The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives
Abstract
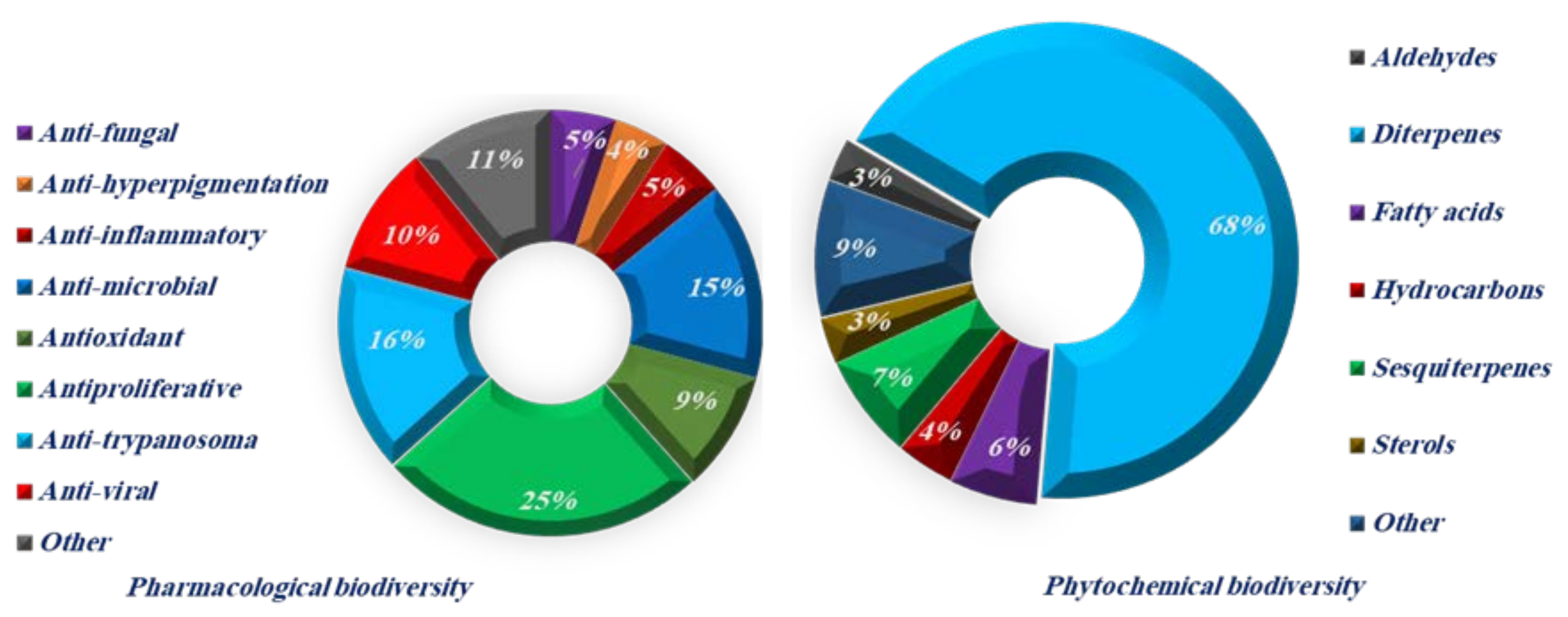
1. Introduction
2. The Pharmacological and Phytochemical Potential of the Genus Dictyota
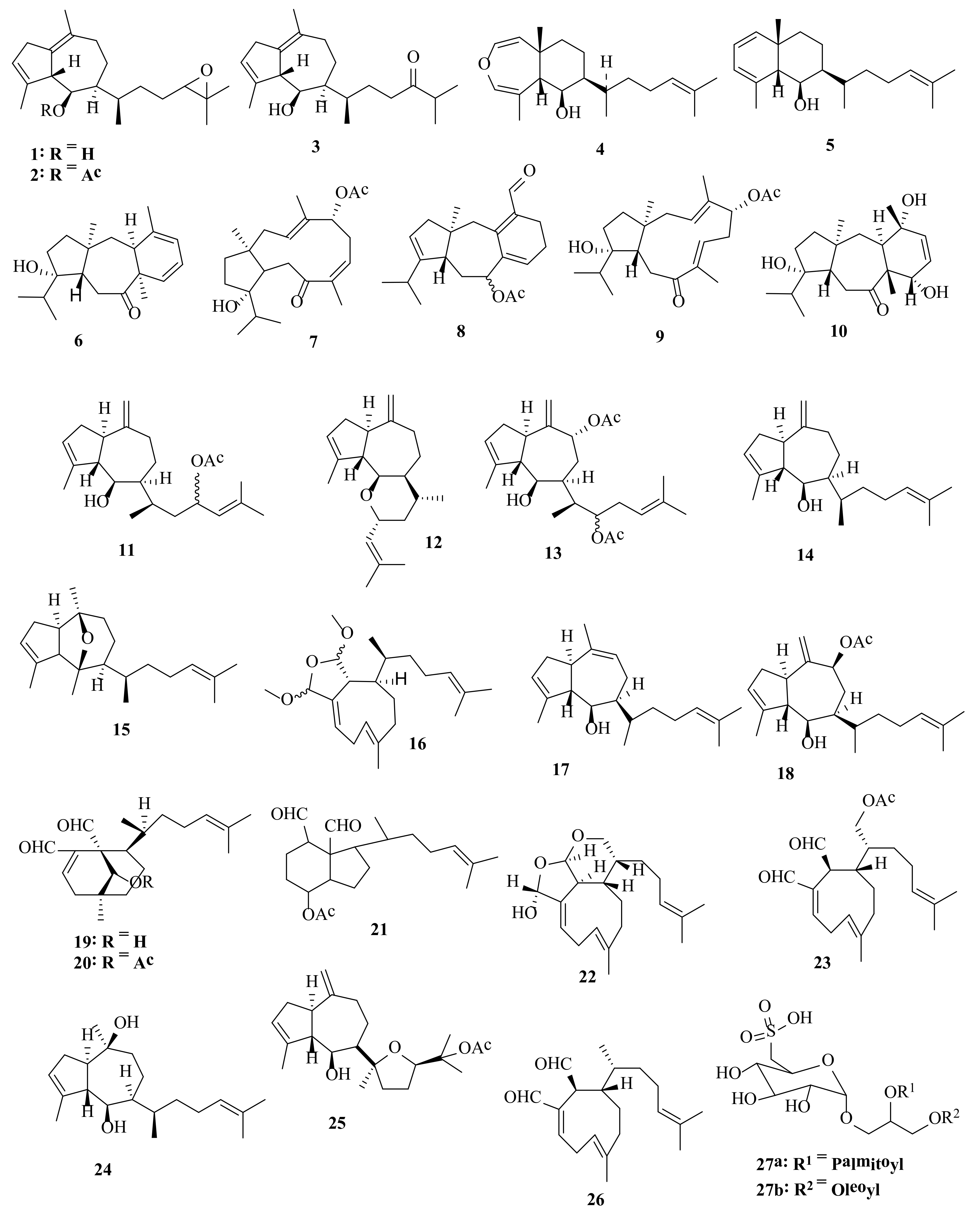
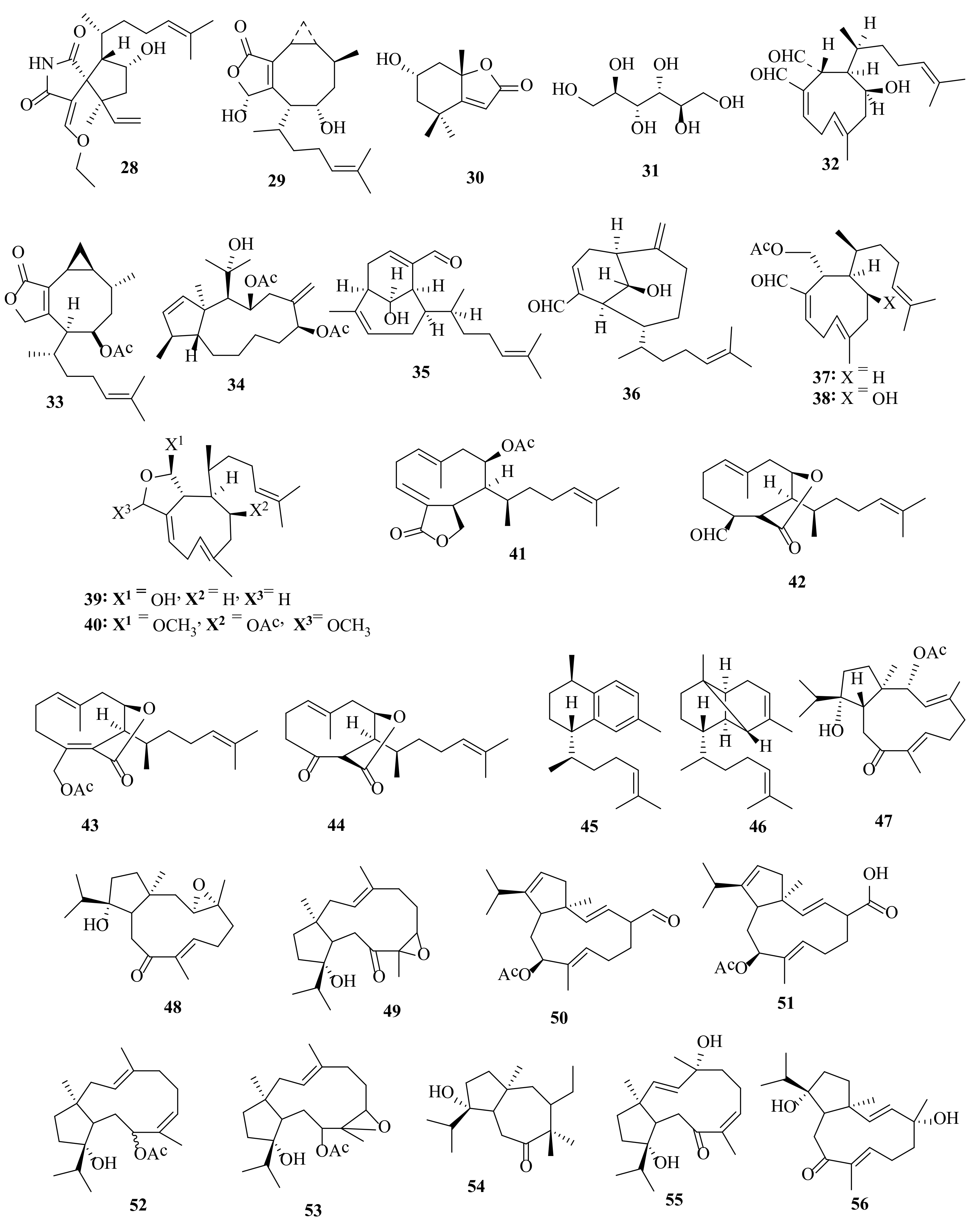
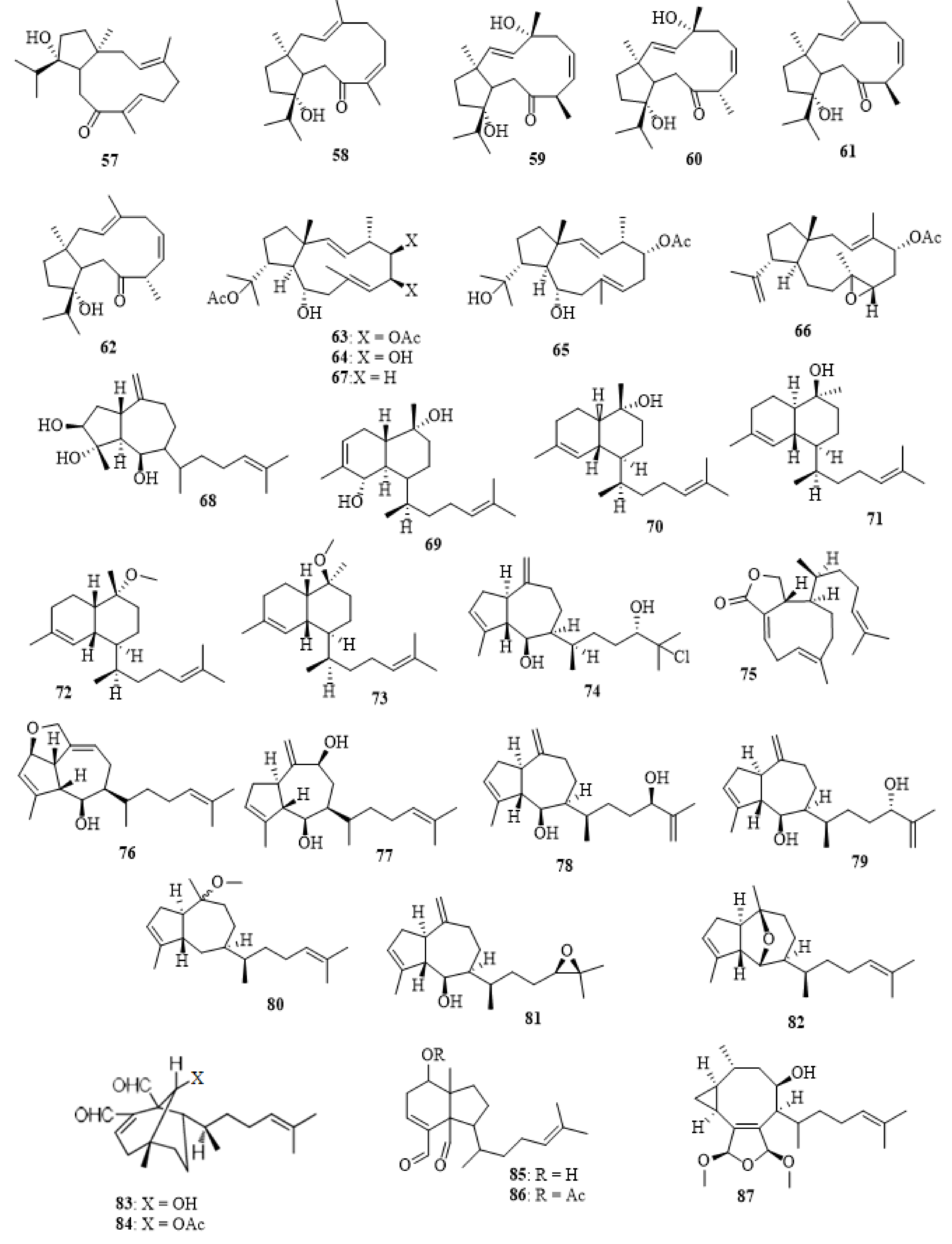
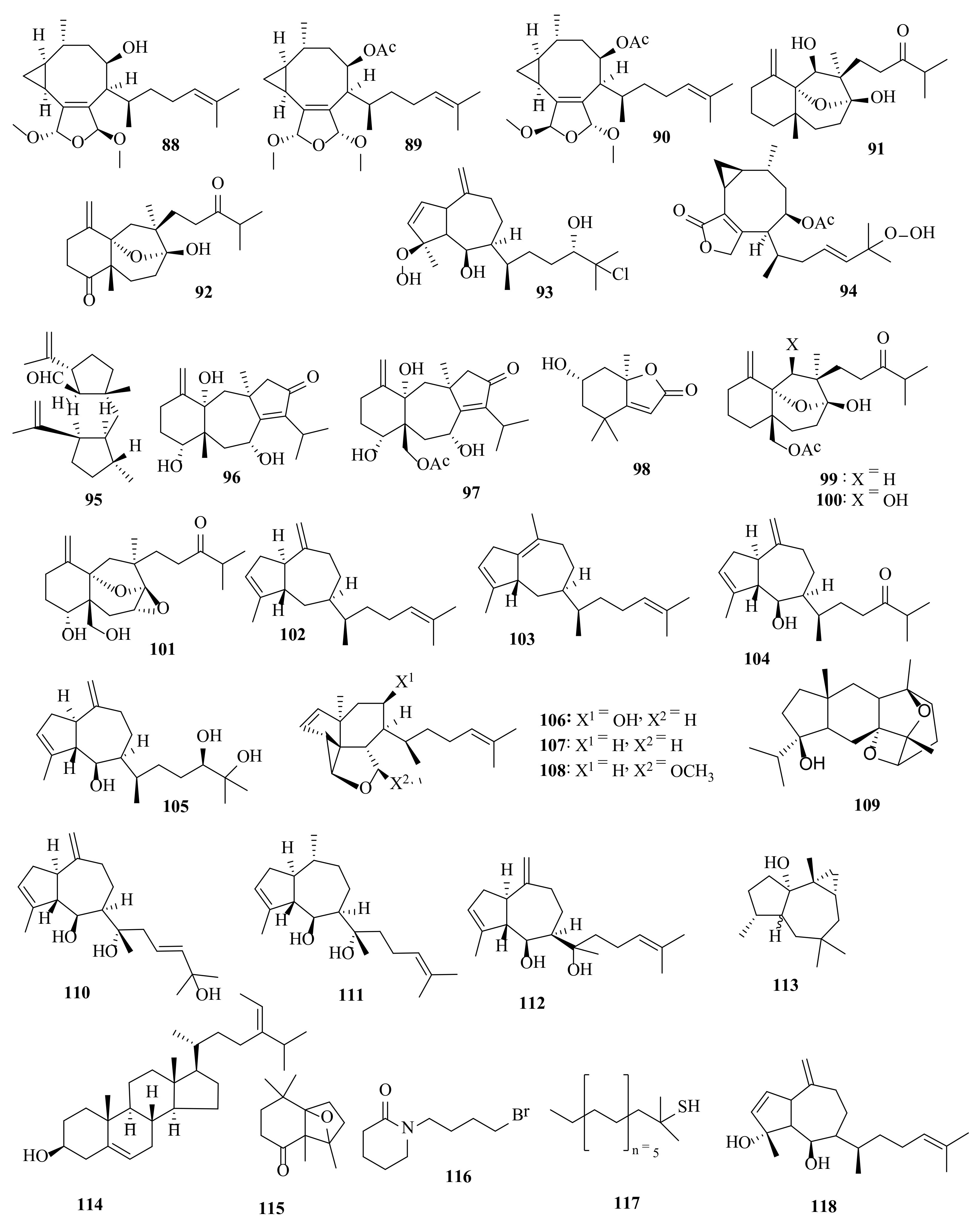
2.1. Dictyota acutiloba J. Agardh 1848
2.2. Dictyota bartayresiana J.V. Lamouroux 1809
2.3. Dictyota binghamiae J. Agardh 1894
2.4. Dictyota caribaea Hörnig and Schnetter 1992
2.5. Dictyota ciliolata Sonder ex Kützing 1859
2.6. Dictyota coriacea (Holmes) I.K. Wang, H.S. Kim and W.J. Lee 2004
2.7. Dictyota crenulata J. Agardh 1847
