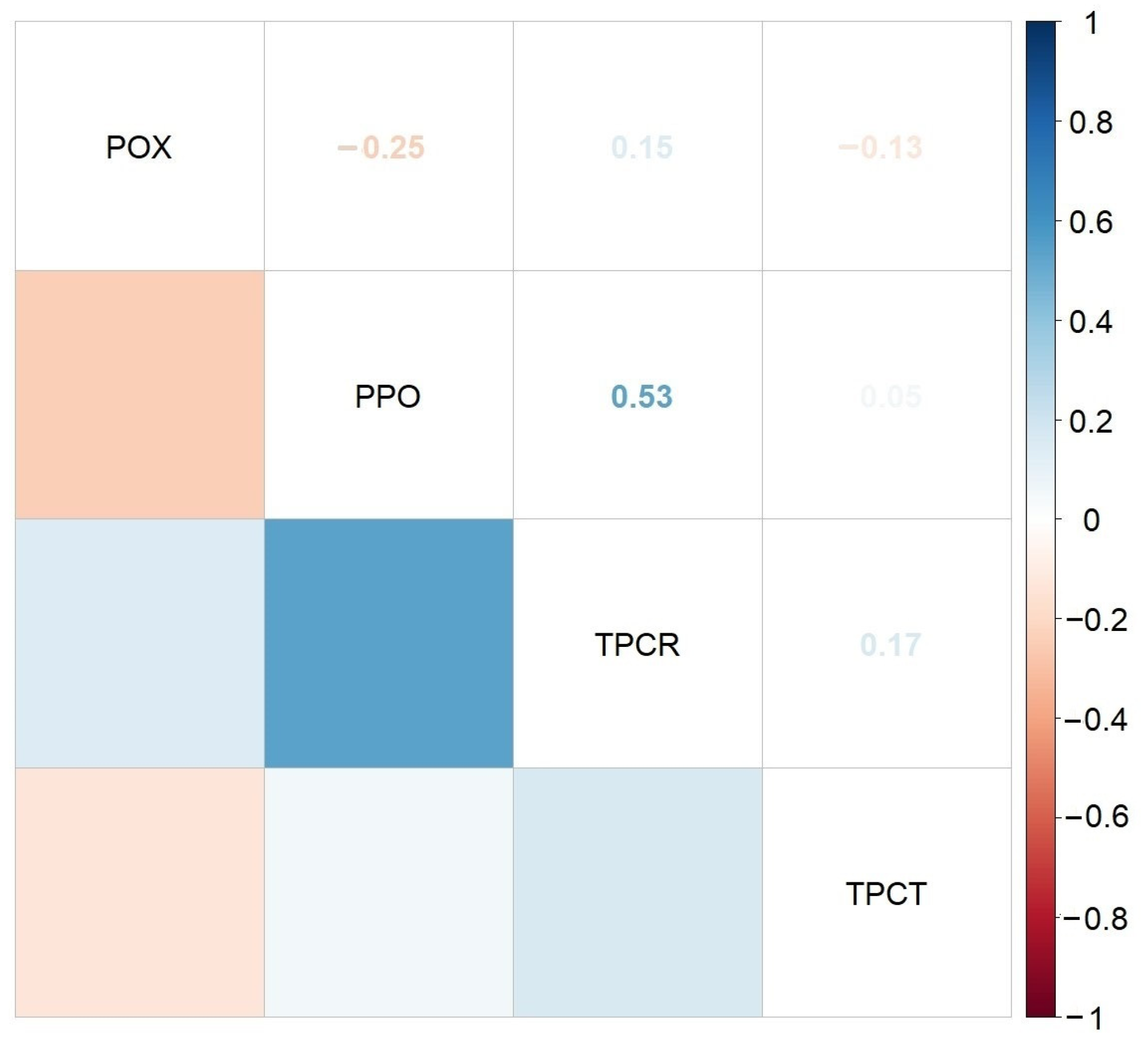
2.2. HA-MNP Genotoxicity on Maize
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Assay of Genotoxicity
4.3. Assay of Phytotoxicity
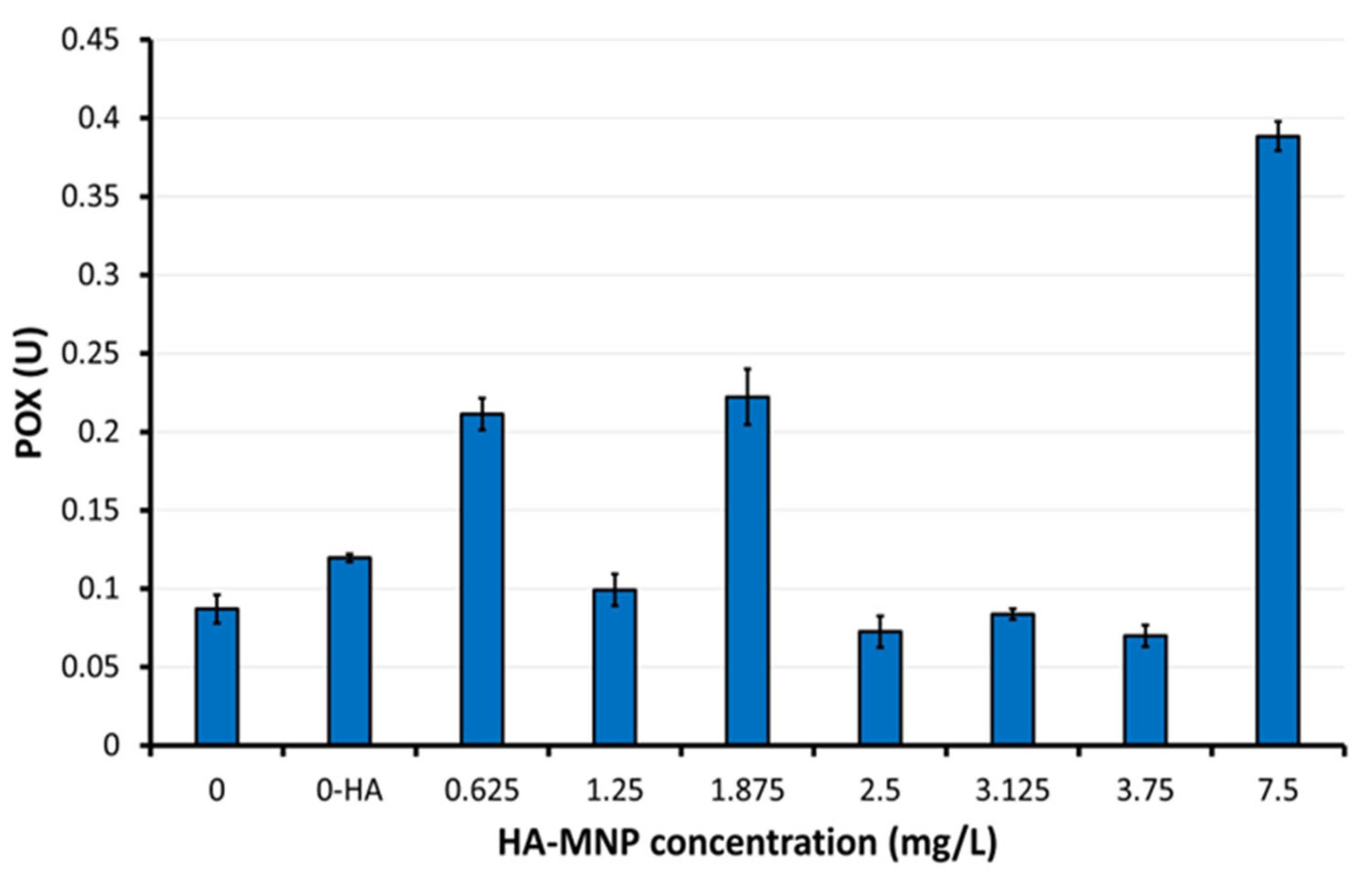
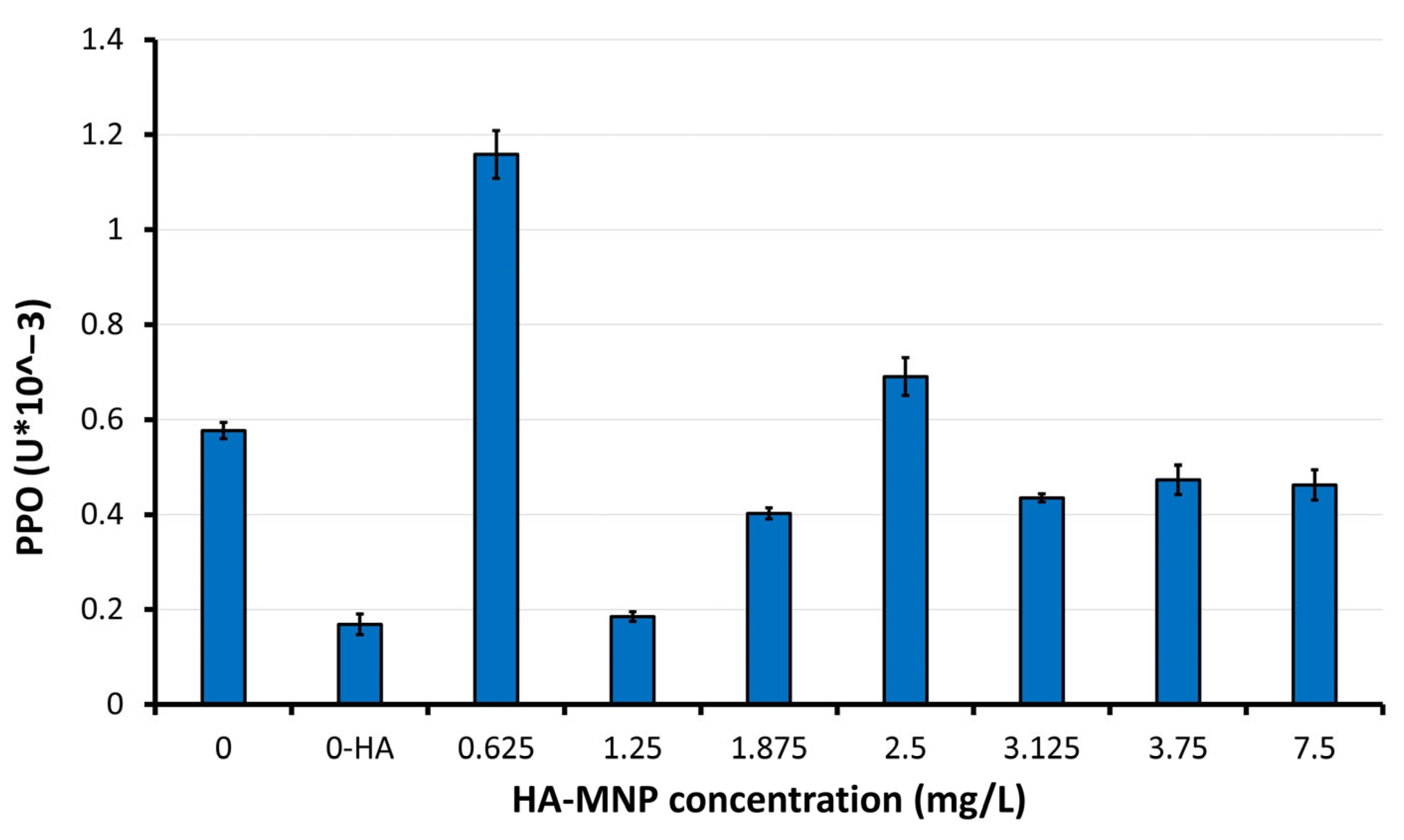
4.4. Assay of Enzymes Activity
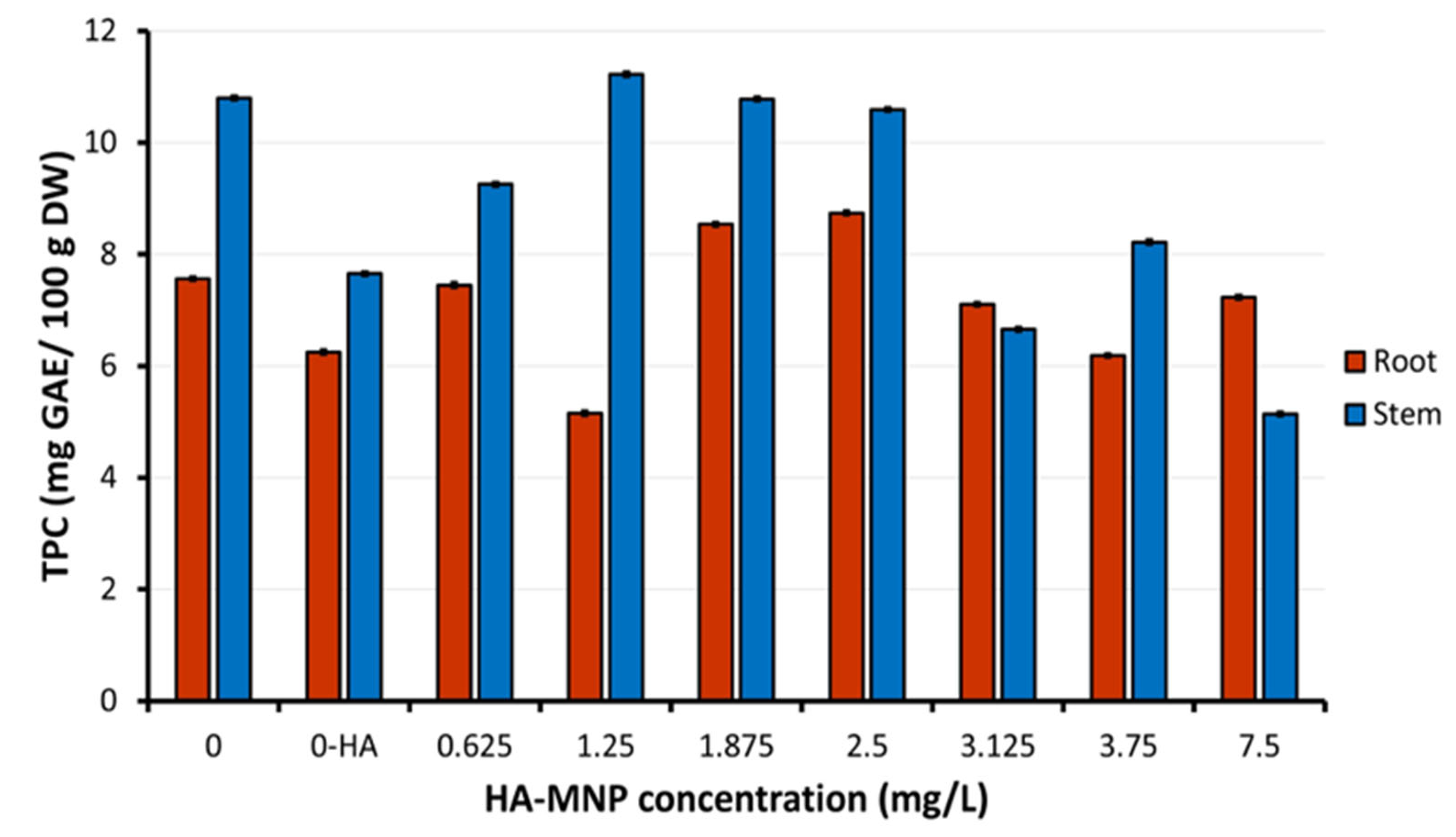
4.5. Assay of Phenolic Compounds
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ur-Rehman, M.; Mfarrej, M.F.B.; Usman, M.; Anayatullah, S.; Rizwan, M.; Alharby, H.F.; Abu Zeid, I.M.; Alabdallah, N.M.; Ali, S. Effect of iron nanoparticles and conventional sources of Fe on growth, physiology and nutrient accumulation in wheat plants grown on normal and salt-affected soils. J. Hazard. Mater. 2023, 458, 131861. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Anwari, A.A.S.; Forghani, A.H.; Mirmazloum, I. Application of iron oxide nanoparticles improves growth and phytochemical constituents of in vitro cultured Carum copticum L. J. Agric. Food Res. 2024, 18, 101402. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Wang, L.; Bi, H.; Peng, Z.; Li, J.; Guo, C.; Bi, Y.; Lai, Y.; Guo, D. Enhanced Germination and Growth of Alfalfa with Seed Presoaking and Hydroponic Culture in Fe2O3 Magnetic Nanoparticles. J. Nanomater. 2023, 2023, 9783977. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Khan, S.U.; Koerniati, S.; Hastilestari, B.R.; Ningrum, R.A.; Wahab, R.; Djalovic, I.; Prasad, P.V.V. Iron oxide nanoparticles alleviate salt-alkaline stress and improve growth by modulating antioxidant defense system in cherry tomato. J. Plant Interact. 