Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay
Abstract
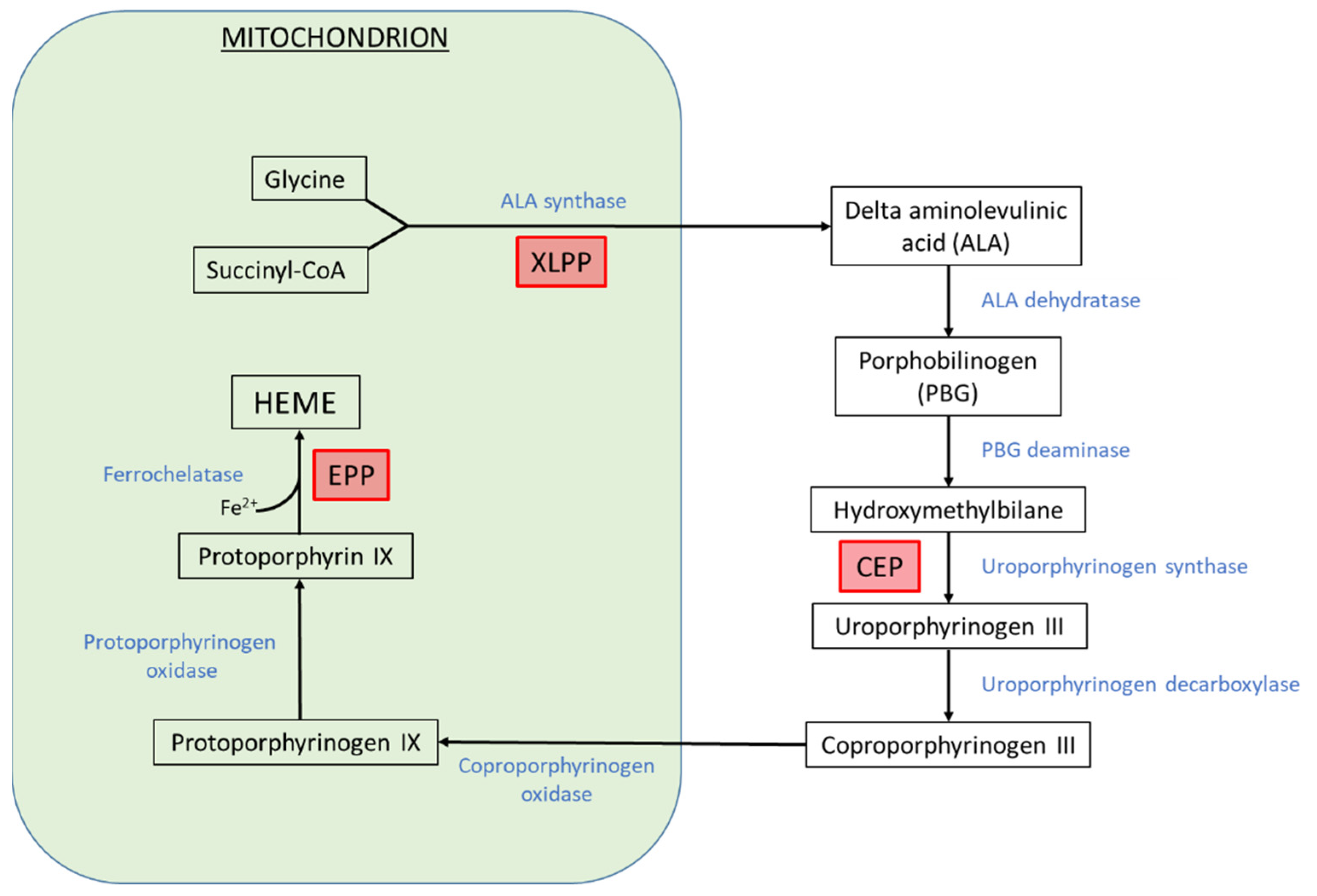
:1. Introduction
2. Iron Supplementation in Erythropoietic Porphyrias
2.1. Erythropoietic Protoporphyrias
2.2. Iron Deficiency in CEP
3. Modulation of Heme Biosynthesis Pathway Activity by Iron Availability in the Erythron
3.1. IRE/IRP System
3.2. Iron-Sulfur Proteins
3.3. FECH and ALAS2 Regulation by Iron in EPP Patients
4. FECH Regulation at the Protein Level
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puy, H.; Gouya, L.; Deybach, J.C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Erwin, A.L.; Desnick, R.J. Congenital erythropoietic porphyria: Recent advances. Mol. Genet. Metab. 2019, 128, 288–297. [Google Scholar] [CrossRef]
- Katugampola, R.P.; Badminton, M.N.; Finlay, A.Y.; Whatley, S.; Woolf, J.; Mason, N.; Deybach, J.C.; Puy, H.; Ged, C.; De Verneuil, H.; et al. Congenital erythropoietic porphyria: A single-observer clinical study of 29 cases. Br. J. Dermatol. 2012, 167, 901–913. [Google Scholar] [CrossRef]
- Phillips, J.D.; Steensma, D.P.; Pulsipher, M.A.; Spangrude, G.J.; Kushner, J.P. Congenital erythropoietic porphyria due to a mutation in GATA1: The first trans-acting mutation causative for a human porphyria. Blood 2007, 109, 2618–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouya, L.; Puy, H.; Lamoril, J.; Da Silva, V.; Grandchamp, B.; Nordmann, Y.; Deybach, J.C. Inheritance in erythropoietic protoporphyria: A common wild-type ferrochelatase allelic variant with low expression accounts for clinical manifestation. Blood 1999, 93, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Whatley, S.D.; Ducamp, S.; Gouya, L.; Grandchamp, B.; Beaumont, C.; Badminton, M.N.; Elder, G.H.; Holme, S.A.; Anstey, A.V.; Parker, M.; et al. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am. J. Hum. Genet. 2008, 83, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balwani, M.; Naik, H.; Anderson, K.E.; Bissell, D.M.; Bloomer, J.; Bonkovsky, H.L.; Phillips, J.D.; Overbey, J.R.; Wang, B.; Singal, A.K.; et al. Clinical, biochemical, and genetic characterization of north American patients with erythropoietic protoporphyria and x-linked protoporphyria. JAMA Dermatol. 2017, 153, 789–796. [Google Scholar] [CrossRef]
- Silva, B.; Faustino, P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Taketani, S.; Adachi, Y.; Nakahashi, Y. Regulation of the expression of human ferrochelatase by intracellular iron levels. Eur. J. Biochem. 2000, 267, 4685–4692. [Google Scholar] [CrossRef]
- Egan, D.N.; Yang, Z.; Phillips, J.; Abkowitz, J.L. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood 2015, 126, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, A.; Poli, A.; Ged, C.; Schmitt, C.; Lefebvre, T.; Manceau, H.; Daher, R.; Moulouel, B.; Peoc’h, K.; Simonin, S.; et al. Phlebotomy as an efficient long-term treatment of congenital erythropoietic porphyria. Haematologica 2020, 106, 913–917. [Google Scholar] [CrossRef] [Green Version]