2.8. Dictyota dichotoma (Hudson) J.V. Lamouroux 1809
2.9. Dictyota dumosa Børgesen 1935
2.10. Dictyota fasciola (Roth) J.V. Lamouroux 1809
2.11. Dictyota fenestrata J. Agardh 1894
2.12. Dictyota friabilis Setchell 1926
2.13. Dictyota flabellata (Collins) Setchell and N.L. Gardner 1924
2.14. Dictyota furcellata (C. Agardh) Greville 1830
2.15. Dictyota guineënsis (Kützing) P. Crouan and H. Crouan 1878
2.16. Dictyota hauckiana Nizamuddin 1975
2.17. Dictyota masonii Setchell and N.L. Gardner 1930
2.18. Dictyota menstrualis (Hoyt) Schnetter, Hörning and Weber-Peukert 1987
2.19. Dictyota mertensii (C. Martius) Kützing 1859
2.20. Dictyota pinnatifida Kützing 1859
2.21. Dictyota plectens (Allender and Kraft) Kraft 2009
2.22. Dictyota pulchella Hörnig and Schnetter 1988
2.23. Dictyota sandvicensis Sonder 1859
2.24. Dictyota spinulosa J.D. Hooker and Arnott 1838
2.25. Dictyota spiralis Montagne 1846
3. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Attia, E.Z.; Abdelraheem, W.M.; Saber, H.; Madkour, H.A.; Amin, E.; Hassan, H.M.; Abdelmohsen, U.R. A review on the diversity, chemical and pharmacological potential of the green algae genus Caulerpa. S. Afr. J. Bot. 2020, 132, 226–241. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Guiry, M.; Guiry, G. AlgaeBase. World. 2022. Available online: https://www.algaebase.org/ (accessed on 8 January 2022).
