Progress in Hyaluronan-Based Nanoencapsulation Systems for Smart Drug Release and Medical Applications
Abstract
1. Introduction
- Physical methods: Spray drying, spray cooling, air suspension, envelope-combination, extrusion, supercritical solution processing, porous centrifugal, electrostatic binding, solvent evaporation, and rotary separation.
- Chemical methods: This category encompasses interfacial polymerization, in situ polymerization, and piercing-solidifying.
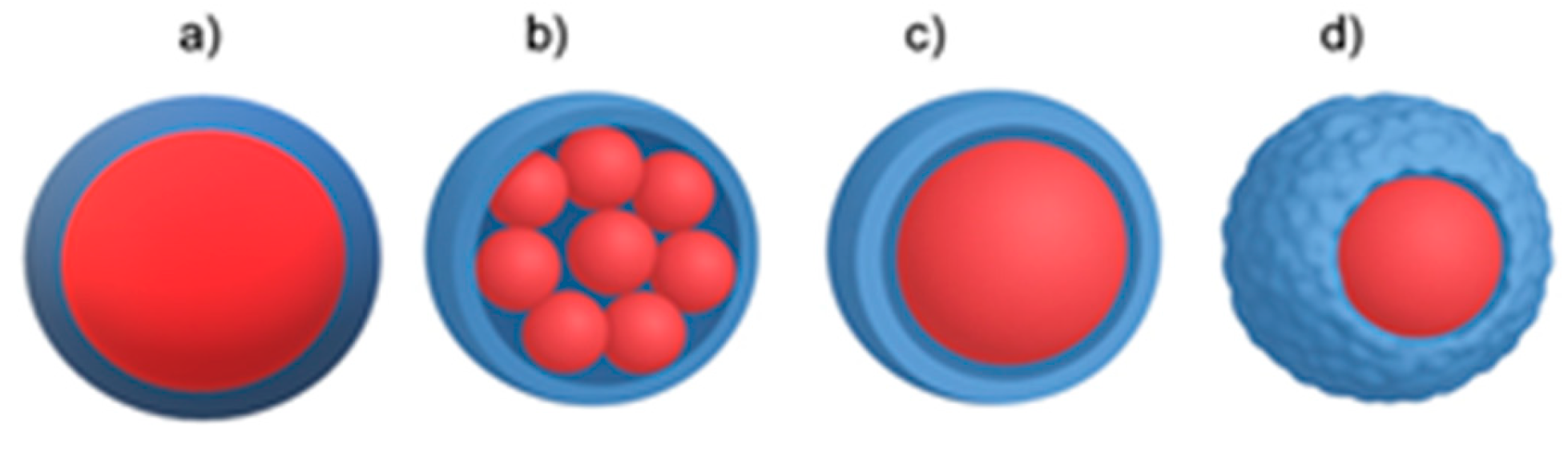
- Physical–chemical methods: Simple and complex coacervation, phase separation, drying bath, powder bed grinding, melting-dispersion condensation, and capsule-core exchange [7,10]. Figure 1 illustrates the various modes of how a core material (depicted in red) can be encapsulated into coating materials.
2. Encapsulated Materials
2.1. Stem Cells
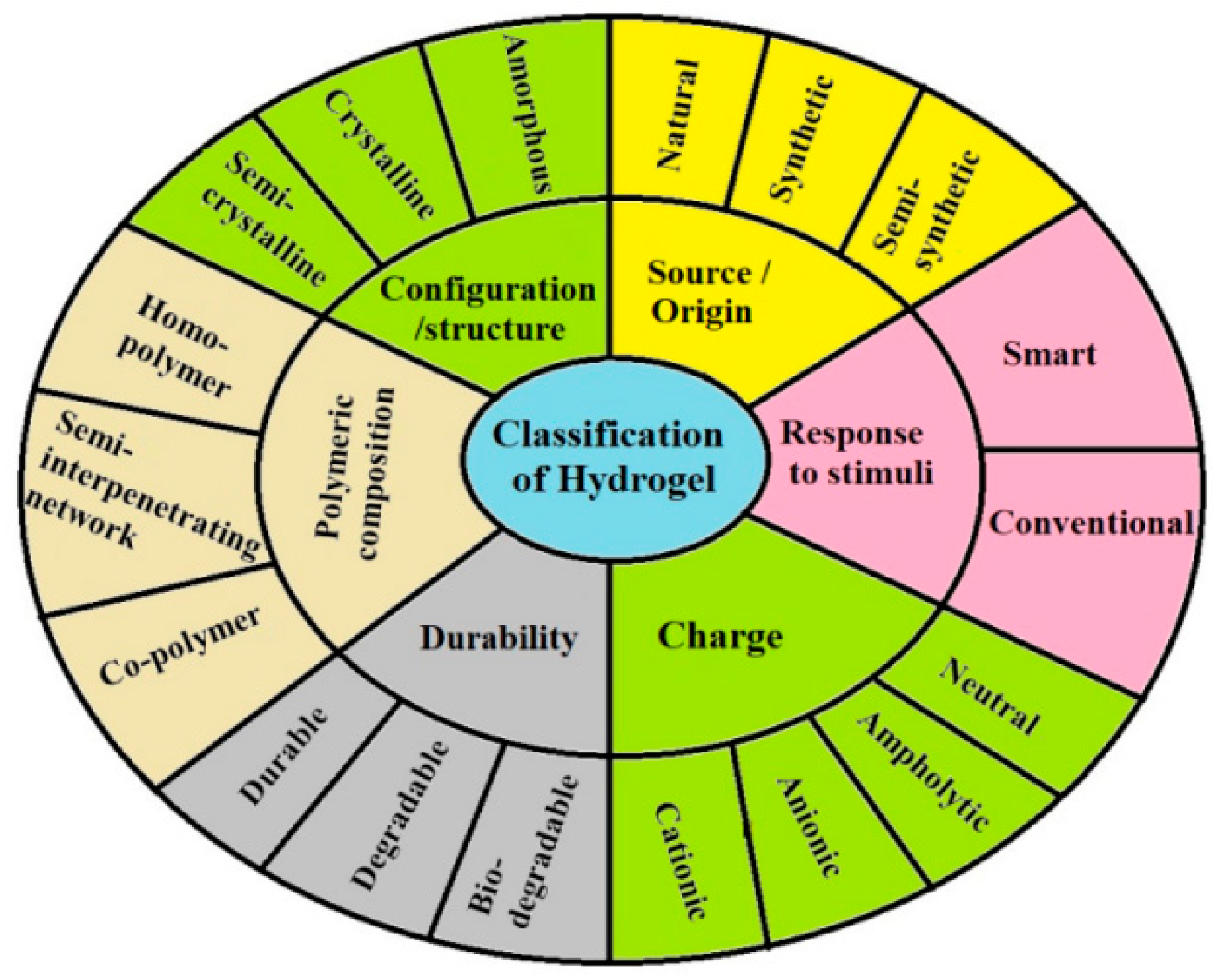
2.2. Hydrogels and Nanoparticles
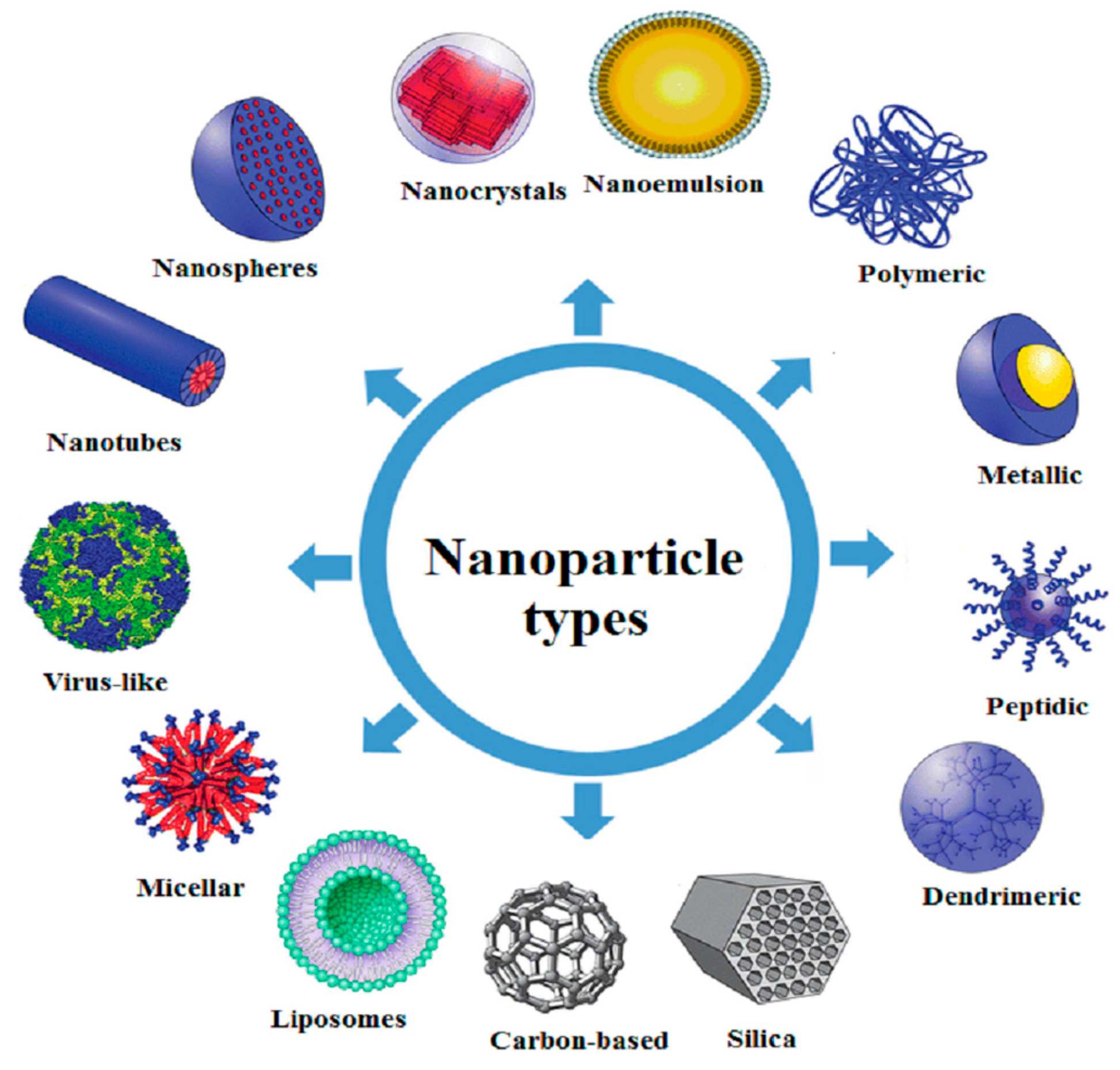
2.3. Nanoparticles–Hydrogel Structures
3. Hyaluronic Acid
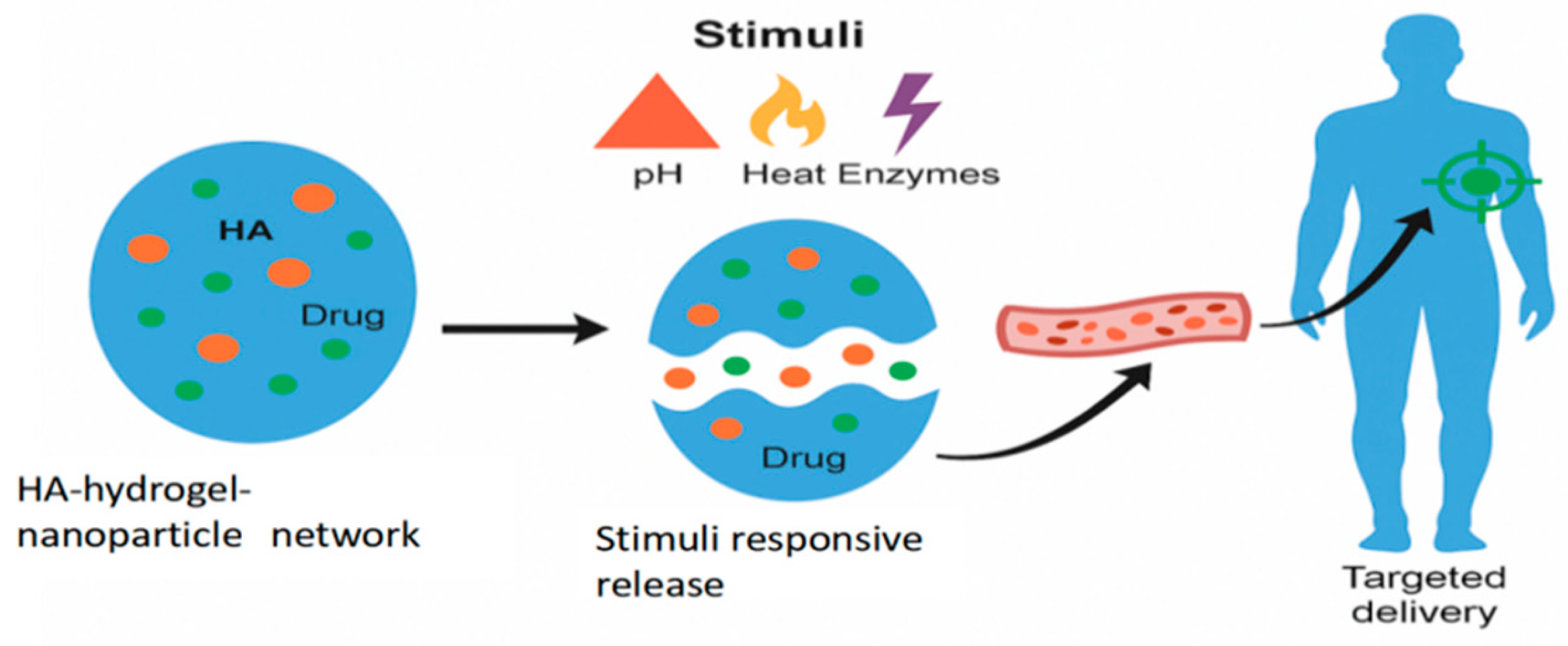
- pH-Responsive systems, which exploit the natural pH gradients in the body, e.g., pH 2–3 in the stomach, pH 6.5–7.4 in the small intestine [50]. For this purpose, pH-sensitive polymers or nanocarriers are often used. These materials contain ionizable groups (e.g., carboxyl or amino groups) that undergo protonation or deprotonation in response to a pH change. This change alters the polymer’s solubility or structure, causing it to swell, dissolve, or undergo a conformational change that results in releasing the encapsulated drug.
