Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
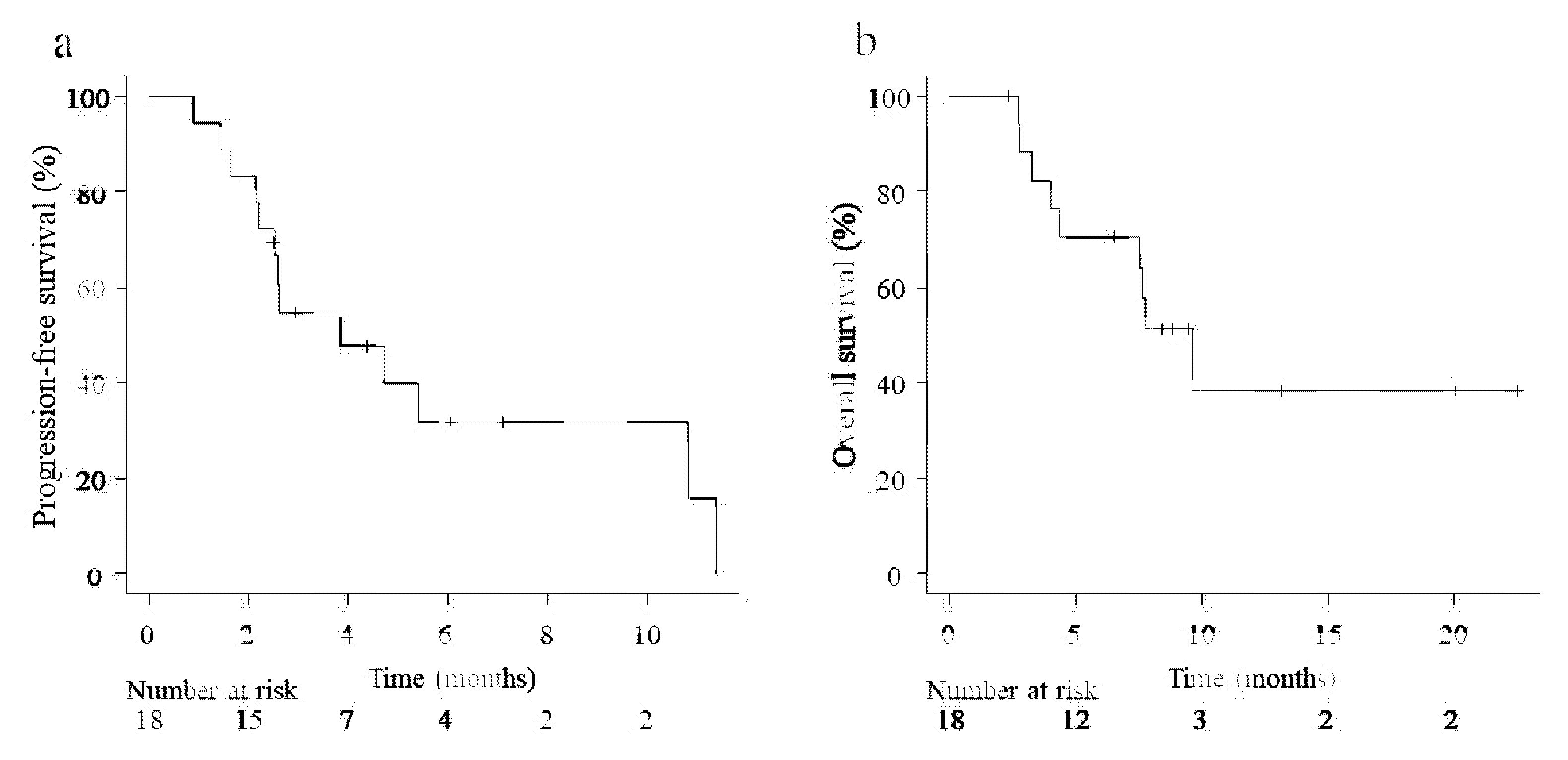
3.2. Efficacy of Chemotherapy after ICI Therapy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Braakhuis, B.J.M.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23 (Suppl. 1), x173–x177. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laura, Q.M.; Chow, M.D. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Park, S.E.; Lee, S.H.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Sun, J.-M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J. Thorac. Oncol. 2018, 13, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arigami, T.; Matsushita, D.; Okubo, K.; Yanagita, S.; Ehi, K.; Sasaki, K.; Noda, M.; Kita, Y.; Mori, S.; Kurahara, H.; et al. response rate and prognostic impact of salvage chemotherapy after nivolumab in patients with advanced gastric cancer. Oncology 2020, 98, 630–636. [Google Scholar] [CrossRef]
- Suzuki, S.; Toyoma, S.; Tomizawa, H.; Yamada, T.; Iikawa, N.; Shiina, K.; Saito, H.; Koizumi, K.; Kawasaki, Y.; Yamada, T. Efficacy of chemotherapy after progression with nivolumab in squamous cell carcinoma of the head and neck. Auris Nasus Larynx 2020, 47, 485–488. [Google Scholar] [CrossRef]
- Saleh, K.; Daste, A.; Martin, N.; Pons-Tostivint, E.; Auperin, A.; Gómez, R.G.H.; Baste-Rotllan, N.; Bidault, F.; Guigay, J.; Le Tourneau, C.; et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur. J. Cancer 2019, 121, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pestana, R.C.; Becnel, M.; Rubin, M.L.; Torman, D.K.; Crespo, J.; Phan, J.; Hanna, E.; Bell, D.; Glisson, B.S.; Johnson, J.; et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020, 101, 104523. [Google Scholar] [CrossRef] [PubMed]
- Hitt, R.; Irigoyen, A.; Cortes-Funes, H.; Grau, J.J.; Garcia-Saenz, J.A.; Cruz-Hernandez, J. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann. Oncol. 2012, 23, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Enokida, T.; Okano, S.; Fujisawa, T.; Ueda, Y.; Uozumi, S.; Tahara, M. Paclitaxel plus cetuximab as 1st line chemotherapy in platinum-based chemoradiotherapy-refractory patients with squamous cell carcinoma of the head and neck. Front. Oncol. 2018, 8, 339. [Google Scholar] [CrossRef]
- Fushimi, C.; Baba, D.; Masubuchi, T.; Yamazaki, M.; Kitani, Y.; Kitajima, T.; Tanaka, J.; Hanyu, K.; Tanaka, N.; Miura, K.; et al. Weekly cetuximab and paclitaxel for recurrent or metastatic head and neck squamous cell carcinoma. In Vivo 2020, 34, 2653–2657. [Google Scholar] [CrossRef]