- Blouin, J.-M.; Ged, C.; Bernardo-Seisdedos, G.; Cabantous, T.; Pinson, B.; Poli, A.; Puy, H.; Millet, O.; Gouya, L.; Morice-Picard, F.; et al. Identification of novel UROS mutations in a patient with congenital erythropoietic porphyria and efficient treatment by phlebotomy. Mol. Genet. Metab. Rep. 2021, 27, 100722. [Google Scholar] [CrossRef] [PubMed]
- Blouin, J.-M.; Ged, C.; Lalanne, M.; Lamrissi-Garcia, I.; Morice-Picard, F.; Costet, P.; Daher, R.; Moreau-Gaudry, F.; Bedel, A.; Puy, H.; et al. Iron chelation rescues hemolytic anemia and skin photosensitivity in congenital erythropoietic porphyria. Blood 2020, 136, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.P.; Meek, E.M. Clinical and biochemical improvement following low-dose intravenous iron therapy in a patient with erythropoietic protoporphyria. Br. J. Haematol. 2013, 163, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Barman-Aksözen, J.; Minder, E.I.; Schubiger, C.; Biolcati, G.; Schneider-Yin, X. In ferrochelatase-deficient protoporphyria patients, ALAS2 expression is enhanced and erythrocytic protoporphyrin concentration correlates with iron availability. Blood Cells Mol. Dis. 2015, 54, 71–77. [Google Scholar] [CrossRef]
- Landefeld, C.; Kentouche, K.; Gruhn, B.; Stauch, T.; Rößler, S.; Schuppan, D.; Whatley, S.D.; Beck, J.F.; Stölzel, U. X-linked protoporphyria: Iron supplementation improves protoporphyrin overload, liver damage and anaemia. Br. J. Haematol. 2016, 173, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, S.; Floderus, Y.; Stål, P.; Harper, P. Erythropoietic protoporphyria in Sweden: Demographic, clinical, biochemical and genetic characteristics. J. Intern. Med. 2011, 269, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.B. Erythropoietic protoporphyria. JAMA 1970, 214, 1060. [Google Scholar] [CrossRef]
- Gordeuk, V.R.; Brittenham, G.M.; Hawkins, C.W.; Mukhtar, H.; Bickers, D.R. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann. Intern. Med. 1986, 105, 27–31. [Google Scholar] [CrossRef]
- Graham-Brown, R.A.C.; Scheuer, P.J.; Sarkany, I. Histiocytosis X and erythropoietic protoporphyria. J. R. Soc. Med. 1984, 77, 238–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, A.; Graham-Brown, R.A.C.; Sarkany, I.; Baker, H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br. J. Dermatol. 1988, 119, 63–66. [Google Scholar] [CrossRef]
- McClements, B.M.; Bingham, A.; Callender, M.E.; Trimble, E.R. Erythropoietic protoporphyria and iron therapy. Br. J. Dermatol. 1990, 122, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.J.; Callender, M.E.; Mayne, E.E.; Walsh, M.; Burrows, D. Erythropoietic protoporphyria, transfusion therapy and liver disease. Br. J. Dermatol. 1992, 127, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Lyoumi, S.; Ducamp, S.; Martin-Schmitt, C.; Gouya, L.; Deybach, J.C.; Beaumont, C.; Puy, H. Excessive erythrocyte ppix influences the hematologic status and iron metabolism in patients with dominant erythropoietic protoporphyria. Cell. Mol. Biol. 2009, 55, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Thomas, C.L.; Whatley, S.D.; Bentley, D.P.; Anstey, A.V.; Badminton, M.N. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J. Am. Acad. Dermatol. 2007, 56, 1070–1072. [Google Scholar] [CrossRef]