- Temperature-responsive systems are triggered by changes in temperature during, e.g., fever, inflammation, or tumors, or are induced externally (e.g., hyperthermia therapy). The most common materials are thermo-responsive polymers that undergo a reversible phase transition at a specific temperature, known as the lower critical solution temperature. Below this temperature, the polymer is hydrophilic and swells with water, but above it, it becomes hydrophobic and shrinks, expelling the encapsulated drug [51].
- Enzyme-responsive systems are based on using specific enzymes that are overexpressed or uniquely present in certain diseases. The drug is often linked to the carrier via a bond that can be specifically cleaved by a target enzyme. When the system encounters the enzyme at the diseased site, the enzyme degrades the carrier or breaks the linker, thus releasing the drug [52].
- Redox-responsive systems are designed to respond to the differences in redox potential between healthy and diseased cells. The drug carrier is cross-linked with a redox-sensitive bond, most commonly a disulfide bond (-S-S-). This bond remains stable in the oxidative extracellular environment but is cleaved by the high concentration of glutathione inside the target cell, e.g., in a tumor. The cleavage leads to the degradation of the carrier and the rapid release of the drug [53].
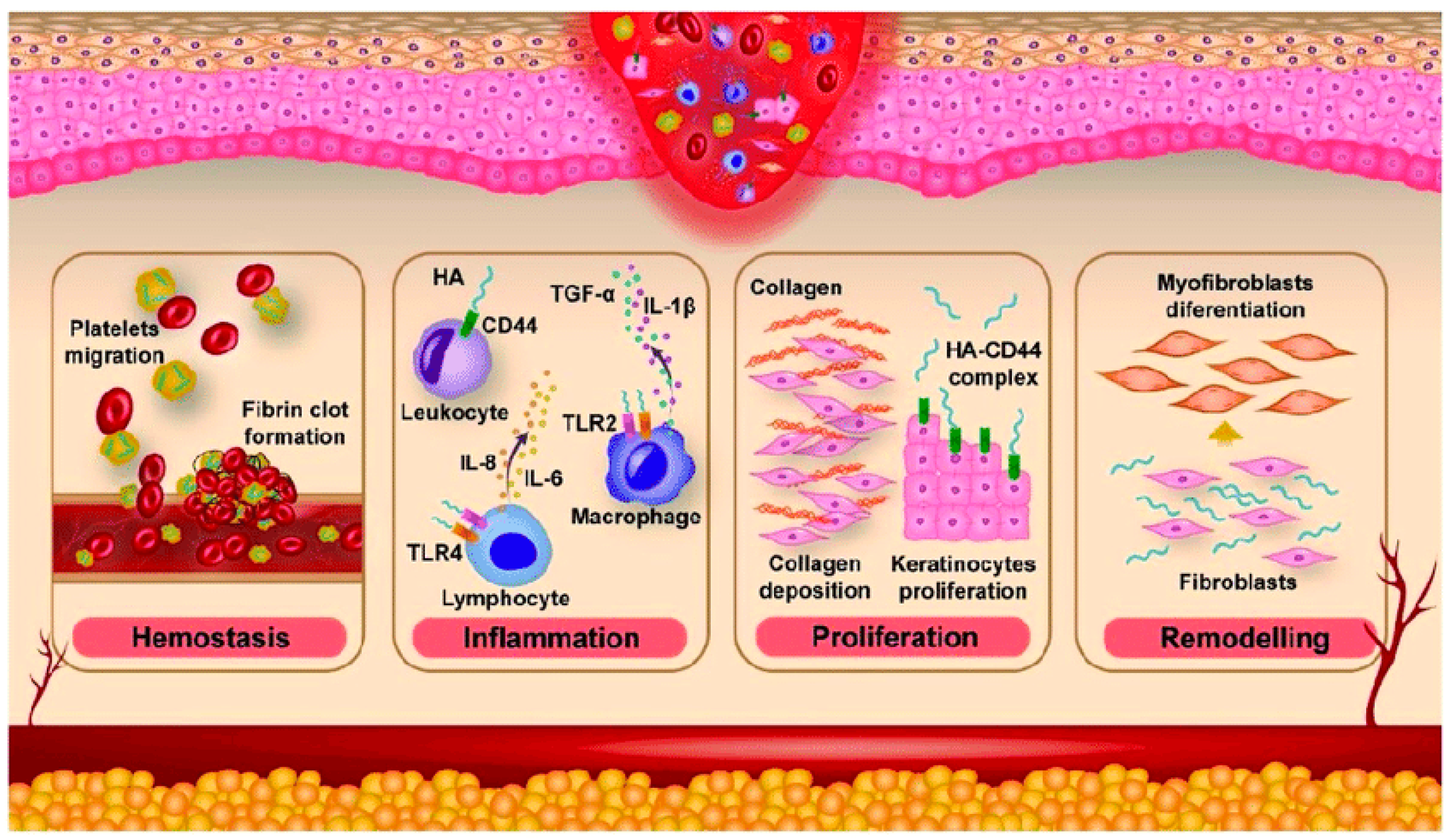
4. Treatment of Skin Wounds
5. Treatment of Diabetes Mellitus
6. Treatment of Eye Diseases
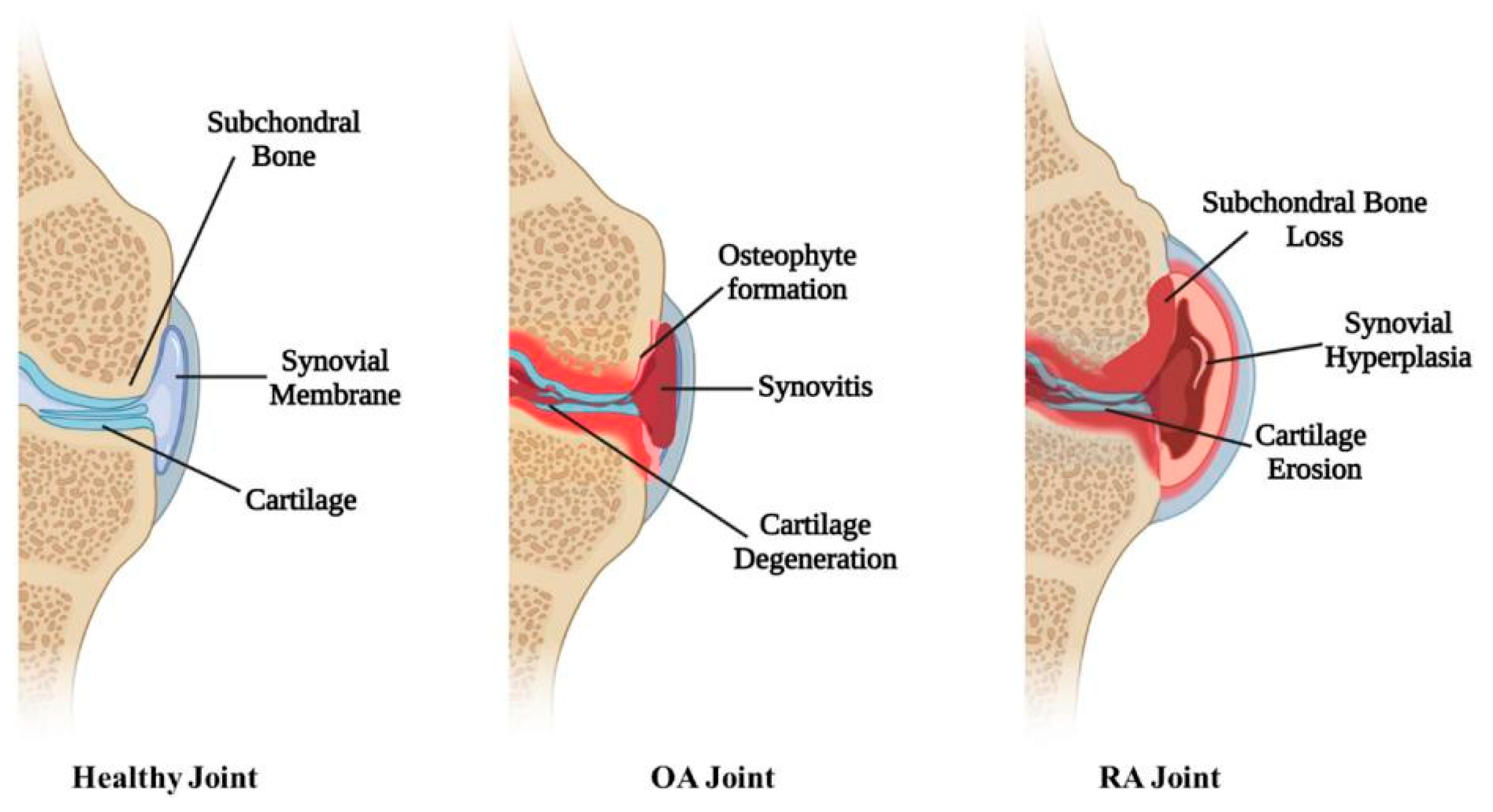
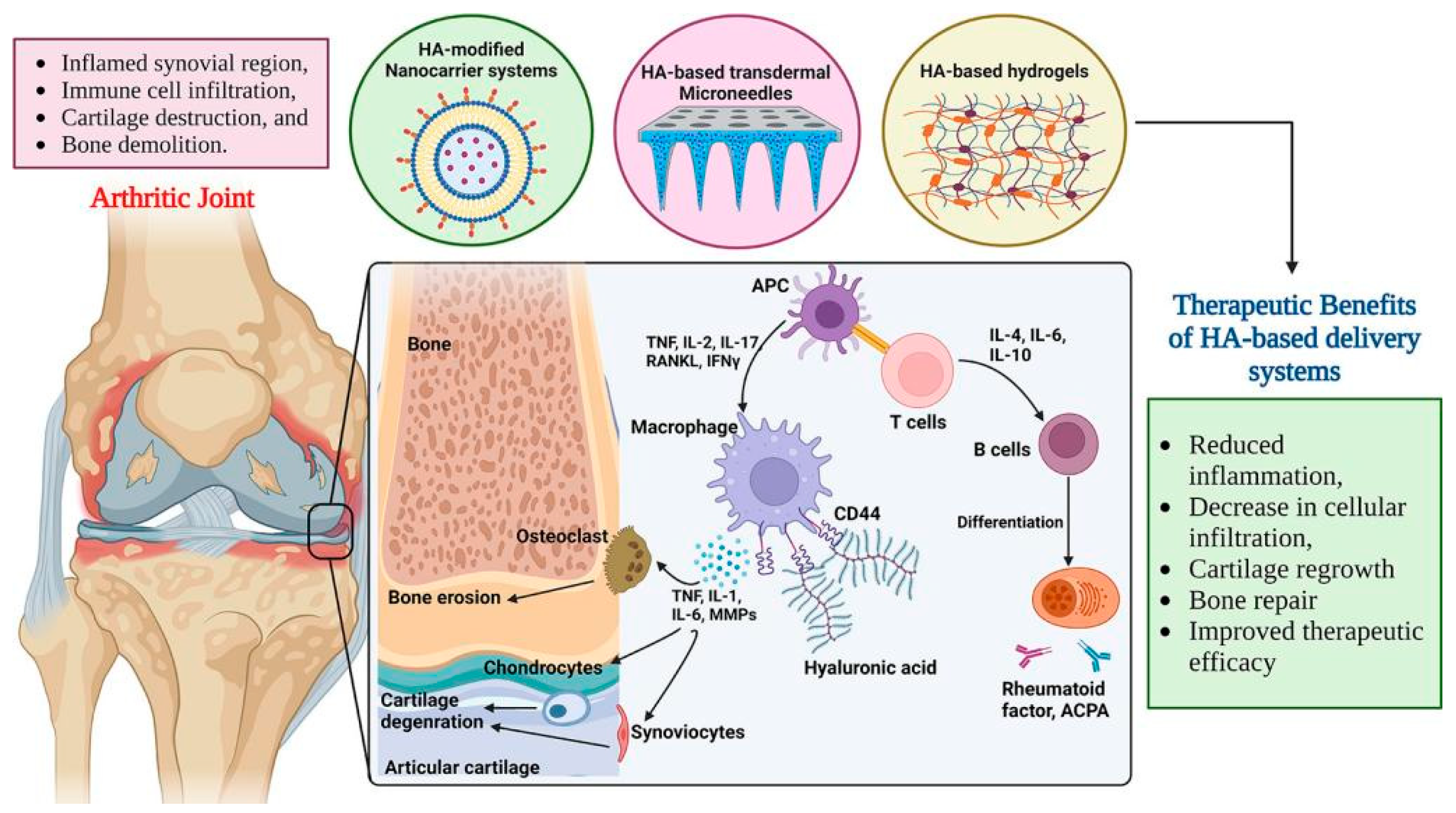
7. Treatment of Osteoarthritis and Rheumatoid Arthritis
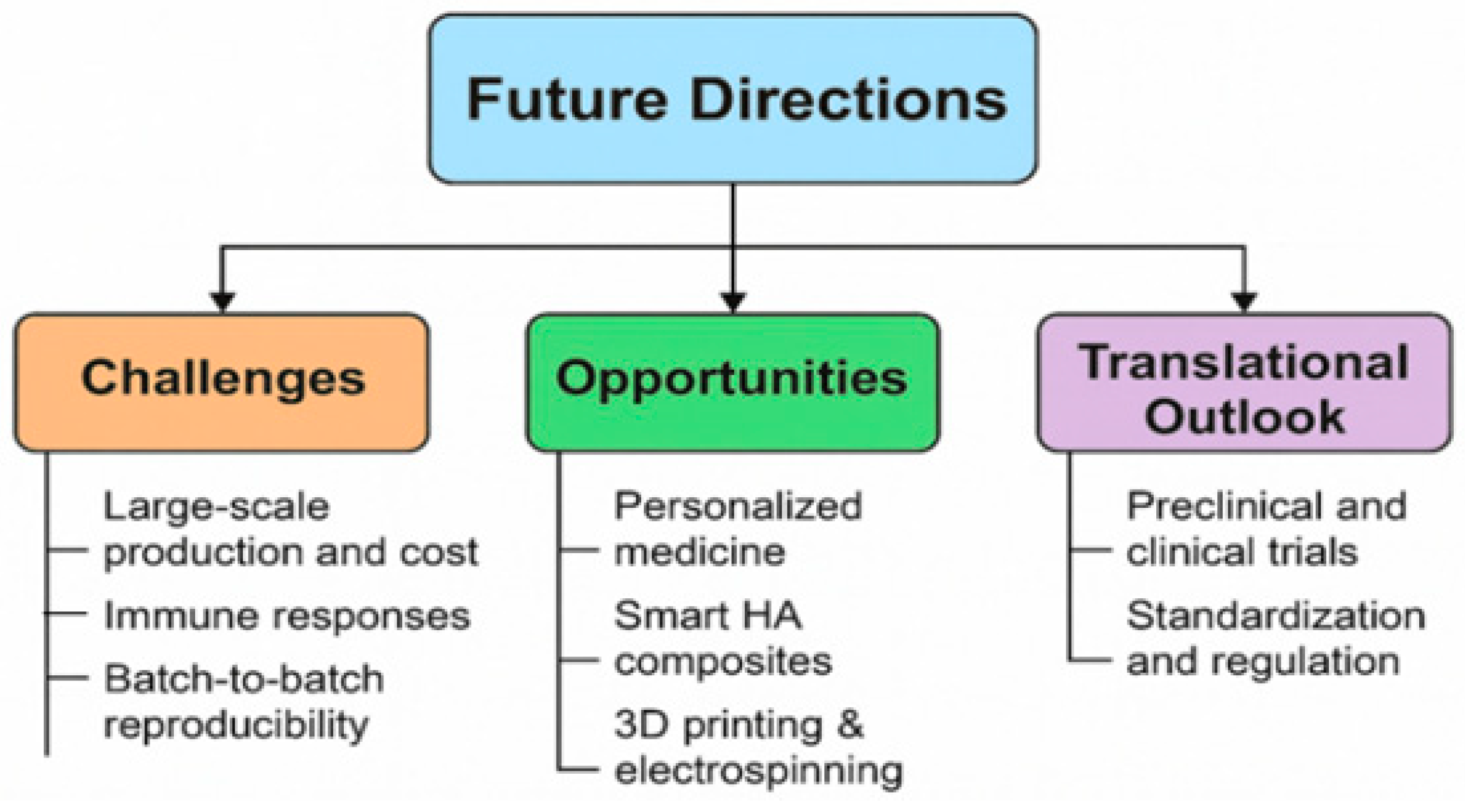
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ALG-POL | Alginate-poloxamer |
| AMD | Age-related macular degeneration |
| AHAMA | Anhydride and methacrylic-modified hyaluronan hydrogel |
| BMP-7 | Bone morphogenic protein 7 |
| BSA | Bovine serum albumin |
| CD | Cyclodextrin |
| DES | Dry eye syndrome |
| DFO | Deferoxamine |
| DR | Diabetic retinopathy |
| DSP | Dexamethasone sodium phosphate |
| ECM | Extracellular matrix |
| Exos | Exosome |
| GelMA | Methacrylated gelatin |
| GOx | Glucose oxidase |
| HA | Hyaluronan |
| HAMA | Hyaluronan methacrylate |
| HMW | High molecular weight |
| HUVEC | Human umbilical vein endothelial cell |
| IGF-1 | Insulin-like growth factor |
| IL | Interleukin |
| LAT-HA-LIP | Latanoprost-loaded phosphatidylcholine liposomes with hyaluronan |
| LMW | Low molecular weight |
| MEL | Melatonin |
| MMP | Metalloproteinase |
| MSc | Mesenchymal stem cells |
| NF-κB | Nuclear factor kappa B |
| NH | Nanohydrogel |
| NIR | Near-infrared radiation |
| NPs | Nanoparticles |
| OA | Osteoarthritis |
| ODDs | Ocular drug delivery systems |
| PAE | Paeonol |
| PBA | Phenylboronic acid |
| PEG | Polyethylene glycol |
| PCO | Collagen thermo-sensitive poloxamer |
| PEGDA | Poly(ethylene glycol)diacrylate |
| PLGA | Poly(lactic-co-glycolid acid) |
| PVA | Polyvinylalcohol |
| RA | Rheumatoid arthritis |
| RGD | Arginylglycylaspartic acid |
| ROS | Reactive oxygen species |
| RNV | Retinal neovascularization |
| SCN | Curcumin/silk/HA |
| SH | Silk/HA |
| siRNA | Silent interfering RNA |
| SNAs | Spherical nucleic acid |
| SrRan | Strontium ranelate |
| TLR-4 | Toll-like receptor |
| TNF-α | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Berkland, C.; Kipper, M.J.; Narasimhan, B.; Kim, K.Y.; Pack, D.W. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J. Control. Release 2004, 94, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Oryan, A. Application of encapsulated probiotics in health care. J. Exp. Pathol. 2020, 1, 16–21. [Google Scholar]
- Ayyaril, S.S.; Rawas-Qalaji, M.; Shanableh, A.; Cagliani, R.; Bhattacharjee, S.; Cagliani, R.; Shahib, A.G.; Khan, M.I. Recent progress in micro and nano-encapsulation techniques for environmental applications: A review. Results Eng. 2023, 18, 101094. [Google Scholar] [CrossRef]
- Tischer, W.; Wedekind, F. Immobilized Enzymes: Methods and Applications Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1999; Volume 200, pp. 96–126. [Google Scholar]
- Dubey, R.; Shami, T.C.; Bhasker Rao, K.U. Microencapsulation technology and applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A review of applications in the food and pharmaceutical industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Garg, F.A.; Chhipa, K.; Kumar, L. Microencapsulation techniques in pharmaceutical formulation. Eur. J. Pharm. Med. Res. 2018, 5, 199–206. [Google Scholar]
- Green, L.J.; Bhatia, N.D.; Toledano, O.; Erlich, M.; Spizuoco, A.; Goodyear, B.C.; York, J.P.; Jakus, J. Silica-based microencapsulation used in topical dermatologic applications. Arch. Dermatol. Res. 2023, 315, 2787–2793. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Sharma, P. A review on microencapsulation as method of drug delivery. BIO Web Conf. 2024, 86, 01033. [Google Scholar] [CrossRef]
- Fu, F.; Hu, L. Temperature sensitive colour changed composites. In Advanced High Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 405–423. [Google Scholar]
- Trojanowska, A.; Nogalska, A.; Garcia Valls, R.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. In Polymer Engineering; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 171–201. [Google Scholar]
- da Silva, P.T.; Fries, M.; de Menezes, L.L.; Holkem, C.R.; Schwan, A.T.; Wigmann, C.L.; de Oliveira Bastos, É.F.; de Bona da Silva, J.C. Microencapsulation: Concepts, mechanisms, methods and some applications in food technology. Ciência Rural 2014, 44, 1304–1311. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials—A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Gurruchaga, H.; Saenz del Burgo, L.; Orive, G.; Hernandez, R.M.; Ciriz, J.; Pedraz, J.L. Low molecular-weight hyaluronan as a cryoprotectant for the storage of microencapsulated cells. Int. J. Pharm. 2018, 548, 206216. [Google Scholar] [CrossRef] [PubMed]
- Marikar, S.N.; El Osta, A.; Johnston, A.; Such, G.; Al Hasani, K. Microencapsulation-based cell therapies. Cell. Mol. Life Sci. 2022, 79, 351. [Google Scholar] [CrossRef]
- Pezhman, A. Biomaterial in microencapsulation: How microencapsulation is changing the medicine world. In Biomaterials in Microencapsulation; Sharma, A., Ed.; Intechopen: Rijeka, Croatia, 2024; ISBN 978-0-85466-106-0. [Google Scholar]
- Rokkam, M.; Vadaga, A.K. A brief review on the current trends in microencapsulation. J. Pharma Insights Res. 2024, 2, 108114. [Google Scholar] [CrossRef]
- Fravel, D.R.; Marois, J.J.; Lumsden, R.D.; Conick, W.J. Encapsulation of potential biocontrol agents in an alginate-clay matrix. Phytopathology 1985, 75, 774–777. [Google Scholar] [CrossRef]
- Salerno, A.; Causa, F.; Di Natale, C.; Domingo, C.; Vecchione, R. Editorial: Microencapsulation for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 891981. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell encapsulation within alginate microcapsules: Immunological challenges and outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef]
- Jahanbekam, S.; Mozafari, N.; Bagheri-Alamooti, A.; Mohammadi Samani, S.; Daneshamouz, S.; Heidari, R.; Azarpira, N.; Ashrafi, H.; Azadi, A. Ultrasound-responsive hyaluronic acid hydrogel of hydrocortisone to treat osteoarthritis. Int. J. Biol. Macromol. 2023, 240, 124449. [Google Scholar] [CrossRef]
- Beninatto, R.; Barbera, C.; De Lucchi, O.; Borsato, G.; Serena, E.; Guarise, C.; Pavan, M.; Luni, C.; Martewicz, S.; Galesso, D.; et al. Photocrosslinked hydrogels from coumarin derivatives of hyaluronic acid for tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 625–634. [Google Scholar] [CrossRef]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic acid biomaterials for central nervous system regenerative medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Aslam, M.A.; Bin Abdullah, M.F.; Al-Arjan, W.S.; Stojanovic, G.M.; Hasan, A. Hydrogels: Classifications, fundamental properties, applications, and scopes in recent advances in tissue engineering and regenerative medicine—A comprehensive review. Arab. J. Chem. 2024, 17, 105968. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Luo, Y.; Liang, Q.; Yu, Y.; Chen, F.; Yao, J. Enhancing stem cell therapy for cartilage repair in osteoarthritis—A hydrogel focused approach. Gels 2021, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.-T.; Liu, T.-Y.; Chiang, M.-Y.; Chen, C.-Y.; Chang, S.-J.; Chen, S.-Y. Cartilage tissue-mimetic pellets with multifunctional magnetic hyaluronic acid-graft-amphiphilic gelatin microcapsules for chondrogenic stimulation. Polymers 2020, 12, 785. [Google Scholar] [CrossRef]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Emerging fabrication strategies of hydrogels and its applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef]