- Durán, R.; Zubía, E.; Ortega, M.J.; Salvá, J. New diterpenoids from the alga Dictyota dichotoma. Tetrahedron 1997, 53, 8675–8688. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelmohsen, U.R. The natural products and pharmacological biodiversity of brown algae from the genus Dictyopteris. J. Mex. Chem. Soc. 2022, 66, 154–180. [Google Scholar] [CrossRef]
- Pasqualetti, M.; Giovannini, V.; Barghini, P.; Gorrasi, S.; Fenice, M. Diversity and ecology of culturable marine fungi associated with Posidonia oceanica leaves and their epiphytic algae Dictyota dichotoma and Sphaerococcus coronopifolius. Fungal Ecol. 2020, 44, 100906. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Delva, S.; De Clerck, O. Concise review of the genus Dictyota J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 1521–1543. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the marine algae of the genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef]
- Hardt, I.H.; Fenical, W.; Cronin, G.; Hay, M.E. Acutilols, potent herbivore feeding deterrents from the tropical brown alga, Dictyota acutiloba. Phytochemistry 1996, 43, 71–73. [Google Scholar] [CrossRef]
- Sun, H.H.; Waraszkiewicz, S.M.; Erickson, K.L.; Finer, J.; Clardy, J. Dictyoxepin and dictyolene, two new diterpenes from the marine alga Dictyota acutiloba (Phaeophyta). J. Am. Chem. Soc. 1977, 99, 3516–3517. [Google Scholar] [CrossRef]
- Solomon, R.D.J.; Santhi, V.S. Purification of bioactive natural product against human microbial pathogens from marine sea weed Dictyota acutiloba J. Ag. World J. Microbiol. Biotechnol. 2008, 24, 1747–1752. [Google Scholar] [CrossRef]
- Rao, C.B.; Trimurtulu, G.; Sreedhara, C.; Rao, D.V.; Bobzin, S.C.; Faulkner, D.J. Diterpenes from the brown alga Dictyota bartayresiana. Phytochemistry 1994, 37, 509–513. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sudha, S. Biosynthesis of silver nanoparticles from Dictyota bartayresiana extract and their antifungal activity. Nano Biomed. Engl. 2013, 5, 72–75. [Google Scholar]
- Antonysamy, J.M.A.; Velayutham, K.; Mani, N.; Thangaiah, S.; Irullappan, R. Antibacterial, cytotoxic and larvicidal potential of Dictyota bartayresiana Lamour. J. Coast. Life Med. 2015, 3, 352–355. [Google Scholar]
- Palanisamy, S.; Sellappa, S.; Stella, C. Antioxidant properties of methanolic extract of Dictyota batresiana from south east coast of India. J. Pharm. Res. 2010, 3, 2974–2976. [Google Scholar]
- Bharathi, D.S.; Boopathyraja, A.; Nachimuthu, S.; Kannan, K. Green Synthesis, Characterization and antibacterial activity of SiO2–ZnO nanocomposite by Dictyota bartayresiana extract and its cytotoxic effect on HT29 Cell Line. J. Clust. Sci. 2021, 3, 1–17. [Google Scholar] [CrossRef]
- Pathirana, C.; Andersen, R.J. Diterpenoids from the brown alga Dictyota binghamiae. Can. J. Chem. 1984, 62, 1666–1671. [Google Scholar] [CrossRef]
- Assef, A.N.B.; da Costa, B.B.; Moreira, T.A.; do Carmo, L.D.; de Souza, T.d.F.G.; Alencar, N.M.N.; Alves, A.P.N.N.; Cinelli, L.P.; Wilke, D.V. Antitumor and immunostimulating sulfated polysaccharides from brown algae Dictyota caribaea. Carbohydr. Polym. Technol. Appl. 2021, 2, 100142. [Google Scholar] [CrossRef]
- Brígido Assef, A.N.; da Costa, B.B.; Moreira, T.A.; do Carmo, L.D.; de Fátima Goebel de Souza, T.; Nunes Alencar, N.M.; Negreiros Nunes, A.P.; Cinelli, L.P.; Wilke, D.V. Antitumor sulfated polysaccharides from brown algae Dictyota caribaea. bioRxiv 2020, 2, 100142–100149. [Google Scholar]
- Simas, D.L.R.; Kaiser, C.R.; Gestinari, L.M.; Duarte, H.M.; de Paula, J.C.; Soares, A.R. Diterpenes from the brown seaweed Dictyota caribaea (Dictyotaceae, Phaeophyceae): The ecological and taxonomic significance. Biochem. Syst. Ecol. 2014, 52, 33–37. [Google Scholar] [CrossRef]
- Farias de Mesquita, M.M.; Baddini, A.L.d.Q.; Pereira Netto, A.D.; Magalhães de Araujo, J.; Salgueiro, F.; Lopes Filho, E.A.P.; De-Paula, J.C.; Fleury, B.G.; Cavalcanti, D.N.; Teixeira, V.L. Chemical similarity between Dictyota caribaea and Dictyota menstrualis (Dictyotaceae, Phaeophyceae) from the coast of Rio de Janeiro, Brazil. Biochem. Syst. Ecol. 2015, 58, 97–101. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Bakkas, S.; Villani, G.; Varcamonti, M.; Zanfardino, A.; Gavagnin, M. Diterpene content of the alga Dictyota ciliolata from a Moroccan lagoon. Phytochem. Lett. 2009, 2, 211–215. [Google Scholar] [CrossRef]
- Bourne, D.J.; Pilchowski, S.E.; Murphy, P.T. A novel sulfonoglycolipid from the brown alga Dictyota ciliolata. Aust. J. Chem. 1999, 52, 69–70. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrín, Y.; Robledo, D. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1244–1248. [Google Scholar] [CrossRef]
- Yan, P.; Li, G.; Wang, C.; Wu, J.; Sun, Z.; Martin, G.E.; Wang, X.; Reibarkh, M.; Saurí, J.; Gustafson, K.R. Characterization by Empirical and Computational Methods of Dictyospiromide, an Intriguing Antioxidant Alkaloid from the Marine Alga Dictyota coriacea. Org. Lett. 2019, 21, 7577–7581. [Google Scholar] [CrossRef]
- Ko, R.K.; Kang, M.-C.; Kim, S.S.; Oh, T.H.; Kim, G.-O.; Hyun, C.-G.; Hyun, J.W.; Lee, N.H. Anti-melanogenesis Constituents from the Seaweed Dictyota coriacea. Nat. Prod. Commun. 2013, 8, 427–428. [Google Scholar] [CrossRef]
- Kang, J.-I.; Oh, T.H.; Kim, J.; No, H.; Lee, N.H.; Yoo, E.-S.; Kang, H.-K. The effect of 1,9-dihydroxycrenulide and epiloliolide from Dictyota coriacea on the hair growth. J. Herb. Med. 2021, 52, 134–142. [Google Scholar]