- Bossi, K.; Lee, J.; Schmeltzer, P.; Holburton, E.; Groseclose, G.; Besur, S.; Hwang, S.; Bonkovsky, H.L. Homeostasis of iron and hepcidin in erythropoietic protoporphyria. Eur. J. Clin. Investig. 2015, 45, 1032–1041. [Google Scholar] [CrossRef]
- Bechtel, M.A.; Bertolone, S.J.; Hodge, S.J. Transfusion therapy in a patient with erythropoietic protoporphyria. Arch. Dermatol. 1981, 117, 99–101. [Google Scholar] [CrossRef]
- Dobozy, A.; Csató, M.; Siklósi, C.; Simon, N. Transfusion therapy for erythropoietic protoporphyria. Br. J. Dermatol. 1983, 109, 571–576. [Google Scholar] [CrossRef]
- Baker, H. Erythropoietic protoporphyria provoked by iron therapy. J. R. Soc. Med. 1971, 64, 610–611. [Google Scholar] [CrossRef] [Green Version]
- Weiss, Y.; Balwani, M.; Chen, B.; Yasuda, M.; Nazarenko, I.; Desnick, R.J. Congenital erythropoietic porphyria and erythropoietic protoporphyria: Identification of 7 uroporphyrinogen III synthase and 20 ferrochelatase novel mutations. Mol. Genet. Metab. 2019, 128, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Mleczko-Sanecka, K.; Silvestri, L. Cell-type-specific insights into iron regulatory processes. Am. J. Hematol. 2021, 96, 110–127. [Google Scholar] [CrossRef]
- Rouault, T.A. The indispensable role of mammalian iron sulfur proteins in function and regulation of multiple diverse metabolic pathways. BioMetals 2019, 32, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maio, N.; Jain, A.; Rouault, T.A. Mammalian iron–sulfur cluster biogenesis: Recent insights into the roles of frataxin, acyl carrier protein and ATPase-mediated transfer to recipient proteins. Curr. Opin. Chem. Biol. 2020, 55, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, S.; Puccio, H. Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Hum. Mol. Genet. 2010, 19, R103–R110. [Google Scholar] [CrossRef] [Green Version]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Wu, C.K.; Medlock, A.E.; Rose, J.P.; Wang, K.F. Ferrochelatase at the millennium: Structures, mechanisms and [2Fe-2S] clusters. Cell. Mol. Life Sci. 2000, 57, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Sellers, V.M.; Wang, K.F.; Johnson, M.K.; Daileyt, H.A. Evidence that the fourth ligand to the [2Fe-2S] cluster in animal ferrochelatase is a cysteine: Characterization of the enzyme from Drosophila melanogaster. J. Biol. Chem. 1998, 273, 22311–22316. [Google Scholar] [CrossRef] [Green Version]
- Crouse, B.R.; Sellers, V.M.; Finnegan, M.G.; Dailey, H.A.; Johnson, M.K. Site-directed mutagenesis and spectroscopic characterization of human ferrochelatase: Identification of residues coordinating the [2Fe-2S] cluster. Biochemistry 1996, 35, 16222–16229. [Google Scholar] [CrossRef]