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Rao, S.; Zhou, M.; Lu, J.; Liang, D.; Zhu, B.; Meng, L.; Lin, J.; Ding, X.; et al. Recent development of micro-nano carriers for oral antineoplastic drug delivery. Mater. Today Bio. 2025, 30, 101445. [Google Scholar] [CrossRef]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef]
- Spleis, H.; Sandmeier, M.; Claus, V.; Bernkop-Schnürch, A. Surface design of nanocarriers: Key to more efficient oral drug delivery systems. Adv. Colloid Interface Sci. 2023, 313, 102848. [Google Scholar] [CrossRef]
- Vishwakarma, N.; Jain, A.; Sharma, R.; Mody, N.; Vyas, S.; Vyas, S.P. Lipid-based nanocarriers for lymphatic transportation. AAPS PharmSciTech 2019, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Morakul, B. Self-nanoemulsifying drug delivery systems (SNEDDS): An advancement technology for oral drug delivery. Pharm. Sci. Asia 2020, 47, 205–220. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Jain, V. An overview of nanostructured lipid carriers and its application in drug delivery through different routes. Adv. Pharm. Bull. 2022, 13, 446–460. [Google Scholar] [CrossRef]
- Liposome Encapsulation of Hydrophilic and Hydrophobic Drugs. Available online: https://www.protocols.io/view/liposome-encapsulation-of-hydrophilic-and-hydropho-rm7vzymorlx1/v1 (accessed on 16 September 2025).
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524539. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Alayli, A.; Ince, S. Synthesis of nanoparticles by green synthesis method. Int. J. Innov. Res. Rev. 2017, 1, 69. [Google Scholar]
- Kim, M.; Jung, M.-Y.; Lee, D.-Y.; Ahn, S.M.; Lee, G.M.; Park, C.Y. How to fabricate hyaluronic acid for ocular drug delivery. Pharmaceutics 2024, 16, 1604. [Google Scholar] [CrossRef]
- Rah, M.J. A review of hyaluronan and its ophthalmic applications. Optometry 2011, 82, 38–43. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millenium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Chylinska, N.; Maciejczyk, M. Hyaluronic acid and skin: Its role in aging and wound-healing processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97 Pt B, 400416. [Google Scholar] [CrossRef] [PubMed]
- John, H.E.; Price, R.D. Perspectives in the selection of hyaluronic acid fillers for facial wrinkles and aging skin. Patient Prefer. Adherence 2009, 3, 225–230. [Google Scholar]
- Khan, M.Z.I.; Prebeg, Ž.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Zhu, S.; Wang, S.; Su, J.; Xu, C. Enzyme-responsive liposomes for controlled drug release. Drug Discov. Today 2024, 29, 104014. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Ang, L.F.; Darwis, Y.; Koh, R.Y.; Leong, K.V.G.; Yew, M.Y.; Por, L.Y.; Yam, M.F. Wound healing property of curcuminoids as a microcapsule-incorporated cream. Pharmaceutics 2019, 11, 205. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in wound-healing products based on natural compounds, stem cells, and microRNA-based biopolymers in the European, USA, and Asian markets: Opportunities, barriers, and regulatory issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burns Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Dalmedico, M.M.; Meier, M.J.; Cestari Felix, J.V.; Pott, F.S.; de Fátima Cordeiro Petz, F.; Santos, M.C. Hyaluronic acid covers in burn treatment: A systematic review. Rev. Esc. Enferm. USP 2016, 50, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.D. Overview: Acute and chronic wounds. Nurs. Clin. N. Am. 2002, 40, 191205. [Google Scholar] [CrossRef]
- Kim, D.S.; Seong, K.-Y.; Lee, H.; Kim, M.J.; An, S.-M.; Jeong, J.S.; Kim, S.Y.; Kang, H.-G.; Jang, S.; Hwang, D.-Y.; et al. Antiadhesive hyaluronic acid-based wound dressings promote wound healing by preventing re-injury: An in vivo investigation. Biomedicines 2024, 12, 510. [Google Scholar] [CrossRef]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for chronic wounds. Dermatol. Ther. 2013, 26, 197206. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 2014, 58, 186–192. [Google Scholar] [CrossRef]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2012, 11, 159–163. [Google Scholar] [CrossRef]
- Tolg, C.; Telmer, P.; Turley, E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS ONE 2014, 9, e88479. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Versatile use of chitosan and hyaluronan in medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef]
- Bourguignon, L. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef]
- Ghier, T. Role of myofibroblasts in wound healing and tissue repair. Curr. Opin. 2023, 15, 11–12. [Google Scholar]
- Guan, A.Y.; Chen, Y.; Tseng, S.C.; Lin, Q. CD44 signaling in skin wound healing and regeneration. J. Transl. Med. 2025, 7, 880. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-J.; Yang, S.-J.; Shieh, M.-J.; Young, T.-H. Development of a chitosan-silver nanocomposite/β-1,3-glucan/hyaluronic acid composite as an antimicrobial system for wound healing. Polymers 2025, 17, 350. [Google Scholar] [CrossRef]
- Hong, H.J.; Gwon, K.; Park, G.; Yu, J.-H.; Lee, S.; Yu, J.-S.; Lee, D.N. Antibacterial and bioadhesive characteristics of mussel-inspired hyaluronic acid hydrogels encapsulated with sea urchin-shaped copper-coated silicon dioxide nanoparticles. Carbohydr. Polym. Technol. Appl. 2025, 10, 100781. [Google Scholar] [CrossRef]
- Gonçalves, R.R.; Peixoto, D.; Costa, R.R.; Franco, A.R.; Castro, V.I.B.; Pires, R.A.; Reis, R.L.; Pashkuleva, I.; Maniglio, D.; Tirella, A.; et al. Antibacterial properties of photo-crosslinked chitosan/methacrylated hyaluronic acid nanoparticles loaded with bacitracin. Int. J. Biol. Macromol. 2024, 277 Pt 4, 134250. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, L.; Krystyjan, M.; Lenart-Boron, A.; Krzan, M.; Kulik, K.; Białecka, A.; Grabacka, M.; Nowak, N.; Khachatryan, K. Preparation of nano/microcapsules of ozonated olive oil in hyaluronan matrix and analysis of physicochemical and microbiological (biological) properties of the obtained biocomposite. Int. J. Mol. Sci. 2022, 23, 14005. [Google Scholar] [CrossRef]
- Wang, J.; Di Risola, D.; Mattioli, R.; Zoratto, N.; Mosca, L.; Di Meo, C.; Matricardi, P. Hyaluronan-cholesterol nanogels embedding betamethasone for the treatment of skin inflammatory conditions. Int. J. Pharm. 2025, 668, 124978. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, L.; Jiang, X.; Hu, Y.; Jin, Y.; Hu, H.; Li, W. Self-assembled hyaluronic acid nanoparticles delivered by polymeric microneedles for targeted and long-acting therapy of psoriasis. Int. J. Pharm. 2025, 669, 125073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, J.; Cheng, L.; Xue, Y.; Yang, J.; Sun, Z.; Liu, J. Hyaluronic acid modified liposomes with enhanced transdermal delivery of methotrexate for psoriasis treatment. Colloids Surf. B Biointerfaces 2025, 247, 114457. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, Y.; Xu, Q.; Li, Z.; Liu, Z.; Dai, F. Hyaluronic acid/silk fibroin nanoparticles loaded with methotrexate for topical treatment of psoriasis. Int. J. Pharm. 2025, 9, 100312. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Z.; Chen, Y.; Lu, H.; Hu, P. Resina draconis particles encapsulated in a hyaluronic-acid-based hydrogel to treat complex burn wounds. Pharmaceutics 2022, 14, 2087. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fu, X.; Lun, L.; Jiang, W.; Situ, X.; Huang, X.; Xiong, Y.; Liu, C.; Wang, F. Engineering a halloysite nanotube-enhanced hydrogel 3D skin model for modulated inflammation and accelerated wound healing. Bioact. Mater. 2025, 45, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhang, J.N.; Wu, S.W.; Deng, Y.; Wang, S.H.; Xie, L.; Li, X.P.; Yang, L. Exosome/antimicrobial peptide laden hydrogel wound dressings promote scarless wound healing through miR-21-5p-mediated multiple functions. Biomaterials 2024, 308, 122558. [Google Scholar] [CrossRef] [PubMed]