- Kang, M.-C.; Lee, J.-Y.; Ko, R.-K.; Kim, H.-B.; Hong, S.-H.; Kim, G.-O. Melanin inhibitory effect and anti-inflammatory effects of Dictyota coriacea extracts derived from adjacent sea of the Jeju Island. KSBB J. 2008, 23, 311–316. [Google Scholar]
- Kirkup, M.P.; Moore, R.E. Two minor diterpenes related to dictyodial A from the brown alga Dictyota crenulata. Phytochemistry 1983, 22, 2539–2541. [Google Scholar] [CrossRef]
- Soto, H.; Rovirosa, J. A new diterpene from Dictyota crenulata. Z. Naturforsch. B 2003, 58, 795–798. [Google Scholar] [CrossRef]
- Kirkup, M.P.; Moore, R.E. Identity of sanadaol with β-crenulal, a diterpene from the brown alga Dictyota crenulata. Phytochemistry 1983, 22, 2527–2529. [Google Scholar] [CrossRef]
- De-Paula, J.C.; Bueno, L.B.; Cavalcanti, D.N.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from the brown alga Dictyota crenulata. Molecules 2008, 13, 1253–1262. [Google Scholar] [CrossRef]
- Nagaoka, H.; Kobayashi, K.; Yamada, Y. Total synthesis of (−)- and (+)-sanadaol: The absolute configuration of sanadaol and dictyodial. Tetrahedron Lett. 1988, 29, 5945–5946. [Google Scholar] [CrossRef]
- El-Shenody, R.A.; Ashour, M.; Ghobara, M.M.E. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz. J. Food Technol. 2019, 22, 1–15. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Usoltseva, R.V.; Zvyagintseva, T.N.; Ermakova, S.P. Laminaran from brown alga Dictyota dichotoma and its sulfated derivative as radioprotectors and radiosensitizers in melanoma therapy. Carbohydr. Polym. 2019, 206, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef]
- Beula, J.M.; Ravikumar, S.; Ali, M.S. Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asian Pac. J. Trop. Biomed. 2011, 1, S143–S146. [Google Scholar] [CrossRef]
- Bakar, K.; Mohamad, H.; Latip, J.; Tan, H.S.; Herng, G. Fatty acids compositions of Sargassum granuliferum and Dictyota dichotoma and their anti-fouling activities. J. Sustain. Sci. Manag. 2017, 12, 8–16. [Google Scholar]
- Araki, S.; Eichenberger, W.; Sakurai, T.; Sato, N. Distribution of Diacylglycerylhydroxymethyltrimethyl-β-alanine (DGTA) and Phosphatidylcholine in Brown Algae. Plant Cell Physiol. 1991, 32, 623–628. [Google Scholar] [CrossRef]
- El-Shaibany, A.; Al-Habori, M.; Al-Maqtari, T.; Al-Mahbashi, H. The Yemeni brown algae Dictyota dichotoma exhibit high in vitro anticancer activity independent of its antioxidant capability. Biomed Res. Int. 2020, 2425693. [Google Scholar] [CrossRef] [PubMed]
- Enoki, N.; Ishida, R.; Matsumoto, T. Structures and conformations of new nine-membered ring diterpenoids from the marine alga Dictyota dichotoma. Chem. Lett. 1982, 11, 1749–1752. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Kusumi, T.; Kakisawa, H. Antitumor xenicane and norxenicane lactones from the brown alga Dictyota dichotoma. J. Org. Chem. 1988, 53, 5010–5013. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Kalinovsky, A.I.; Fedorov, S.N.; Shubina, L.K.; Stonik, V.A. Diterpenes from the Far-eastern brown alga Dictyota dichotoma. Phytochemistry 2006, 67, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.B.; Pullaiah, K.C.; Surapaneni, R.K.; Sullivan, B.W.; Albizati, K.F.; Faulkner, D.J.; Cun-heng, H.; Clardy, J. The diterpenes of Dictyota dichotoma from the Indian Ocean. J. Org. Chem. 1986, 51, 2736–2742. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Kusumi, T.; Ichikawa, A.; Kakisawa, H. Bicyclic diterpenes from two species of brown algae of the Dictyotaceae. Phytochemistry 1990, 29, 605–2610. [Google Scholar] [CrossRef]
- Kim, J.Y.; Alamsjah, M.A.; Hamada, A.; Fujita, Y.; Ishibashi, F. Algicidal Diterpenes from the Brown Alga Dictyota dichotoma. Biosci. Biotechnol. Biochem. 2006, 70, 2571–2574. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C.; Sica, D.; Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C. Dictyol A and B, two novel diterpene alcohols from the brown alga Dictyota dichotoma. J. Chem. Soc. Chem. Commun. 1976, 14, 575–576. [Google Scholar] [CrossRef]
- Enoki, N.; Tsuzuki, K.; Omura, S.; Ishida, R.; Matsumoto, T. New antimicrobial diterpenes, dictyol F and epidictyol F, from the brown alga Dictyota dichotoma. Chem. Lett. 1983, 12, 1627–1630. [Google Scholar] [CrossRef]
- Blount, J.; Dunlop, R.; Erickson, K.; Wells, R. Two diterpenes with new carbocyclic ring systems from an Australian collection of the brown alga Dictyota dichotoma. Aust. J. Chem. 1982, 35, 145–163. [Google Scholar] [CrossRef]
- Kusumi, T.; Muanza-Nkongolo, D.; Goya, M.; Ishitsuka, M.; Iwashita, T.; Kakisawa, H. Structures of crenulacetals A, B, C, and D. The new diterpenoids from the brown algae of Dictyotaceae. J. Org. Chem. 1986, 51, 384–387. [Google Scholar] [CrossRef]
- Ali, M.S.; Pervez, M.K. seco-Dolastanes from the marine brown alga Dictyota dichotoma (Huds.) amour. Z. Naturforsch. B 2003, 58, 438–442. [Google Scholar]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Stonik, V.A. Diterpenoid Hydroperoxides from the Far-Eastern Brown Alga Dictyota dichotoma. Aust. J. Chem. 2009, 62, 1185–1188. [Google Scholar] [CrossRef]
- Segawa, M.; Enoki, N.; Ikura, M.; Hikichi, K.; Ishida, R.; Shirahama, H.; Matsumoto, T. Dictymal, a new Seco-fusicoccin type diterpene from the brown alga Dictyota dichotoma. Tetrahedron Lett. 1987, 2, 3703–3704. [Google Scholar] [CrossRef]
- Ali, M.S.; Pervez, M.K.; Saleem, M.; Ahmed, F. Dichotenone-A and -B: Two New Enones from the Marine Brown Alga Dictyota Dichotoma (Hudson) Lamour. Nat. Prod. Res. 2003, 17, 301–306. [Google Scholar] [CrossRef]