2024, 19, 2375508. [Google Scholar] [CrossRef]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Understanding the Effect of the interaction of nanoparticles with roots on the uptake in plants. In Environmental Nanotechnology Volume 3, Environmental Chemistry for a Sustainable World; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 277–304. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Singh, R.; Srinivas, S.P.; Kumawat, M.; Daima, H.K. Ligand-based surface engineering of nanomaterials: Trends, challenges, and biomedical perspectives. OpenNano 2023, 15, 100194. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Daya, R.; Xu, C.; Nguyen, N.T.; Liu, H.H. Angiogenic Hyaluronic Acid Hydrogels with Curcumin-Coated Magnetic Nanoparticles for Tissue Repair. ACS Appl. Mater. Interfaces 2022, 14, 11051–11067. [Google Scholar] [CrossRef]
- Thomas, R.G.; Moon, M.J.; Lee, H.; Sasikala, A.R.K.; Kim, C.S.; Park, I.K.; Jeong, Y.Y. Hyaluronic acid conjugated superparamagnetic iron oxide nanoparticle for cancer diagnosis and hyperthermia therapy. Carbohydr. Polym. 2015, 131, 439–446. [Google Scholar] [CrossRef]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids—Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry (CPFA), Unit F4.3; John Wiley: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M.; et al. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Selvakesavan, R.K.; Kruszka, D.; Shakya, P.; Mondal, D.; Franklin, G. Impact of Nanomaterials on Plant Secondary Metabolism. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Al-Khayri, J.M., Alnaddaf, L.M., Jain, S.M., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, J.; Zhang, S.; Xiao, W.; Guan, C.; Wang, G.; Wang, Y. Changes in the phenolic compound content and antioxidant activity in developmental maize kernels and expression profiles of phenolic biosynthesis-related genes. J. Cereal Sci. 2020, 96, 103113. [Google Scholar] [CrossRef]