- Politi, F.A.S.; Carvalho, S.G.; Rodero, C.F.; Dos Santos, K.; Meneguin, A.B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. Piperine-loaded nanoparticles incorporated into hyaluronic acid/sodium alginate-based membranes for the treatment of inflammatory skin diseases. Int. J. Biol. Macromol. 2023, 227, 736748. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, Y.; Kong, X.; Luo, Y.; Zhang, Y.; Wu, Y.; Tan, J.; Chen, J.; Xu, T.; Zhu, L. Enhanced burn wound healing by controlled-release 3D ADMSC-derived exosome-loaded hyaluronan hydrogel. Regen. Biomater. 2024, 11, rbae035. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 2032. [Google Scholar] [CrossRef]
- Celani, L.M.S. Effect of hyaluronic acid on skin healing in diabetic rats. J. Surg. Clin. Res. 2019, 10, 76–87. [Google Scholar] [CrossRef]
- Basta, G.; Montanucci, P.; Calafiore, R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: The actual state and future perspectives between promise and progress. J. Diabetes Investig. 2021, 12, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Li, J.; Guo, J.; Yu, Y.; Li, L. Pancreatic islet cells in microfluidic-spun hydrogel microfibers for the treatment of diabetes. Acta Biomater. 2024, 187, 149160. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic -cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef]
- Calafiore, R.; Basta, G.; Montanucci, P. Microencapsulation of islets for the treatment of type 1 diabetes mellitus (T1D). In Cell Microencapsulation. Methods and Protocols; Opara, E.C., Ed.; Humana Press: New York, NY, USA, 2017. [Google Scholar]
- Omami, M.; McGarrigle, J.J.; Reedy, M.; Isa, D.; Ghani, S.; Marchese, E.; Bochenek, M.A.; Longi, M.; Xing, Y.; Joshi, I.; et al. Islet microencapsulation: Strategies and clinical status in diabetes. Curr. Diab. Rep. 2017, 17, 47. [Google Scholar] [CrossRef]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McCarrigle, J.J.; Meirigeng, Q.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza, E.J.; Nourmohammadzadeh, M.; et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cell transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2018, 2, 810–821. [Google Scholar] [CrossRef]
- Cañibano-Hernández, A.; del Burgoa, L.S.; Espona-Noguera, A.; Orive, G.; Hernández, R.M.; Ciriza, J.; Pedraz, J.L. Hyaluronic acid enhances cell survival of encapsulated insulin-producing cells in alginate-based microcapsules. Int. J. Pharm. 2019, 557, 192–198. [Google Scholar] [CrossRef]
- Scheiner, K.C.; Coulter, F.; Maas-Bakker, R.F.; Ghersi, G.; Nguyen, T.T.; Steendam, R.; Duffy, G.P.; Hennink, W.E.; O’Cearbhaill, E.D.; Kok, R.J. Vascular endothelial growth factor-releasing microspheres based on poly(ε-caprolactone-PEG-ε-caprolactone)-b-poly(l-lactide) multiblock copolymers incorporated in a three-dimensional printed poly(dimethylsiloxane) cell macroencapsulation device. J. Pharm. Sci. 2020, 109, 863870. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2023, 165, 86–101. [Google Scholar] [CrossRef]
- Li, H.; Shang, Y.; Feng, Q.; Liu, Y.; Chen, J.; Dong, H. A novel bioartificial pancreas fabricated via islets microencapsulation in anti-adhesive core-shell microgels and macroencapsulation in a hydrogel scaffold prevascularized in vivo. Bioact. Mater. 2023, 27, 362376. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhao, C.; Li, X.; Chen, M.; Liu, Z.; Liu, J.; Xiao, Y.; Fan, Y.; Jiang, Q.; et al. Polysaccharide hybrid scaffold encapsulated endogenous factors for microfracture enhancement by sustainable release and cell recruitment. Compos. Part B 2024, 273, 111235. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Shakya, S.; Wang, Y.; Mack, J.A.; Maytin, E.V. Hyperglycemia-induced changes in hyaluronan contribute to impaired skin wound healing in diabetes: Review and perspective. Int. J. Cell Biol. 2015, 2015, 701738. [Google Scholar] [CrossRef]
- Alissa, M.; Alghamdi, A.; Alshehri, M.A. Curcumin nanoparticles loaded in a bioengineering and biodegradable silk-hyaluronan scaffold triggered wound healing by regulating inflammation and accelerating proliferation in a diabetic rat model. Tissue Cell 2025, 95, 102840. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Y.; Wang, Y.; Walter, M.; Chirume, D.; Yao, W.; Lan, Z.; Zhao, Z.; Xu, X.; Zhang, W.; et al. A smart drug delivery microgel system with phased intervention capabilities and dual physical state of use promotes healing of diabetic infected wounds. J. Mater. Chem. B 2025, 13, 4138–4156. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; You, J.; Zhang, Y.; Zhang, L.; Quni, S.; Wang, H.; Zhou, Y. Glucose-responsive self-healing bilayer drug microneedles promote diabetic wound healing via a trojan-horse strategy. ACS Appl. Mater. Interfaces 2024, 16, 24351–24371. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Wang, Y.; Xiang, H.; He, J.; Sun, Q.; Yong, Y.; Chen, L.; Jiang, K.; Li, Y. Zinc-based polyoxometalate nanozyme functionalized hydrogels for optimizing the hyperglycemic-immune microenvironment to promote diabetic wound regeneration. J. Nanobiotechnol. 2024, 22, 611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, H.; Gan, T.; Ma, X.; Geng, Q.; Yin, S.; Zhang, H.; Wu, Y. Smart hydrogel dressing enhances the healing of chronic infectious diabetic wounds through dual-barrier drug delivery action. Biomacromolecules 2024, 25, 6814–6829. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Yang, S.; Guo, Q.; Zhang, Y.; Wang, Z.; Xu, S.; Qiao, D.; Ma, M.G.; Zheng, P.; et al. Targeting starvation therapy for diabetic bacterial infections with endogenous enzyme-triggered hyaluronan-modified nanozymes in the infection microenvironment. Int. J. Biol. Macromol. 2024, 270 Pt 1, 77. [Google Scholar] [CrossRef]
- Luo, Q.; Yan, C.; Ma, R.; Dai, L.; Shu, W.; Asghar, A.; Jia, Z.; Zhu, X.; Yu, S. E-PL/MnO2 nanozymes/gellan gum/hyaluronic acid-based multifunctional hydrogel to promote diabetic wound healing. Int. J. Biol. Macromol. 2025, 304 Pt 1, 140777. [Google Scholar] [CrossRef]
- Wu, J.; Yang, L.; Shi, G.; Zou, L.; He, J.; Li, J.; Zhang, A.; Wang, X.; Liu, Z.; Tang, K.; et al. Carvacrol/cyclodextrin/ceria nanoparticle/hyaluronate hybrid microneedle for promoted diabetic wound healing through the modulation of microenvironment. Int. J. Biol. Macromol. 2025, 291, 139126. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Yu, H.; Wang, L.; Wang, Y.; Liu, X.; Huang, Y.; Ouyang, C.; Hong, Y.; Ren, S.; et al. Lauric acid-mediated gelatin/hyaluronic acid composite hydrogel with effective antibacterial and immune regulation for accelerating MRSA-infected diabetic wound healing. Int. J. Biol. Macromol. 2025, 290, 138792. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; He, X.; Liu, H.; Liu, Y.; Wang, H.; Zhou, Z.; Chen, L.; Ji, X.; Yang, R.; Xie, J. Multifunctional bioactive nanozyme systems for enhanced diabetic wound healing. Adv. Healthc. Mater. 2025, 14, 2401580. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qin, B.; Tang, X.; Cui, T.; Yin, S.; Dong, H.; Liu, Y.; Deng, S.; Zhang, H.; Feng, G.; et al. Enzyme-responsive microneedle patch for bacterial infection and accelerated healing of diabetic wounds. Chem. Eng. J. 2023, 466, 26. [Google Scholar] [CrossRef]
- Hua, J.; Huang, R.; Yu, M.; You, R.; Wang, L.; Yan, S.; Huang, Y.; Zhang, Q. High-performance silk fibroin/hyaluronic acid interpenetrating network hydrogel microneedles for diabetes management. Int. J. Biol. Macromol. 2025, 298, 140357. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, L.; Lin, M.; Bao, X.; Zhong, H.; Ke, P.; Dai, Q.; Yang, Q.; Tang, X.; Xu, W.H.; et al. Double layer spherical nanoparticles with hyaluronic acid coating to enhance oral delivery of exenatide in T2DM rats. Eur. J. Pharm. Biopharm. 2023, 191, 205–218. [Google Scholar] [CrossRef]
- Tu, Q.A.; Thi, P.L.; Oh, D.H.; Lee, S.; Park, K.D. Injectable multifunctional hyaluronan-based hydrogel suppressing the excessive glucose and reactive oxygen species in diabetic wound treatment. Mater. Today Commun. 2025, 42, 98. [Google Scholar] [CrossRef]
- Radwan, S.E.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef]