- Schneider-Yin, X.; Gouya, L.; Dorsey, M.; Rüfenacht, U.; Deybach, J.C.; Ferreira, G.C. Mutations in the iron-sulfur cluster ligands of the human ferrochelatase lead to erythropoietic protoporphyria. Blood 2000, 96, 1545–1549. [Google Scholar] [CrossRef]
- Crooks, D.R.; Ghosh, M.C.; Haller, R.G.; Tong, W.-H.; Rouault, T.A. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 2010, 115, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Barman-Aksözen, J.; Halloy, F.; Iyer, P.S.; Schümperli, D.; Minder, A.E.; Hall, J.; Minder, E.I.; Schneider-Yin, X. Delta-aminolevulinic acid synthase 2 expression in combination with iron as modifiers of disease severity in erythropoietic protoporphyria. Mol. Genet. Metab. 2019, 128, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Barman-Aksözen, J.; Béguin, C.; Dogar, A.M.; Schneider-Yin, X.; Minder, E.I. Iron availability modulates aberrant splicing of ferrochelatase through the iron- and 2-oxoglutarate dependent dioxygenase Jmjd6 and U2AF65. Blood Cells Mol. Dis. 2013, 51, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ducamp, S.; Luscieti, S.; Ferrer-Cortès, X.; Nicolas, G.; Manceau, H.; Peoc’h, K.; Yien, Y.Y.; Kannengiesser, C.; Gouya, L.; Puy, H.; et al. A mutation in the iron-responsive element of ALAS2 is a modifier of disease severity in apatient suffering from CLPX associated erythropoietic protoporphyria. Haematologica 2021, 106, 2030. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J.; Ducamp, S.; Clayton, J.; Badenas, C.; Delaby, C.; Ged, C.; Lyoumi, S.; Gouya, L.; De Verneuil, H.; Beaumont, C.; et al. ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood 2011, 118, 1443–1451. [Google Scholar] [CrossRef]
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA interference therapy for acute intermittent porphyria. N. Engl. J. Med. 2019, 380, 549–558. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Agarwal, S.; Simon, A.R.; Goel, V.; Habtemariam, B.A.; Clausen, V.A.; Kim, J.B.; Robbie, G.J. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin. Pharmacol. Ther. 2020, 108, 63–72. [Google Scholar] [CrossRef]
- Medlock, A.E.; Najahi-Missaoui, W.; Shiferaw, M.T.; Albetel, A.N.; Lanzilotta, W.N.; Dailey, H.A. Insight into the function of active site residues in the catalytic mechanism of human ferrochelatase. Biochem. J. 2021, 478, 3239–3252. [Google Scholar] [CrossRef]
- Chung, J.; Wittig, J.G.; Ghamari, A.; Maeda, M.; Dailey, T.A.; Bergonia, H.; Kafina, M.D.; Coughlin, E.E.; Minogue, C.E.; Hebert, A.S.; et al. Erythropoietin signaling regulates heme biosynthesis. eLife 2017, 6, e24767. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef]
- Taketani, S.; Kakimoto, K.; Ueta, H.; Masaki, R.; Furukawa, T. Involvement of ABC7 in the biosynthesis of heme in erythroid cells: Interaction of ABC7 with ferrochelatase. Blood 2003, 101, 3274–3280. [Google Scholar] [CrossRef] [Green Version]