- Ahmed, B.; Shahid, M.; Khan, M.S.; Musarrat, J. Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics 2018, 10, 1315–1327. [Google Scholar] [CrossRef]
- Pandey, A.K.; Shahi, A.K.; Srivastava, N.; Kumar, G.; Gopal, R. Synthesis and cytogenetic effect of magnetic nanoparticles. Adv. Mater. Lett. 2015, 6, 954–960. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.E. Cytogenetic changes induced by β-cyclodextrin coated nanoparticles in plant seeds. Rom. J. Phys. 2009, 54, 125–131. [Google Scholar]
- Răcuciu, M.; Creangă, D. Cytogenetic changes induced by aqueous ferrofluids in agricultural plants. J. Magn. Magn. Mater. 2007, 311, 288–291. [Google Scholar] [CrossRef]
- Haq, I.; Kumari, V.; Kumar, S.; Raj, A.; Lohani, M.; Bhargava, R.N. Evaluation of the phytotoxic and genotoxic potential of pulp and paper mill effluent using Vigna radiata and Allium cepa. Adv. Biol. 2016, 2016, 8065736. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Khan, I.; Tanveer, M.; Shahid, M.; Shakoor, A.; Wang, L. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 2017, 27, 262–273. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.; Webb, K.J.; Kingston-Smith, A.H. Detection of potential chloroplastic substrates for polyphenol oxidase suggests a role in undamaged leaves. Front. Plant Sci. 2017, 8, 237. [Google Scholar] [CrossRef]