- Elmotasem, H.; Salama, A.A.A.; Shalaby, E.S. Hyaluronate-functionalized span-labrasol nanovesicular transdermal therapeutic system of ferulic acid targeting diabetic nephropathy. Int. J. Biol. Macromol. 2024, 279, 135292. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Leinonen, S.; Immonen, I.; Kotaniemi, K. Fluocinolone acetonide intravitreal implant (Retisert®) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol. 2018, 96, 648–651. [Google Scholar] [CrossRef]
- Cabrera, F.J.; Wang, D.C.; Reddy, K.; Acharya, G.; Shin, C.S. Challenges and opportunities for drug delivery to the posterior of the eye. Ther. Deliv. 2017, 8, 641–653. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Udaondo, P.; Cunha-Vaz, J.; Sivaprasad, S.; Bandello, F.; Lanzetta, P.; Kodjikian, L.; Goldstein, M.; Habot-Wilner, Z.; Loewenstein, A. A collaborative retrospective study on the efficacy and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: The European DME registry study. Ophthalmology 2020, 127, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Woo, S.J. Ocular drug delivery to the retina: Current innovations and future perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Casey-Power, S.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. Hyaluronic acid: Its versatile use in ocular drug delivery with a specific focus on hyaluronic acid-based polyelectrolyte complexes. Pharmaceutics 2022, 14, 1479. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.B.; Zhang, H. The emerging role of topical ocular drugs to target the posterior eye. Ophthalmol. Ther. 2021, 10, 465–494. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H.H. Applications of hyaluronic acid in ophthalmology and contact lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Zadło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: Asystematic review. J. Cosmet. Dermatol. 2016, 4, 520–526. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a prominent biomolecule with numerous applications in medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Sakpal, D.; Mhase, M.; Momin, M.; Gharat, S.; Sawarkar, S.; Boddu, S.H.S.; Al Tabakha, M. Design, formulation, and evaluation of opthalmic multilayer nanofber insert for management of dry eye disease. Bionanoscience 2025, 15, 97. [Google Scholar] [CrossRef]
- Ngo, H.V.; Nguyen, H.D.; Lee, B.J. Hyaluronic acid conjugates with controlled oleic acid substitution as new nanomaterials for improving ocular co-delivery of cyclosporine A and oleic acid. Asian J. Pharm. Sci. 2025, 20, 101009. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked hyaluronan electrospun nanofibers for ferulic acid ocular delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, A.; Zhang, D.; Zhang, M.; Ma, L.; Li, Y.; Wang, W.; Nan, K.; Chen, H.; Li, L. Injectable double-network hydrogel for corneal repair. Chem. Eng. J. 2023, 455, 140698. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Wu, X.; Guan, L. Enhanced corneal repair with hyaluronic acid/proanthocyanidins nanoparticles. ACS Omega 2025, 10, 2222–2230. [Google Scholar] [CrossRef]
- Ribeiro, M.C.S.; de Miranda, M.C.; Cunha, P.D.S.; Andrade, G.F.; Fulgêncio, G.D.O.; Gomes, D.A.; Fialho, S.L.; Pittella, F.; Charrueau, C.; Escriou, V.; et al. Neuroprotective effect of siRNA entrapped in hyaluronic acid-coated lipoplexes by intravitreal administration. Pharmaceutics 2021, 13, 845. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Zhao, L.; Fu, X.; Peng, S.; Zhang, W.; Wang, R.; Yuan, W.; Luo, R.; Wang, X.; et al. Retinal cell-targeted liposomal ginsenoside Rg3 attenuates retinal ischemia-reperfusion injury via alleviating oxidative stress and promoting microglia/macrophage M2 polarization. Free Rad. Biol. Med. 2023, 206, 162–179. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, D.; Mo, L.; Jing, G.; Zeng, L.; Cheng, H.; Guo, Q.; Dai, M.; Wang, Y.; Chen, J.; et al. Multifunctional nanogel loaded with cerium oxide nanozyme and CX3CL1 protein: Targeted immunomodulation and retinal protection in uveitis rat model. Biomaterials 2024, 309, 2617. [Google Scholar] [CrossRef]
- Duan, N.; Mei, L.; Hu, L.; Yin, X.; Wei, X.; Li, Y.; Li, Q.; Zhao, G.; Zhou, Q.; Du, Z. Biomimetic, injectable, and self-healing hydrogels with sustained release of ranibizumab to treat retinal neovascularization. ACS Appl. Mater. Interfaces 2023, 15, 6371–6384. [Google Scholar] [CrossRef]
- Miyagawa, T.; Chen, Z.-Y.; Chang, C.-Y.; Chen, K.-H.; Wang, Y.-K.; Liu, G.-S.; Tseng, C.-L. Topical application of hyaluronic acid-RGD peptide-coated gelatin/epigallocatechin-3 gallate (EGCG) nanoparticles inhibits corneal neovascularization viainhibition of VEGF production. Pharmaceutics 2020, 12, 404. [Google Scholar] [CrossRef]
- Durak, S.; Sutova, H.E.; Ceylan, R.; Aciksari, A.; Yetisgin, A.A.; Tokuc, E.O.; Kutlu, O.; Karabas, V.L.; Cetinel, S. A nanogel formulation of anti-VEGF peptide for ocular neovascularization treatment. CS Appl. Bio Mater. 2024, 7, 6001–6013. [Google Scholar] [CrossRef]
- Baker, A.E.G.; Cui, H.; Ballios, B.G.; Ing, S.; Yan, P.; Wolfer, J.; Wright, T.; Dang, M.; Gan, N.Y.; Cook, M.J.; et al. Stable oxime-crosslinked hyaluronan-based hydrogel as a biomimetic vitreous substitute. Biomaterials 2021, 271, 120750. [Google Scholar] [CrossRef]
- Brugnera, M.; Vicario-de-la-Torre, M.; González-Cela Casamayor, M.A.; López-Cano, J.J.; Bravo-Osuna, I.; Huete-Toral, F.; González Rubio, M.L.; Carracedo, G.; Molina-Martínez, I.T.; Andrés-Guerrero, V.; et al. Enhancing the hypotensive effect of latanoprost by combining synthetic phosphatidylcholine liposomes with hyaluronic acid and osmoprotective agents. Drug Deliv. Transl. Res. 2024, 14, 2804–2822. [Google Scholar] [CrossRef]
- Chaharband, F.; Daftarian, N.; Kanavi, M.R.; Varshochian, R.; Hajiramezanali, M.; Norouzi, P.; Arefian, E.; Atyabi, F.; Dinarvand, R. Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal VEGFR-2 siRNA delivery: Formulation and in vivo efficacy evaluation. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102181. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.U.; Luyten, F.P. Osteoarthritis, a disease bridging development and regeneration. BoneKEy Rep. 2012, 1, 136. [Google Scholar] [CrossRef] [PubMed]
- Arhebamen, E.P.; Teodoro, M.T.; Blonka, A.B.; Matthew, H.W.T. Long-term culture performance of a polyelectrolyte complex microcapsule platform for hyaline cartilage repair. Bioengineering 2023, 10, 467. [Google Scholar] [CrossRef]
- Katoh, S.; Yoshioka, H.; Senthilkumar, R.; Preethy, S.; Abraham, S.J.K. Enhanced expression of hyaluronic acid in osteoarthritis affected knee-cartilage chondrocytes during three-dimensional in vitro culture in a hyaluronic-acid-retaining polymer scaffold. Knee 2021, 29, 365–373. [Google Scholar] [CrossRef]
- Juránek, I.; Stern, R.; Šoltés, L. Hyaluronan peroxidation is required for normal synovial function: A hypothesis. Med. Hypotheses 2014, 82, 662–666. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Sprott, H.; Fleck, C. Hyaluronic acid in rheumatology. Pharmaceutics 2023, 15, 2247. [Google Scholar] [CrossRef]
- Šoltés, L.; Steiner, B.; Machová, E.; Kogan, G.; Bystrický, S.; Mendichi, R. Clathrate Complexes Formed by Hyaluronic Acid Derivatives and Use Thereof as Pharmaceuticals. International Patent C08B 37/08; WO 01/66601 A1, 12 March 2001. [Google Scholar]
- Šoltés, L.; Bystrický, S.; Steiner, B.; Machová, E.; Mendichi, R.; Bauer, V.; Kogan, G.; Alföldi, J.; Stratilová, E.; Mach, M. Method of Preparation of Ultra-High Molecular Weight Hyaluronans by Physico-Chemical Crosslinking of Derivatives of High Molecular Weight Hyaluronic Acid or of Its Salts. Slovak Patent No. 282717, 10 March 2000. [Google Scholar]
- Pradal, J.; Mauden, P.; Gabay, C.; Seemayer, C.A.; Jordan, O.; Allémann, E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int. J. Pharm. 2016, 498, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Acar, K.; Bedir, S.; Kayitmazer, A.B.; Kose, G.T. Chondro-inductive hyaluronic acid/chitosan coacervate-based scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2021, 188, 300–312. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Hou, Y.; Meng, X.; Wang, D.; Liu, J.; Sun, F.; Li, Y. Hyaluronic acid coated acid-sensitive nanoparticles for targeted therapy of adjuvant-induced arthritis in rats. Molecules 2019, 24, 146. [Google Scholar] [CrossRef]
- Liu, L.; Guo, W.; Liang, X.J. Move to nano-arthrology: Targeted stimuli-responsive nanomedicines combat adaptive treatment tolerance (ATT) of rheumatoid arthritis. Biotechnol. J. 2019, 14, 1800024. [Google Scholar] [CrossRef]