- Maio, N.; Kim, K.S.; Holmes-Hampton, G.; Singh, A.; Rouault, T.A. Dimeric ferrochelatase bridges ABCB7 and ABCB10 homodimers in an architecturally defined molecular complex required for heme biosynthesis. Haematologica 2019, 104, 1756–1767. [Google Scholar] [CrossRef]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the mitochondrial heme metabolism complex. PLoS ONE 2015, 10, e0135896. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Paradkar, P.N.; Li, L.; Pierce, E.L.; Langer, N.B.; Takahashi-Makise, N.; Hyde, B.B.; Shirihai, O.S.; Ward, D.M.; Kaplan, J.; et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16263–16268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.; Cowan, J.A. Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J. Biol. Chem. 2004, 279, 25943–25946. [Google Scholar] [CrossRef] [Green Version]
- Yien, Y.Y.; Shi, J.; Chen, C.; Cheung, J.T.M.; Grillo, A.S.; Shrestha, R.; Li, L.; Zhang, X.; Kafina, M.D.; Kingsley, P.D.; et al. FAM210B is an erythropoietin target and regulates erythroid heme synthesis by controlling mitochondrial iron import and ferrochelatase activity. J. Biol. Chem. 2018, 293, 19797–19811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.; Farrell, C.; Wang, Y.; Singal, A.K.; Anderson, K.; Balwani, M.; Bissell, M.; Bonkovsky, H.; Seay, T.; Paw, B.; et al. Strong correlation of ferrochelatase enzymatic activity with Mitoferrin-1 mRNA in lymphoblasts of patients with protoporphyria. Mol. Genet. Metab. 2019, 128, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Langer, N.B.; Shaw, G.C.; Yang, G.; Li, L.; Kaplan, J.; Paw, B.H.; Bloomer, J.R. Abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. Exp. Hematol. 2011, 39, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Pandolfo, M.; Pastore, A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J. Neurol. 2009, 256, 9–17. [Google Scholar] [CrossRef]
- Lesuisse, E.; Santos, R.; Matzanke, B.F.; Knight, S.A.B.; Camadro, J.M.; Dancis, A. Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1). Hum. Mol. Genet. 2003, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Bencze, K.Z.; Yoon, T.; Millán-Pacheco, C.; Bradley, P.B.; Pastor, N.; Cowan, J.A.; Stemmler, T.L. Human frataxin: Iron and ferrochelatase binding surface. Chem. Commun. 2007, 18, 1798–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Gakh, O.; O’Neill, H.A.; Mangravita, A.; Nichol, H.; Ferreira, G.C.; Isaya, G. Yeast frataxin sequentially chaperones and stores iron by coupling protein assembly with iron oxidation. J. Biol. Chem. 2003, 278, 31340–31351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, M.; Funk, J.; Körner, A.; Alberati, D.; Christen, F.; Schmitt, G.; Altmann, B.; Pospischil, A.; Singer, T. Effects of GlyT1 inhibition on erythropoiesis and iron homeostasis in rats. Exp. Hematol. 2016, 44, 964–974. [Google Scholar] [CrossRef]
- Matte, A.; Federti, E.; Winter, M.; Koerner, A.; Harmeier, A.; Mazer, N.; Tomka, T.; Di Paolo, M.L.; Defalco, L.; Andolfo, I.; et al. Bitopertin, a selective oral GLYT1 inhibitor, improves anemia in a mouse model of β-thalassemia. JCI Insight 2019, 4, e130111. [Google Scholar] [CrossRef]