- Plaksenkova, I.; Jermalonoka, M.; Bankovska, L.; Gavarane, I.; Gerbreders, V.; Sledevskis, E.; Snikeris, J.; Kokina, I. Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J. Nanomater. 2019, 2019, 2678247. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mohhamed, M.A.; Khater, M.S.; Osman, Y.A.H.; Elsherbini, E. Effect of magnetite nano–fertilizer on growth and yield of Ocimum basilicum L. Int. J. Indig. Med. Plants 2013, 46, 1286–1293. [Google Scholar]
- Kokina, I.; Plaksenkova, I.; Jermalonoka, M.; Petrova, A. Impact of iron oxide nanoparticles on yellow medick (Medicago falcata L.) plants. J. Plant Interact. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe2O3 and Fe3O4 nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 2016, 159, 326–334. [Google Scholar] [CrossRef]
- Souza, L.R.R.; Bernardes, L.E.; Barbetta, M.F.S.; da Veiga, M.A.M.S. Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: Effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Pollut. Res. 2019, 26, 24121–24131. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; van Raap, M.B.F.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; Zawoznik, M.S.; Coral, D.F.; Fernández van Raap, M.B.; Benavides, M.P. Magnetite nanoparticles coated with citric acid are not phytotoxic and stimulate soybean and alfalfa growth. Ecotoxicol. Environ. Saf. 2021, 211, 111942. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, O. Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Xie, Y. Stimulatory Effect of Fe3O4 Nanoparticles on the Growth and Yield of Pseudostellaria heterophylla via Improved Photosynthetic Performance. HortScience 2021, 56, 753–761. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Barroso, D.; Komárek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut. Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bai, X.; Ye, Z.; Ma, L.; Liang, L. Toxicological responses of Fe3O4 nanoparticles on Eichhornia crassipes and associated plant transportation. Sci. Total Environ. 