- Zewail, M.; Nafee, N.; Helmy, M.W.; Boraie, N. Synergistic and receptor-mediated targeting of arthritic joints via intra-articular injectable smart hydrogels containing leflunomide-loaded lipid nanocarriers. Drug Deliv. Transl. Res. 2021, 11, 2496–2519. [Google Scholar] [CrossRef]
- Wen, J.; Li, H.; Dai, H.; Hua, S.; Long, X.; Li, H.; Ivanovski, S.; Xu, C. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio 2023, 19, 100597. [Google Scholar] [CrossRef]
- Priya, S.; Daryani, J.; Desai, V.M.; Singhvi, G. Bridging the gap in rheumatoid arthritis treatment with hyaluronic acid-based drug delivery approaches. Int. J. Biol. Macromol. 2024, 271 Pt 1, 132586. [Google Scholar] [CrossRef]
- Greco, V.; Lanza, V.; Tomasello, B.; Naletova, I.; Cairns, W.R.L.; Sciuto, S.; Rizzarelli, E. Copper complexes with new glycyl-l-histidyl-l-lysine–hyaluronan conjugates show antioxidant properties and osteogenic and angiogenic synergistic effects. Bioconjugate Chem. 2025, 36, 662675. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2020, 6, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, Z.; Nasrollahzadeh, M.; Heidari, F.; Nasrabadi, D. A drug-loaded nano chitosan/hyaluronic acid hydrogel system as a cartilage tissue engineering scaffold for drug delivery. Int. J. Biol. Macromol. 2024, 283 Pt 1, 137314. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Liu, J.; Zhang, Y.; Yang, B.; Wan, Y.; Wu, J. Morphogenic protein-7-loaded hyaluronic acid complex nanoparticles for inducing chondrogenic differentiation of synovium-derived mesenchymal stem cells. Pharmaceutics 2020, 12, 613. [Google Scholar] [CrossRef]
- Sharma, R.; Jain, H.; Pratibha; Godugu, C.; Chella, N. Formulation and optimization of aceclofenac loaded hyaluronic-oleic acid based micellar gel for the management of osteoarthritis. J. Drug Deliv. Sci. Technol. 2023, 84, 104560. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.; Li, X.; Xu, Y.; Lu, G.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold. Acta Biomater. 2020, 111, 197–207. [Google Scholar] [CrossRef]
- Yin, Z.; Qin, C.; Pan, S.; Shi, C.; Wu, G.; Feng, Y.; Zhang, J.; Yu, Z.; Liang, B.; Gui, J. Injectable hyperbranched PEG crosslinked hyaluronan hydrogel microparticles containing mir-99a-3p modified subcutaneous ADSCs-derived exosomes was beneficial for long-term treatment of osteoarthritis. Mater. Today Bio 2023, 23, 100813. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, F.; Zhang, H.; Cheng, L.; Chen, K.; Shen, J.; Qi, J.; Deng, L.; He, C.; Santos, H.A.; et al. DNA-grafted hyaluronic acid system with enhanced injectability and biostability for photo-controlled osteoarthritis gene therapy. Adv. Sci. 2021, 8, 2004793. [Google Scholar] [CrossRef]
- Svarca, A.; Grava, A.; Dubnika, A.; Ramata-Stunda, A.; Narnickis, R.; Aunina, K.; Rieksta, E.; Boroduskis, M.; Jurgelane, I.; Locs, J.; et al. Calcium phosphate/hyaluronic acid composite hydrogels for local antiosteoporotic drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 917765. [Google Scholar] [CrossRef]
- Vu, B.T.H.; Le, A.M.M.; Tang, T.N.; Dang, N.N.T.; Duong, T.T.; Hua, H.T.N.; Phan, T.B.; Ta, H.T.K.; Pham, V.H.; Tran, Q.N.; et al. Fabrication of novel bone substitute alginate–N,O-carboxymethyl chitosan–aldehyde hyaluronic acid–biphasic calcium phosphate for bone regeneration. React. Funct. Polym. 2023, 191, 105691. [Google Scholar] [CrossRef]
- Makled, S.; Abbas, H.; Ali, M.E.; Zewail, M. Melatonin hyalurosomes in collagen thermosensitive gel as a potential repurposing approach for rheumatoid arthritis management via the intra-articular route. Int. J. Pharm. 2024, 661, 124449. [Google Scholar] [CrossRef] [PubMed]
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Monteiro, M.; Grandjean, C.; Alonso, M.J. An in situ hyaluronic acid-fibrin hydrogel containing drug-loaded nanocapsules for intra-articular treatment of inflammatory joint diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Abbas, H.; Elsheikh, M.A. Innovative rheumatoid arthritis management using injection replacement approach via dual therapeutic effects of hyalurosomes-encapsulated luteolin and dexamethasone. Colloids Surf. B Biointerfaces 2025, 249, 114497. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, K.; Wan, G.; Liao, W.; Jin, J.; Wang, P.; Sun, X.; Wang, W.; Jiang, Q. A ROS-responsive hydrogel encapsulated with matrix metalloproteinase-13 siRNA nanocarriers to attenuate osteoarthritis progression. J. Nanobiotechnol. 2025, 23, 18. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, T.; Chen, J.; Cui, Y.; Zhang, X.; Lee, R.J.; Sun, F.; Li, Y.; Teng, L. PLGA/PCADK composite microspheres containing hyaluronic acid–chitosan siRNA nanoparticles: A rational design for rheumatoid arthritis therapy. Int. J. Pharm. 2021, 596, 120204. [Google Scholar] [CrossRef]
- Hussain, Z.; Pandey, M.; Thu, H.E.; Kaur, T.; Jia, G.W.; Ying, P.C.; Xian, T.M.; Abourehab, M.A.S. Hyaluronic acid functionalization improves dermal targeting of polymeric nanoparticles for management of burn wounds: In vitro, ex vivo and in vivo evaluations. Biomed. Pharmacother. 2022, 150, 112992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Guo, X.; Zhao, Y.; Ren, S.; Zhang, Z.; Lv, H. Hyaluronic acid-cyclodextrin encapsulating paeonol for treatment of atopic dermatitis. Int. J. Pharm. 2022, 623, 121916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhou, H.; Shu, J.; Fu, S.; Yang, Z. Skin wound healing promoted by novel curcumin-loaded micelle hydrogel. Ann. Transl. Med. 2021, 9, 1152. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Zhang, Z.; Wang, L.; Fan, Y.; Liu, X.; Liu, X.; Ya, H.; Zhang, Y.; Xu, Y. Hyaluronic acid micelles for promoting the skin permeation and deposition of curcumin. Int. J. Nanomed. 2022, 17, 4009–4022. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Song, Y.; Chen, Y.; Zhu, D.; Yu, L.; Huang, Q.; Zhang, Z.; Xue, Z.; Hua, Z.; et al. Hyaluronic acid hydrogels hybridized with Au-triptolide nanoparticles for intraarticular targeted multi-therapy of rheumatoid arthritis. Front. Pharmacol. 2022, 13, 849101. [Google Scholar] [CrossRef]
- Zewail, M.; El-Deeb, N.M.; Mousa, M.R.; Abbas, H. Hyaluronic acid coated teriflunomide (A771726) loaded lipid carriers for the oral management of rheumatoid arthritis. Int. J. Pharm. 2022, 623, 121939. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Khattab, M.A.; Abd-Allah, H. Intra-articular multifunctional celecoxib loaded hyaluronan nanocapsules for the suppression of inflammation in an osteoarthritic rat model. Int. J. Pharm. 2020, 583, 119378. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, Y.C.; Yang, K.C.; Ling, Y.F.; Wu, A.T.H.; Tseng, C.L. Development and functional evaluation of a hyaluronic acid coated nano-formulation with kaempferol as a novel intra-articular agent for knee osteoarthritis treatment. Biomed. Pharmacother. 2024, 175, 11671. [Google Scholar] [CrossRef]
- Mammella, A.; Bhavana, V.; Sandeep Chary, P.; Anuradha, U.; Mehra, N.K. Modulation of chondroprotective hyaluronic acid and poloxamer gel with ketoprofen loaded transethosomes: Quality by design-based optimization, characterization, and preclinical investigations in osteoarthritis. Int. J. Biol. Macromol. 2024, 280 Pt 4, 135919. [Google Scholar] [CrossRef]
- Hsu, X.L.; Wu, L.C.; Hsieh, J.Y.; Huang, Y.Y. Nanoparticle-hydrogel composite drug delivery system for potential ocular applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Ricci, F.; Racaniello, G.F.; Laquintana, V.; Arduino, I.; Lopedota, A.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Sigurdsson, H.H.; Denora, N. Chitosan/sulfobutylether-β-cyclodextrin based nanoparticles coated with thiolated hyaluronic acid for indomethacin ophthalmic delivery. Int. J. Pharm. 2022, 622, 121905. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Marto, J.; São Braz, B.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef]
- Cardoso, J.F.; Perasolia, F.B.; Almeida, T.C.; De Freitas Marques, M.B.; Toledo, C.R.; Gil, P.O.; Da Silva Tavares, H.; Da Paz, M.C.; Da Nova-Mussel, W.; Magalhães, J.T.; et al. Vancomycin-loaded N,N-dodecyl, methyl-polyethylenimine nanoparticles coated with hyaluronic acid to treat bacterial endophthalmitis: Development, characterization, and ocular biocompatibility. Int. J. Biol. Macromol. 2021, 169, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Oliveira, B.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Ciprofloxacin-loaded zein/hyaluronic acid nanoparticles for ocular mucosa delivery. Pharmaceutics 2022, 14, 1557. [Google Scholar] [CrossRef]