- Taher, A.T.; Viprakasit, V.; Cappellini, M.D.; Kraus, D.; Cech, P.; Volz, D.; Winter, E.; Nave, S.; Dukart, J.; Khwaja, O.; et al. Haematological effects of oral administration of bitopertin, a glycine transport inhibitor, in patients with non-transfusion-dependent β-thalassaemia. Br. J. Haematol. 2021, 194, 474–477. [Google Scholar] [CrossRef]


| Authors | Year | Number of Patients | Sex | Type | Molecular Diagnosis | Biochemical Diagnosis | Intervention | Clinical Outcome | Biochemical Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Reed et al. | 1970 | 1 | F | NA | no | yes | oral iron therapy | worsening | NA |
| Baker et al. | 1971 | 1 | F | NA | no | yes | oral iron therapy | worsening | PPIX increase |
| Bechtel et al. | 1981 | 1 | M | NA | no | yes | repeated transfusions | improvement | PPIX decrease |
| Dobozy et al. | 1983 | 5 | 1 F and 4 M | NA | no | yes | repeated transfusions | improvement | PPIX decrease |
| Graham-Brown et al. | 1984 | 1 | F | NA | no | yes | oral iron therapy | worsening | PPIX decrease after iron therapy discontinuation |
| Gordeuk et al. | 1986 | 1 | F | NA | no | yes | oral iron therapy * | NA | PPIX decrease |
| Milligan et al. | 1988 | 2 ** | F | NA | no | yes | oral iron therapy | worsening | PPIX increase |
| McClements et al. | 1990 | 1 | F | NA | no | yes | oral iron therapy | worsening | PPIX decrease after iron therapy discontinuation |
| Todd et al. | 1992 | 1 | M | NA | no | yes | repeated transfusions | worsening | PPIX increase |
| Holme et al. | 2007 | 1 | M | EPP | yes | yes | oral iron therapy | improvement | stable PPIX |
| Whatley et al. | 2008 | 1 | M | XLPP | yes | yes | oral iron therapy | improvement | PPIX decrease |
| Whalin et al. | 2011 | 1 | F | EPP | yes | yes | oral iron therapy | worsening | stable PPIX |
| Bentley et al. | 2013 | 1 *** | M | EPP | yes | yes | IV iron therapy | improvement | PPIX decrease |
| Barman-Aksözen et al. | 2015 | 2 | F | EPP | yes | yes | IV or oral iron therapy | worsening | PPIX increase (one patient) stable PPIX (one patient) |
| Balwani et al. | 2017 | 8 | F | XLPP | yes | yes | oral iron therapy | improvement (7/8) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poli, A.; Schmitt, C.; Moulouel, B.; Mirmiran, A.; Puy, H.; Lefèbvre, T.; Gouya, L. Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay. Metabolites 2021, 11, 798. https://doi.org/10.3390/metabo11120798
Poli A, Schmitt C, Moulouel B, Mirmiran A, Puy H, Lefèbvre T, Gouya L. Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay. Metabolites. 2021; 11(12):798. https://doi.org/10.3390/metabo11120798
Chicago/Turabian StylePoli, Antoine, Caroline Schmitt, Boualem Moulouel, Arienne Mirmiran, Hervé Puy, Thibaud Lefèbvre, and Laurent Gouya. 2021. "Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay" Metabolites 11, no. 12: 798. https://doi.org/10.3390/metabo11120798