2019, 671, 558–567. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant Cell Tissue Organ. Cult. 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of stevia rebaudiana bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Varduny, T.V.; Lysenko, V.S.; Kapralova, O.A.; Chokheli, V.A.; Sereda, M.M.; Dmitriev, P.A.; Varduny, V.M. Effect of Nano and Crystalline Metal Oxides on Growth, Gene and Cytotoxicity of Plants in Vitro and Ex Vitro. Turczaninowia 2018, 21, 207–214. [Google Scholar]
- Giorgetti, L.; Castiglione, M.R.; Bernabini, M.; Geri, C. Nanoparticles effects on growth and differentiation in cell culture of carrot (Daucus carota L.). Agrochimica 2011, 55, 45–53. [Google Scholar]
- Chandra Sekhar Singh, B. Toxicity Analysis of Hybrid Nanodiamond/Fe3O4 Nanoparticles on Allium cepa L. J. Toxicol. 2022, 2022, 5903409. [Google Scholar] [CrossRef]
- Gantayat, S.; Nayak, S.P.; Badamali, S.K.; Pradhan, C.; Das, A.B. Analysis on cytotoxicity and oxidative damage of iron nano-composite on Allium cepa L. root meristems. Cytologia 2020, 85, 325–332. [Google Scholar] [CrossRef]
- Răcuciu, M.; Oancea, S.; Barbu-Tudoran, L.; Drăghici, O.; Agavriloaei, A.; Creangă, D. A Study of Hyaluronic Acid’s Theoretical Reactivity and of Magnetic Nanoparticles Capped with Hyaluronic Acid. Materials 2024, 17, 1229. [Google Scholar] [CrossRef] [PubMed]
- Antunes, D.R.; Forini, M.M.L.H.; Coqueiro, Y.A.; Pontes, M.S.; Lima, P.H.C.; Cavalcante, L.A.F.; Sanches, A.O.; Caires, A.R.L.; Santiago, E.F.; Grillo, R. Effect of hyaluronic acid-stabilized silver nanoparticles on lettuce (Lactuca sativa L.) seed germination. Chemosphere 2024, 364, 143080. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Wetter, L.R. Plant Tissue Culture Methods; National Research Council of Canada, Prairie Regional Laboratory: Saskatoon, SK, Canada, 1975. [Google Scholar]