- Zingale, E.; Weaver, E.; Bertelli, P.M.; Lengyel, I.; Pignatello, R.; Lamprou, D.A. Development of dual drug loaded-hydrogel scaffold combining microfluidics and coaxial 3D-printing for intravitreal implantation. Int. J. Pharm. 2024, 665, 124700. [Google Scholar] [CrossRef]
- Frayssinet, A.; Petta, D.; Illoul, C.; Haye, B.; Markitantova, A.; Eglin, D.; Mosser, G.; D’Este, M.D.; Hélary, C. Extracellular matrix-mimetic composite hydrogels of cross-linked hyaluronan and fibrillar collagen with tunable properties and ultrastructure. Carbohydr. Polym. 2020, 236, 116042. [Google Scholar] [CrossRef] [PubMed]
- Chocarro-Wrona, C.; de Andres, J.L.; Rioboó-Legaspi, P.; Pleguezuelos-Beltrán, P.; Antich, C.; De Vicente, J.; Gálvez-Martín, P.; López-Ruiz, E.; Marchal, J.A. Design and evaluation of a bilayered dermal/hypodermal 3D model using a biomimetic hydrogel formulation. Biomed. Pharmacother. 2024, 177, 117051. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Fang, J.; Zhou, S.; Xie, M.; Zhang, K.; Li, J.; Yin, G. Enzyme-crosslinked hyaluronic acid hydrogel scaffolds for BMSCs microenvironment and wound healing. Int. J. Biol. Macromol. 2025, 95, 139566. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.H.; Na, K.S.; et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef]
- Montero, A.; Atienza, C.; Elvira, C.; Jorcano, J.L.; Velasco, D. Hyaluronic acid-fibrin hydrogels show improved mechanical stability in dermo-epidermal skin substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112352. [Google Scholar] [CrossRef]
- Lengwan, P.; Chanvorachote, P. In situ hyaluronic acid-based hydrogel incorpor ogel incorporated with insulin-like growth factor-1 for three-dimensional encapsulation of keratinocytes. Thai J. Pharm. Sci. 2022, 46, 37–45. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Li, G.; Hu, Z.; Xu, R.; Zhu, T.; Cao, X.; Yao, Y.; Jian, W.; Chen, J.; et al. Micelle-facilitated gelation kinetics and viscoelasticity of dynamic hyaluronan hydrogels for bioprinting of mimetic constructs and tissue repair. Compos. B Eng. 2025, 294, 112151. [Google Scholar] [CrossRef]
- Harrington, S.; Ott, L.; Karanu, F.; Ramachandran, K.; Stehno-Bittel, L. A versatile platform for hyaluronic acid and polyethylene glycol. Tissue Eng. Part A 2021, 27, 153–164. [Google Scholar] [CrossRef]
- Zamboni, F. Hyaluronic Acid 3D Microenvironments for Diabetes Treatment. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2021. [Google Scholar]
- Park, J.; Choe, G.; Oh, S.; Lee, J.Y. In situ formation of proangiogenic mesenchymal stem cell spheroids in hyaluronic acid/alginate core-shell microcapsules. ACS Biomater. Sci. Eng. 2020, 6, 6938–6948. [Google Scholar] [CrossRef]
- Assi, M.M.; Grawish, M.E.; Elsabaal, H.M.; Helal, M.E.; Ezzat, S.K. Therapeutic potential of hyaluronic acid hydrogel combined with bone marrow stem cells-conditioned medium on arthritic rats’ TMJs. Sci. Rep. 2024, 14, 26828. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, L.; Chen, X.; Ran, Y.; Tong, Q.; Tang, L.; Li, X. Injectable hyaluronic acid/hydroxyapatite composite hydrogels as cell carriers for bone repair. Int. J. Biol. Macromol. 2022, 216, 547–557. [Google Scholar] [CrossRef]
- Flégeau, K.; Puiggali-Jou, A.; Zenobi-Wong, M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication 2022, 14, 034105. [Google Scholar] [CrossRef] [PubMed]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef]
| Components | Encapsulated Drug | Matrix Morphology | Targeted Therapy | Effects | Particle Size [nm] | Period of Drug Release | Ref. |
|---|---|---|---|---|---|---|---|
| HA | Curcumin and quercetin | NPs | Skin burns | MC3T3-E1 cell proliferation, improved penetration of curcumin and quercetin through the stratum corneum, 98% wound healing on day 28, granulation tissue was formed. | 177 ± 11 | 72.4% curcumin and 87.7% quercetin in 70 h | [174] |
| HA/cyclodextrin (CD) | Paeonol (PAE, a natural substance) | Topical delivery carrier | Atopic dermatitis | The PAE retention rates of the HACD-PAE group in the stratum corneum and dermis were 3.35 and 1.78 times, respectively, higher than those of the PAE group. HACD could increase the gap of keratinocytes by interacting with corneum lipids and loosening the keratin, with high efficacy on atopic dermatitis mice. | 177 ± 9.19 | 40% in 12 h | [175] |
| HA/polycaprolactone-b-PEG-b-polycaprolactone | Curcumin | Hydrogel | Skin wound | Enhanced angiogenesis, the formation of collagen fibers. | 129 | 72% in 12 h | [176] |
| Octadecylamine-modified HA | Curcumin | Micelles | Skin burns | Significantly increased skin penetration and retention of curcumin, higher analgesic and anti-inflammatory activities in vivo when compared with curcumin solution. Curcumin’s transdermal penetration mechanism may be associated with HA’s hydration of the stratum corneum. | 165.64 | 48 h at 4 °C in 21 days | [177] |
| HA/bilosomes | Au-triptolide | Hydrogel | RA | Excellent cellular uptake and targeted delivery efficiency for triptolide, elongation of circulatory residence time, enhancement of intraarticular bioavailability, and higher in vivo antiarthritic efficacy compared to uncoated triptolide/bilosomes. | 164.2 | 60% release with near-infrared radiation (NIR), 30% release without NIR in 24 h. | [178] |
| HA | Teriflunomide | Lipid carriers | RA | High stability, superior cytotoxicity and binding affinity to CD44 receptors compared with teriflunomide itself, increased teriflunomide bioavailability, reduced TNF-α serum levels, and improved joint healing. | 284.9 ± 3.8 | 100% in 30 h | [179] |
| Lipid carriers coated with chondroitin sulfate, HA, or chitosan | Leflunomide | Hydrogel | OA | Fastest recovery of rats, improved cartilage thickness, chondrocyte proliferation and neovascularization, reduced TNF-α level 4–5-fold relative to positive control, limited chondrocyte apoptosis, and production of pro-inflammatory cytokines. | 101.5−153.8 | 100% in 44 h | [156] |
| HA/oleic acid | Aceclofenac | Micelles | OA | A significant reduction in pain and inflammation and improved radiological and histopathological conditions in animals. | 245 ± 7.68 | 80% in 50 h | [163] |
| HA | Celecoxib | Nanocapsules | OA | Higher efficacy of celecoxib nanocapsules compared to celecoxib suspension in a monoiodoacetate-induced OA rat model. | 254.9 ± 3.06 | 100% in 7 days | [180] |
| HA/gelatin | Kaempferol | NPs | OA | Significant reduction in subchondral sclerosis and the severity of OA in the ACLT rat model, attenuated inflammation and ECM degradation, and restored cartilage thickness. | 88.62 ± 3.90 | 18% over 48 h | [181] |
| HA/poloxamer | Ketoprofen-loaded transethosomes | Hydrogel | OA | The X-ray imaging of the treated group showed intact meniscus, healthy articular joints, and the same normal synovial lining as in the healthy control group, reduced pain and inflammation. | 110.0 ± 1.70 | Approx. 90% in 80 h | [182] |
| PLGA NPs/HA | Bovine serum albumin | Hydrogel | Ocular neovascular diseases − AMD | Retained 75% of its wet weight without losing its integrity, and the release of the model drug at the rate of 0.4 g/day for more than 2 months under physiological conditions improved bioavailability of the drug by penetrating deep into the retinal layers. | 54.81 ± 7.95 | Cumulative rapid release within 24 h, followed by a linear release lasting up to 56 days. | [183] |
| Chitosan/sulfobutylether-β-CD/thiolated HA | Indomethacin | NPs | Ophthalmology (anterior segment inflammation diseases) | Increased residential time in the conjunctival sac, no irritation or toxicity. In contrast, the uncoated NPs displayed better permeating properties since they are smaller and could be further exploited for the treatment of posterior segment diseases. | 340 ± 7 | 80% in 6 h | [184] |
| Chitosan/HA | Erythropoietin | NPs | Ophthalmology | More rapid permeation through porcine conjunctiva, followed by sclera and cornea, and noncytotoxicity on ARPE-19 and HaCaT cell lines enhanced its retention time and permeation through the different ocular membranes. | ≤300 | 80% release of erythropoetin from simulated tear fluid in 6 h | [185] |