- Ali, M.S.; Pervez, M.K.; Ahmed, F.; Saleem, M. Dichotenol-A, B and C: The C-16 oxidized seco-dolastanes from the marine brown alga Dictyota dichotoma (Huds.) Lamour. Nat. Prod. Res. 2004, 18, 543–549. [Google Scholar] [CrossRef]
- Nobuyasu, E.; Ryoichi, I.; Shiro, U.; Masamitsu, O.; Takashi, T.; Takeshi, M. New hydroazulenoid diterpenes from the marine alga Dictyota dichotoma. Chem. Lett. 1982, 11, 1837–1840. [Google Scholar]
- Enoki, N.; Ishida, R.; Urano, S.; Matsumoto, T. New tricarbocyclic cycropropanoid diterpenes from the brown alga Dictyota dichotoma. Tetrahedron Lett. 1985, 26, 1731–1734. [Google Scholar] [CrossRef]
- Pullaiah, K.C.; Surapaneni, R.K.; Rao, C.B.; Albizati, K.F.; Sullivan, B.W.; Faulkner, D.J.; He, C.H.; Clardy, J. Dictyoxetane, a novel diterpene from the brown alga Dictyota dichotoma from the Indian Ocean. J. Org. Chem. 1985, 50, 3665–3666. [Google Scholar] [CrossRef]
- Abou-El-Wafa, G.S.E.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J.; Ravi, B.N.; Finer, J.; Clardy, J. Diterpenes from Dictyota dichotoma. Phytochemistry 1977, 16, 991–993. [Google Scholar] [CrossRef]
- Bakar, K.; Mohamad, H.; Tan, H.S.; Latip, J. Sterols compositions, antibacterial, and antifouling properties from two Malaysian seaweeds: Dictyota dichotoma and Sargassum granuliferum. J. Appl. Pharm. Sci. 2019, 9, 047–053. [Google Scholar]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Madkour, H.A.; Abdelmohsen, U.R. A review on the pharmacological potential of the genus Padina. S. Afr. J. Bot. 2021, 141, 37–48. [Google Scholar] [CrossRef]
- Benfares, R.; Kord, A.; Boudjema, K.; Bouarab, M.; Benrabah, S.; Boudjemaa, K.; Švarc-Gajić, J. Chemical characterization of essential oils and antioxidant activity of Dictyota dichotoma and Dictyopteris membranacea. Acta Period. Technol. 2019, 33–42. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Rao, C.B.; Trimurtulu, G.; Rao, D.V.; Bobzin, S.C.; Kushlan, D.M.; Faulkner, D.J. Diterpenes from the brown alga Dictyota divaricata of the Indian Ocean. Phytochemistry 1991, 30, 1971–1976. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. Diterpenes from the brown alga Dictyota divaricata. Phytochemistry 1991, 30, 3679–3682. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. New xenicane and hydroazulenoid diterpenes from an Australian collection of Dictyota divaricata. Tetrahedron 1991, 47, 1399–1410. [Google Scholar] [CrossRef]
- Sun, H.H.; McConnell, O.J.; Fenical, W.; Hirotsu, K.; Clardy, J. Tricyclic diterpenoids of the dolastane ring system from the marine alga Dictyota divaricata. Tetrahedron 1981, 37, 1237–1242. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Kushlan, D.M.; Faulkner, D.J.; Rao, C.B. Divarinone, a novel diterpene from the brown alga Dictyota divaricata of the indian ocean. Tetrahedron Lett. 1992, 33, 729–732. [Google Scholar] [CrossRef]
- Ayyad, S.-E.N.; Makki, M.S.; Al-kayal, N.S.; Basaif, S.A.; El-Foty, K.O.; Asiri, A.M.; Alarif, W.M.; Badria, F.A. Cytotoxic and protective DNA damage of three new diterpenoids from the brown alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011, 46, 175–182. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Zavodnik, N. Hydroazulenoid diterpenes from the brown alga Dictyota dichotoma var. Implexa. Phytochemistry 1986, 25, 2179–2181. [Google Scholar] [CrossRef]
- Bano, S.; Parveen, S.; Ahmad, V.U. Marine natural products, XIV. secodolastane diterpenoids of Dictyota indica from the Arabian Sea. J. Nat. Prod. 1990, 53, 492–495. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tomita, Y.; Kawamoto, Y.; Ito, H. Highly stereocontrolled total synthesis of secodolastane diterpenoid isolinearol. Org. Biomol. Chem. 2020, 18, 7316–7320. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Parveen, S.; Bano, S.; Shaikh, W.; Shameel, M. Dolastane diterpenoids from the brown alga Dictyota indica. Phytochemistry 1991, 30, 1015–1018. [Google Scholar] [CrossRef]
- Niang, L.L.; Hung, X. Studies on the biologically active compounds of the algae from the Yellow Sea. Hydrobiologia 1984, 116, 168–171. [Google Scholar] [CrossRef]
- Karkhane Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob. J. Environ. Sci. Manag. 2018, 4, 81–90. [Google Scholar]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Abdelmohsen, U.R. The genus Turbinaria: Chemical and pharmacological diversity. Nat. Prod. Res. 2020, 35, 4560–4578. [Google Scholar] [CrossRef]
- Yazdani, A.; Sayadi, M.; Heidari, A. Green biosynthesis of palladium oxide nanoparticles using Dictyota indica seaweed and its application for adsorption. J. Water Environ. Nanotechnol. 2018, 3, 337–347. [Google Scholar]
- Meashi, D.; Sharma, P.; Sharma, A.K. Bio-functionalized Gold Nanoparticles: A Potent Probe for Profound Antibacterial Efficiency through Drug Delivery System. Asian J. Biol. Life Sci. 2020, 9, 42–50. [Google Scholar]
- Ochi, M.; Watanabe, M.; Miura, I.; Taniguchi, M.; Tokoroyama, T. Amijiol; isoamijiol; and 14-deoxyamijiol; three new diterpenoids from the brown seaweed Dictyota linearis. Chem. Lett. 1980, 9, 1229–1232. [Google Scholar] [CrossRef]
- Ochi, M.; Asao, K.; Kotsuki, H.; Miura, I.; Shibata, K. Amijitrienol and 14-deoxyisoamijiol, two new diterpenoids from the brown seaward Dictyota linearis. Bull. Chem. Soc. Jpn. 1986, 59, 661–662. [Google Scholar] [CrossRef]
- Crews, P.; Klein, T.E.; Hogue, E.R.; Myers, B.L. Tricyclic diterpenes from the brown marine algae Dictyota divaricata and Dictyota linearis. J. Org. Chem. 1982, 47, 811–815. [Google Scholar] [CrossRef]