- Singh, R.J. Plant Cytogenetics, 3rd ed.; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Truta, E.; Vochita, G.; Zamfirache, M.M.; Olteanu, Z.; Rosu, C.M. Copper-induced genotoxic effects in root meristems of Triticum aestivum L. cv. Beti. Carpath. J. Earth Environ. Sci. 2013, 8, 83–92. [Google Scholar]
- Bakare, A.A.; Mosuro, A.A.; Osibanjo, O. Effect of simulated leachate on chromosomes and mitosis in roots of Allium cepa (L.). J. Environ. Biol. 2000, 21, 263–271. [Google Scholar]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Pandiyan, M.; Senthil, N.; Balaji, T.; Veeramani, P.; Savitha, B.K.; Sendhilvel, V.; Gopilkrishnan, A. Studies on performance of drought tolerant genotypes under drought and normal conditions through morpho, physio and biochemical attributes of blackgram (Vigna mungo L.) and green gram (Vigna radiata L.). Int. J. Adv. Res. 2017, 5, 489–496. [Google Scholar] [CrossRef]
- Reddy, K.P.; Subhani, S.M.; Khan, P.A.; Kumar, K.B. Effect of light and benzyladenine and desk treated growing leaves, Changes in the peroxidase activity. Cell Physiol. 1995, 26, 984. [Google Scholar]
- Esterbauer, H.; Schwarzl, E.; Hayn, M. A rapid assay for catechol oxidase and laccase using 2-nitro-5-thiobenzoic acid. Anal. Biochem. 1977, 77, 486–494. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dinno, A. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.6. 2024. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 1 October 2024).