| N,N-dodecyl, methyl-polyethylenimine/HA | Vancomycin | NPs | Ophthalmology (bacterial endophthalmitis) | Nontoxic to ARPE-19 cells, non-irritating to the chorioallantoic membrane, and no changes in retinal functions. | 154 ± 3 | 58% over 96 h | [186] |
| Zein and HA | Ciprofloxacin | NPs | Ophthalmology (bacterial conjunctivitis) | A possible alternative to the current antibacterial topical dosage forms available on the market for treating. | 200 | Approx. 100% within 24 h | [187] |
| HA/chitosan | Curcumin liposomes, resveratrol | Hydrogel | Diabetic retinopathy | The successful integration of liposomes and hydrogels in the creation of 3D-printed hydrogel scaffolds enabled the delivery of resveratrol and curcumin. Microfluidics and 3D bioprinting can be effectively combined to produce versatile carriers capable of accommodating various active pharmaceutical ingredients. | <200 | 75% of resveratrol and 10% of curcumin in 24 h | [188] |
| Components/Coating Material | Encapsulated Cells | Matrix Morphology | Targeted Therapy | Effects, Properties | Ref. |
|---|---|---|---|---|---|
| Collagen/tyramine/HA | Amniotic mesenchymal stem cell metabolite products | Hydrogel | Skin wounds | Highly resistant against enzymatic degradation, with a high degree of hydration and cell viability, collagen improved cell attachment and survival. | [189] |
| Agarose-collagen type I/dermatan sulfate, HA, elastin | NIH-3T3 cells | Hydrogel | Skin wounds | High cytocompatibility and hemocompatibility, supported cell growth and metabolic activity, created 3D mesh structures with potential clinical application as a cellular skin substitute. | [190] |
| HA/dopamine | BMSCs and growth factors | Hydrogel | Skin wounds | Significantly accelerated healing of acute full-thickness skin wounds, resulting in the formation of appendages such as hair follicles and minimal scarring. | [191] |
| HA-CD and HA-adamantane | Human corneal epithelial cells | Hydrogel | Opthalmology | Absorbed within the corneal stroma over time, modulated mesenchymal corneal stromal cell secretome production, reduced cellularity and inflammation of the anterior stroma, and significantly mitigated corneal edema compared to treatment with linear HA and untreated control eyes. | [192] |
| Fibrin and thiolated HA | Primary human fibroblasts | Hydrogel | Skin wound healing | Reduction in contraction, more homogeneous keratin 10 (K10) expressions in the supra-basal layer of the epidermis; enhanced stratum corneum formation for the constructs containing HA. | [193] |
| HAMA/insulin-like growth factor 1 (IGF-1) | Keratinocytes | Hydrogel | Skin wound healing | HAMA (3% w/v) hydrogel was the most appropriate for the 3D cell culture. Incorporating IGF-1 into the hydrogel in a dose-dependent manner significantly enhanced the viability of the encapsulated keratinocytes. The hydrogels were shown to be cytocompatible. The keratinocytes were shown to grow in 3D fashion. | [194] |
| F127 diacrylate/HA | NIH-3T3 cells | Micelles | Tissue repair | Better physical–chemical properties, using a 3D printer led to precise structures with high cell viability. The viscoelastic microenvironment fosters fibroblast spreading within the bioprinted matrices and supports the development of a biomimetic skin construct characterized by multilayer keratinocytes on the surface. The healing was accelerated by inflammation suppression, angiogenesis, and ECM promotion using a full-thickness mouse skin wound model. | [195] |
| PEGDA and HAMA | Canine islets | Hydrogel microspheres | Diabetes mellitus type 1 | In diabetic NOD mice, PEGDA microspheres reversed diabetes for the length of the study (up to 16 weeks). On the contrary, islets encapsulated in HAMA microspheres restored normoglycemia, but only transiently (3–4 weeks). Transplanted nonencapsulated canine islets did not restore normoglycemia for any length of time. | [196] |
| HA of 0.1 and 1.2 MDa crosslinked with bis(β-isocyanatoethyl) disulfide | Pancreatic beta cells from the MIN-6 lineage | Hydrogel | Diabetes mellitus type I | Gels (0.1 MDa HA) had higher crosslinking densities and consequently, higher tensile and storage loss moduli. Both HMW and LMW HAs were biocompatible. Gels maintained cell viability, and they did not activate the immune system. Due to sex dimorphism and hormonal variances, female mice were shown to be more resistant to the inducing effects of streptozotocin, where hyperglycemia was achieved in 48% of the cohort. Moreover, single-cell encapsulation did not revert hyperglycaemia after transplantation due to the lack of cell–cell interactions. | [197] |
| HA/alginate | MSC spheroids | Microcapsules | Stem cell-based therapies | Enhanced secretion of various growth factors was found from MSC spheroids, a significant promotion of angiogenesis by MSC spheroids compared to the controls (i.e., MSCs and MSC spheroids), which is likely because of the higher retention of MSC spheroid forms in the microcapsules. | [198] |
| HA | BMSCs-conditioned media | Hydrogel | OA | Enhanced beneficial effect of HA in treating degenerative changes in articulating surfaces associated with arthritic temporomandibular joints in rats, reduced toxicity and side effects, increased bioavailability, and minimized off-target activity. | [199] |
| HA/chitosan coacervate | Rat BMSCs | Hydrogel | Cartilage repair | Chondrogenic induction of encapsulated BMSCs within coacervate demonstrated remarkable cellular viability in addition to the elevated expression levels of chondrogenic markers such as sex-determining region Y-box 9 protein, aggrecan, cartilage oligomeric matrix protein, and collagen type II. | [151] |
| HA/hydroxyapatite | L929 fibroblasts | Hydrogel | Bone repair | Excellent cytocompatibility and supported adhesion and proliferation of cells under 3D culture conditions. | [200] |
| Enzymatically crosslinked HA | Human auricular chondrocytes | Microgel bioink | Cartilage regeneration | Excellent rheological properties, the granular hydrogels supported the homogeneous development of mature cartilage-like tissues in vitro. After 6 weeks of in vivo implantation, small-diameter microgels formed stable constructs with low immunogenicity and continuous tissue maturation. Conversely, increasing the microgel size resulted in an increased inflammatory response, with limited stability in vivo. | [201] |
| HA/tyramine/silk-fibroin | Articular chondrocytes | Hydrogel | Cartilage repair | Cytocompatible, promoted the expression of cartilage matrix proteins, while the most prominent chondrogenic effects were observed in hydrogels with HA: silk fibroin in the polymeric ratio 20:80. Among the hydrogels loaded with anabolic and anti-inflammatory drugs, the HA20/SF80 hydrogel demonstrated the longest and most sustained release profile over time, which is desirable for the extended treatment duration typically required for OA joints. | [202] |
| HAMA, collagen type I, and chitosan | BMSCs or primary articular chondrocytes | Hydrogel | Cartilage repair | Chondrocytes exhibited superior growth and matrix deposition compared to either chondrogenically induced BMSCs or a mixed polyelectrolyte complex microcapsule culture containing both chondrocytes and BMSCs. | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valachová, K.; Hassan, M.E.; Tamer, T.M.; Šoltés, L. Progress in Hyaluronan-Based Nanoencapsulation Systems for Smart Drug Release and Medical Applications. Molecules 2025, 30, 3883. https://doi.org/10.3390/molecules30193883
Valachová K, Hassan ME, Tamer TM, Šoltés L. Progress in Hyaluronan-Based Nanoencapsulation Systems for Smart Drug Release and Medical Applications. Molecules. 2025; 30(19):3883. https://doi.org/10.3390/molecules30193883
Chicago/Turabian StyleValachová, Katarína, Mohamed E. Hassan, Tamer M. Tamer, and Ladislav Šoltés. 2025. "Progress in Hyaluronan-Based Nanoencapsulation Systems for Smart Drug Release and Medical Applications" Molecules 30, no. 19: 3883. https://doi.org/10.3390/molecules30193883
APA StyleValachová, K., Hassan, M. E., Tamer, T. M., & Šoltés, L. (2025). Progress in Hyaluronan-Based Nanoencapsulation Systems for Smart Drug Release and Medical Applications. Molecules, 30(19), 3883. https://doi.org/10.3390/molecules30193883