- Siamopoulou, P.; Bimplakis, A.; Iliopoulou, D.; Vagias, C.; Cos, P. Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 2004, 65, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; König, G.M.; Sticher, O. New and highly oxidised hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Tetrahedron 1993, 49, 571–580. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O.; Rüegger, H. Four New Hydroazulenoid Diterpenes from the Tropical Marine Brown Alga Dictyota volubilis. Planta Med. 1993, 59, 174–178. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Botić, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Čagalj, M.; Skroza, D. Bioactive Phenolic Metabolites from Adriatic Brown Algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef]
- El-Katony, T.M.; Deyab, M.A.; El-Adl, M.F.; Ward, F.M.E.-N. Extracts of the Brown Alga Dictyota dichotoma (Hudson) J.V. Lamouroux Alleviate Salt Stress in Rice (Oryza sativa L.) During Germination. J. Plant Growth Regul. 2021, 40, 986–999. [Google Scholar] [CrossRef]
- Rachel, D.; Thangaraju, N. Phytochemical screening and comparative analysis of antimicrobial activity of selected species of brown seaweeds from Gulf of Mannar, Tamil Nadu, India. J. Mod. Biotechnol. 2015, 5, 1–7. [Google Scholar]
- Shameel, M.; Shaikh, W.; Khan, R. Comparative fatty acid composition of five species of Dictyota (Phaeophyta). Bot. Mar. 1991, 34, 425–428. [Google Scholar] [CrossRef]
- Ktari, L.; Ismail-Ben Ali, A.; Ben Redjem, Y.; Langar, H.; El Bour, M. Antifouling activity and chemical investigation of the brown alga Dictyota fasciola (Dictyotales) from Tunisian coast. Cah. Biol. Mar. 2010, 51, 109–115. [Google Scholar]
- Vanaltena, I.; Mcgivern, J. New Spatane-Derived Diterpenoids from the Australian Brown Alga Dictyota fenestrata. Aust. J. Chem. 1992, 45, 541–551. [Google Scholar] [CrossRef]
- Stephens, P.R.S.; Cirne-Santos, C.C.; de Souza Barros, C.; Teixeira, V.L.; Carneiro, L.A.D.; Amorim, L.d.S.C.; Ocampo, J.S.P.; Castello-Branco, L.R.R.; de Palmer Paixão, I.C.N. Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J Appl. Phycol. 2017, 29, 775–780. [Google Scholar] [CrossRef]
- Robertson, K.J.; Fenical, W. Pachydictyol-A epoxide, a new diterpene from the brown seaweed Dictyota flabellata. Phytochemistry 1977, 16, 1071–1073. [Google Scholar] [CrossRef]
- Finer, J.; Clardy, J.; Fenical, W.; Minale, L.; Riccio, R.; Battaile, J.; Kirkup, M.; Moore, R.E. Structures of dictyodial and dictyolactone, unusual marine diterpenoids. J. Org. Chem. 1979, 44, 2044–2047. [Google Scholar] [CrossRef]
- Tenorio-Rodríguez, P.; Méndez-Rodrìguez, L.; Serviere-Zaragoza, E.; OHara, T.; Zenteno-Savín, T. Antioxidant substances and trace element content in macroalgae from a subtropical lagoon in the West Coast of the Baja California Peninsula. Vitam. Trace Elem. 2013, 2, 1000108. [Google Scholar]
- Dunlop, R.; Ghisalberti, E.; Jefferies, P.; Skelton, B.; White, A. Structure of a New Dolastane Diterpene from Dictyota furcellata. Aust. J. Chem. 1989, 42, 315–319. [Google Scholar] [CrossRef]
- De-Paula, J.C.; Cavalcanti, D.N.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from marine brown alga Dictyota guineensis (Dictyotaceae, Phaeophyceae). Rev. Bras. Farmacogn. 2012, 22, 736–740. [Google Scholar] [CrossRef][Green Version]
- Shaikh, W.; Shameel, M.; Ahmad, V.U. Phycochemical studies on sterols from three brown seaweeds of northern Arabian Sea. Pak. J. Mar. Sci. 1995, 4, 107–113. [Google Scholar]
- Ayesha, H.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S. In vitro cytotoxicity of seaweeds from Karachi coast on brine shrimp. Pak. J. Bot. 2010, 42, 3555–3560. [Google Scholar]
- Sun, H.H.; Fenical, W. Hydroxydilophol, a new monocyclic diterpenoid from the brown alga Dictyota masonii. J. Org. Chem. 1979, 44, 1354–1356. [Google Scholar] [CrossRef]
- do Nascimento Ávila, F.; da Silva Souza, L.G.; de Macedo Carneiro, P.B.; Santos, F.A.; Sasahara, G.L.; Marinho Filho, J.D.B.; Araújo, A.J.; Barros, A.B.; Vieira Monteiro, N.D.K.; Silveira, E.R.; et al. Anti-inflammatory diterpenoids from the Brazilian alga Dictyota menstrualis. Algal Res. 2019, 44, 101695. [Google Scholar] [CrossRef]
- Pereira, H.; Leão-Ferreira, L.; Moussatché, N.; Teixeira, V.; Cavalcanti, D.; Costa, L.; Diaz, R.; Frugulhetti, I. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2004, 64, 69–76. [Google Scholar] [CrossRef]
- De Andrade Moura, L.; Marqui de Almeida, A.C.; Domingos, T.F.S.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Teixeira, V.L.; Fuly, A.L. Antiplatelet and anticoagulant effects of diterpenes isolated from the marine alga, Dictyota menstrualis. Mar. Drugs 2014, 12, 2471–2484. [Google Scholar] [CrossRef]
- Abrantes, J.L.; Barbosa, J.; Cavalcanti, D.; Pereira, R.C.; Fontes, C.L.F.; Teixeira, V.L.; Souza, T.L.M.; Paixão, I.C. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76, 339–344. [Google Scholar] [CrossRef]
- Gomes, D.L.; Telles, C.B.S.; Costa, M.S.S.P.; Almeida-Lima, J.; Costa, L.S.; Keesen, T.S.L.; Rocha, H.A.O. Methanolic extracts from brown seaweeds Dictyota cilliolata and Dictyota menstrualis induce apoptosis in human cervical adenocarcinoma HeLa cells. Molecules 2015, 20, 6573–6591. [Google Scholar] [CrossRef]
- Albuquerque, I.R.L.; Queiroz, K.C.S.; Alves, L.G.; Santos, E.A.D.; Leite, E.L.; Rocha, H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171. [Google Scholar] [CrossRef]