- R Core Development Team. R: A Language and Environment for Statistical Computing, Version 4.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.r-project.org (accessed on 1 October 2024).
- Harrell, F., Jr. Hmisc: Harrell Miscellaneous. R Package Version 5.1-3. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 1 October 2024).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.94). Available online: https://github.com/taiyun/corrplot (accessed on 1 October 2024).


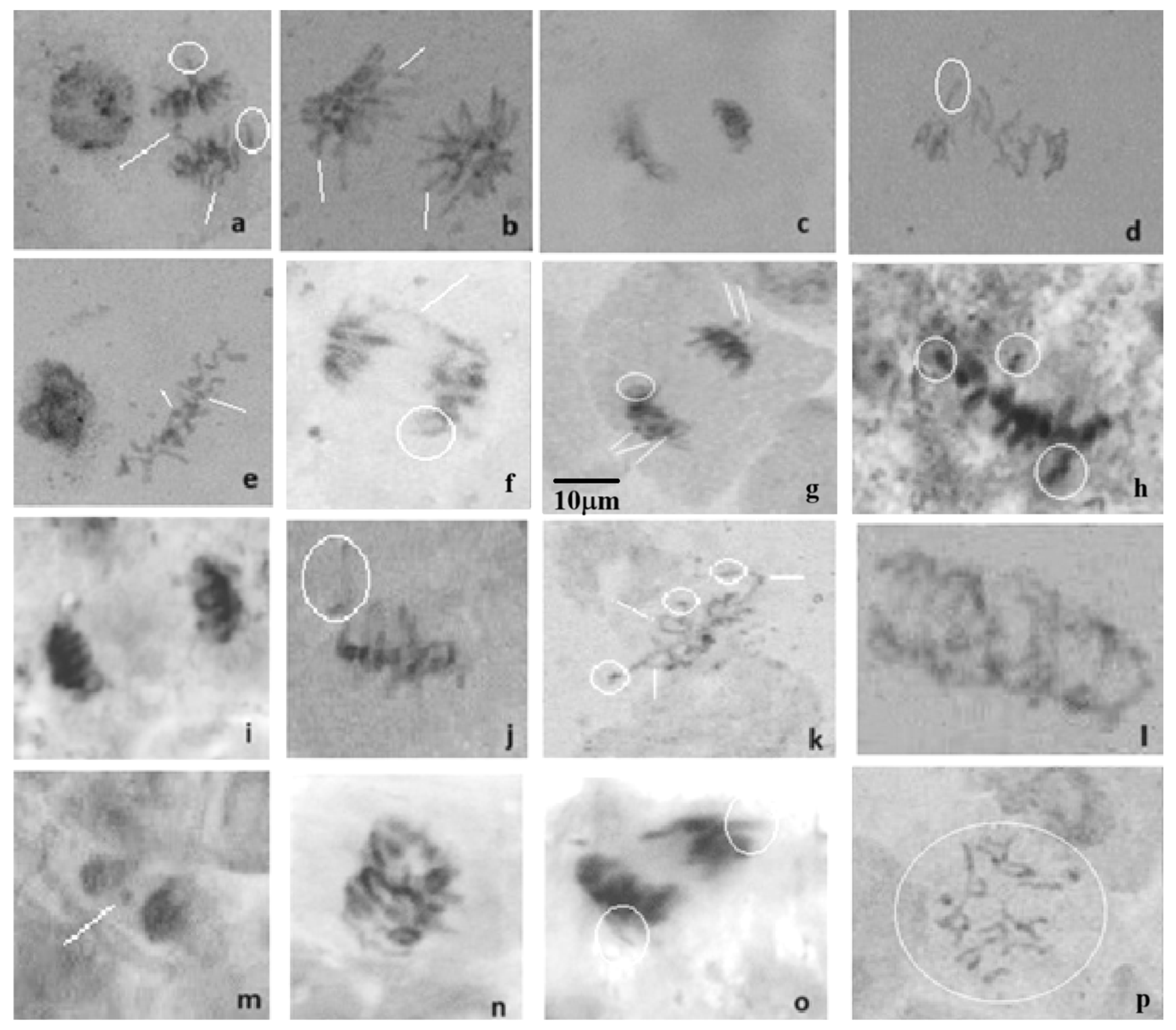
| C (mg/L) | TC | TD | TA | Total Number of Cells with Chromosomal Aberrations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Vg | Lg | DA | Cm | Bg | Bk | Rc | As | St | Pn | Co | ||||
| 0 | 18,419 | 1129 | 149 | 18 | 32 | 5 | 0 | 13 | 7 | 1 | 1 | 23 | 5 | 1 | 43 |
| 0-HA | 17,273 | 1140 | 206 | 22 | 37 | 2 | 2 | 32 | 4 | 11 | 2 | 46 | 6 | 14 | 28 |
| 0.625 | 12,596 | 1300 | 562 | 30 | 141 | 2 | 4 | 24 | 2 | 9 | 3 | 23 | 12 | 10 | 302 |
| 1.25 | 13,070 | 1008 | 532 | 40 | 133 | 6 | 3 | 12 | 6 | 2 | 5 | 25 | 13 | 2 | 285 |
| 1.875 | 12,312 | 911 | 454 | 30 | 184 | 2 | 2 | 17 | 5 | 10 | 7 | 25 | 6 | 0 | 166 |
| 2.5 | 11,034 | 962 | 527 | 53 | 142 | 13 | 8 | 9 | 8 | 2 | 1 | 24 | 5 | 1 | 261 |
| 3.125 | 11,172 | 835 | 489 | 53 | 101 | 2 | 2 | 9 | 2 | 0 | 1 | 27 | 6 | 2 | 284 |
| 3.75 | 11,479 | 968 | 551 | 44 | 150 | 9 | 11 | 3 | 2 | 1 | 3 | 32 | 4 | 3 | 289 |
| 7.5 | 11,774 | 938 | 612 | 34 | 137 | 2 | 3 | 1 | 2 | 0 | 5 | 19 | 3 | 1 | 405 |
| HA-MNP Suspension Volume Fraction (µL/L) | 50 | 100 | 150 | 200 | 250 | 300 | 600 |
| HA-MNP Concentration (mg/L) | 0.625 | 1.25 | 1.875 | 2.5 | 3.125 | 3.75 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răcuciu, M.; Precup, C.-N.; Cocîrlea, M.D.; Oancea, S. Assessment of Potential Toxicity of Hyaluronic Acid-Coated Magnetic Nanoparticles on Maize (Zea mays) at Early Development Stages. Molecules 2025, 30, 1316. https://doi.org/10.3390/molecules30061316
Răcuciu M, Precup C-N, Cocîrlea MD, Oancea S. Assessment of Potential Toxicity of Hyaluronic Acid-Coated Magnetic Nanoparticles on Maize (Zea mays) at Early Development Stages. Molecules. 2025; 30(6):1316. https://doi.org/10.3390/molecules30061316
Chicago/Turabian StyleRăcuciu, Mihaela, Cristina-Nicoleta Precup, Maria Denisa Cocîrlea, and Simona Oancea. 2025. "Assessment of Potential Toxicity of Hyaluronic Acid-Coated Magnetic Nanoparticles on Maize (Zea mays) at Early Development Stages" Molecules 30, no. 6: 1316. https://doi.org/10.3390/molecules30061316
APA StyleRăcuciu, M., Precup, C.-N., Cocîrlea, M. D., & Oancea, S. (2025). Assessment of Potential Toxicity of Hyaluronic Acid-Coated Magnetic Nanoparticles on Maize (Zea mays) at Early Development Stages. Molecules, 30(6), 1316. https://doi.org/10.3390/molecules30061316