- Albuquerque, I.R.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.; Leite, E.L.; Nader, H.B.; Rocha, H.A. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; Barros, C.; Gomes, M.W.L.; Gomes, R.; Cavalcanti, D.N.; Obando, J.M.C.; Ramos, C.J.B.; Villaça, R.C.; Teixeira, V.L.; Paixão, I.C.N.D.P. In vitro antiviral activity against Zika virus from a natural product of the Brazilian brown seaweed Dictyota menstrualis. Nat. Prod. Commun. 2019, 14, 1934578X19859128. [Google Scholar] [CrossRef]
- Fernandes-Negreiros, M.M.; Araújo Machado, R.I.; Bezerra, F.L.; Nunes Melo, M.C.; Alves, M.G.C.F.; Alves Filgueira, L.G.; Morgano, M.A.; Trindade, E.S.; Costa, L.S.; Rocha, H.A.O. Antibacterial, antiproliferative, and immunomodulatory activity of silver nanoparticles synthesized with fucans from the alga Dictyota mertensii. Nanomaterials 2018, 8, 6. [Google Scholar] [CrossRef]
- Nunes Pinheiro, A.D.; Pereira Lopes-Filho, E.A.; De-Paula, J.C.; Pereira Netto, A.D.; Teixeira, V.L. Diterpenes from the brown alga Dictyota mertensii. Biochem. Syst. Ecol. 2019, 86, 103926. [Google Scholar] [CrossRef]
- Rubiano-Buitrago, P.; Duque, F.; Puyana, M.; Ramos, F.A.; Castellanos, L. Bacterial biofilm inhibitor diterpenes from Dictyota pinnatifida collected from the Colombian Caribbean. Phytochem. Lett. 2019, 30, 74–80. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, S.; Yuan, W.; Dong, J.; Huang, K.; Sun, Z.; Yan, P. Further New Xenicanes from a Chinese Collection of the brown Alga Dictyota plectens. Chem. Pharm. Bull. 2015, 63, 1081–1086. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014, 77, 2685–2693. [Google Scholar] [CrossRef]
- Queiroz, T.M.; Machado, N.T.; Furtado, F.F.; Oliveira-Filho, A.A.; Alustau, M.C.; Figueiredo, C.S.; Miranda, G.E.; Barbosa-Filho, J.M.; Braga, V.A.; Medeiros, I.A. Vasorelaxation, induced by Dictyota pulchella (Dictyotaceae), a brown alga, is mediated via inhibition of calcium influx in rats. Mar. Drugs 2011, 9, 2075–2088. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T. Hydroxydictyodial, a new antifeedant diterpene from the brown alga Dictyota spinulosa. Chem. Lett. 1984, 13, 231–232. [Google Scholar] [CrossRef]
- Chiboub, O.; Sifaoui, I.; Lorenzo-Morales, J.; Abderrabba, M.; Mejri, M.; Fernández, J.J.; Piñero, J.E.; Díaz-Marrero, A.R. Spiralyde A, an antikinetoplastid dolabellane from the brown alga Dictyota spiralis. Mar. Drugs 2019, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Othmani, A.; Bouzidi, N.; Viano, Y.; Alliche, Z.; Seridi, H.; Blache, Y.; El Hattab, M.; Briand, J.-F.; Culioli, G. Anti-microfouling properties of compounds isolated from several Mediterranean Dictyota spp. J. Appl. Phycol. 2014, 26, 1573–1584. [Google Scholar] [CrossRef]
- Viano, Y.; Bonhomme, D.; Ortalo-Magné, A.; Thomas, O.P.; Hattab, M.E.; Piovetti, L.; Blache, Y.; Culioli, G. Dictyotadimer A, a new dissymmetric bis-diterpene from a brown alga of the genus Dictyota. Tetrahedron Lett. 2011, 52, 1031–1035. [Google Scholar] [CrossRef]
- Guella, G.; Ndiaye, I.; Chiasera, G.; Pietra, F. Joalin, the first nitrogen-containing xenicane diterpene isolated from a brown seaweed collected off the Senegalese coast. J. Chem. Soc. Perkin Trans. I 1993, 10, 1545–1546. [Google Scholar] [CrossRef]
- Tringali, C.; Oriente, G.; Piattelli, M.; Nicolosi, G. Two minor dolabellane diterpenoid constituents from a Dictyota species. J. Nat. Prod. 1985, 48, 484–485. [Google Scholar] [CrossRef]
- Vazquez, J.T.; Chang, M.; Nakanishi, K.; Manta, E.; Perez, C.; Martin, J.D. Structure of hydroazulenoid diterpenes from a marine alga and their absolute configuration based on circular dichroism. J. Org. Chem. 1988, 53, 4797–4800. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Martin, J.D.; Gonzalez, B.; Ravelo, J.L.; Perez, C.; Rafii, S.; Clardy, J. A new diterpene with a novel carbon skeleton from a marine alga. J. Chem. Soc. Chem. Commun. 1984, 669–670. [Google Scholar] [CrossRef]
- Chiboub, O.; Sifaoui, I.; Abderrabba, M.; Mejri, M.; Fernández, J.J.; Díaz-Marrero, A.R.; Lorenzo-Morales, J.; Piñero, J.E. Apoptosis-like cell death upon kinetoplastid induction by compounds isolated from the brown algae Dictyota spiralis. Parasite Vectors 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xi, Y.; Li, G.; Zheng, Y.; Wang, Z.; Wang, J.; Fang, C.; Sun, Z.; Hu, L.; Jiang, W.; et al. Hydroazulene diterpenes from a Dictyota brown alga and their antioxidant and neuroprotective effects against cerebral ischemia-reperfusion injury. J. Nat. Prod. 2021, 84, 1306–1315. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rushdi, M.I.; Abdel-Rahman, I.A.M.; Attia, E.Z.; Saber, H.; Saber, A.A.; Bringmann, G.; Abdelmohsen, U.R. The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules 2022, 27, 672. https://doi.org/10.3390/molecules27030672
Rushdi MI, Abdel-Rahman IAM, Attia EZ, Saber H, Saber AA, Bringmann G, Abdelmohsen UR. The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules. 2022; 27(3):672. https://doi.org/10.3390/molecules27030672
Chicago/Turabian StyleRushdi, Mohammed I., Iman A. M. Abdel-Rahman, Eman Zekry Attia, Hani Saber, Abdullah A. Saber, Gerhard Bringmann, and Usama Ramadan Abdelmohsen. 2022. "The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives" Molecules 27, no. 3: 672. https://doi.org/10.3390/molecules27030672
APA StyleRushdi, M. I., Abdel-Rahman, I. A. M., Attia, E. Z., Saber, H., Saber, A. A., Bringmann, G., & Abdelmohsen, U. R. (2022). The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules, 27(3), 672. https://doi.org/10.3390/molecules27030672