- Wakasaki, T.; Yasumatsu, R.; Masuda, M.; Takeuchi, T.; Manako, T.; Matsuo, M.; Jiromaru, R.; Uchi, R.; Komune, N.; Noda, T.; et al. Prognostic biomarkers of salvage chemotherapy following nivolumab treatment for recurrent and/or metastatic head and neck squamous cell carcinoma. Cancers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Camarero, S.; Cabrera-Martín, M.N.; Merino-Menéndez, S.; Paz-Cabezas, M.; García-Barberán, V.; Sanz, M.S.; Iglesias-Moreno, M.; Alonso-Ovies, A.; Pérez-Segura, P. Safety and efficacy of cetuximab-based salvage chemotherapy after checkpoint inhibitors in head and neck cancer. Oncology 2021, 26, e1018–e1035. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Kobayashi, H.; Ueyama, Y. Paclitaxel in combination with cetuximab exerts antitumor effect by suppressing NF-κB activity in human oral squamous cell carcinoma cell lines. Int. J. Oncol. 2014, 45, 2439–2445. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Lenz, H.-J.; Trotta, A.M.; García-Foncillas, J.; Schulten, J.; Audhuy, F.; Merlano, M.; Milano, G. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat. Rev. 2018, 63, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, L.; Denaro, N.; Vivenza, D.; Varamo, C.; Strola, G.; Fortunato, M.; Chamorey, E.; Comino, A.; Monteverde, M.; Nigro, C.L.; et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol. Immunother. 2017, 66, 573–579. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Kurosaki, T.; Mitani, S.; Tanaka, K.; Suzuki, S.; Kanemura, H.; Haratani, K.; Fumita, S.; Iwasa, T.; Hayashi, H.; Yoshida, T.; et al. Safety and efficacy of cetuximab-containing chemotherapy after immune checkpoint inhibitors for patients with squamous cell carcinoma of the head and neck: A single-center retrospective study. Anti-Cancer Drugs 2021, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.M.; Montagut, C.; Wainberg, Z.A.; Ronga, P.; Audhuy, F.; Taieb, J.; Stintzing, S.; Siena, S.; Santini, D. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open 2018, 3, e000353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | No. of Patients | Percent |
|---|---|---|
| Age (years), median (range) | 61.5 (40–78) | |
| Sex | ||
| Male | 17 | 94.4 |
| Female | 1 | 5.6 |
| EOCG performance status | ||
| 0 | 6 | 33.3 |
| 1 | 10 | 55.6 |
| 2 | 2 | 11.1 |
| Initial tumor location | ||
| Oral | 5 | 27.8 |
| Oropharynx | 3 | 16.7 |
| Hypopharynx | 7 | 38.9 |
| Others | 3 | 16.7 |
| Site of progression after ICI therapy | ||
| Locoregional | 14 | 77.8 |
| Distant | 4 | 22.2 |
| ICI | ||
| Nivolumab | 14 | 77.8 |
| Pembrolizumab | 4 | 22.2 |
| Cetuximab before ICI therapy | ||
| Yes | 8 | 44.4 |
| No | 10 | 55.6 |
| Patients | CR-PR | SD-PD | ORR (%) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 8 | 9 | 47.1 | 0.36 |
| Female | 0 | 1 | 0.0 | |
| Age (years) | ||||
| >60 | 4 | 6 | 40.0 | 0.67 |
| <60 | 4 | 4 | 50.0 | |
| Performance status | ||||
| 0.1 | 7 | 9 | 43.8 | 0.87 |
| 2 | 1 | 1 | 50.0 | |
| Initial tumor location | ||||
| Hypopharynx | 2 | 5 | 28.6 | 0.71 |
| Oropharynx | 2 | 1 | 66.7 | |
| Oral | 3 | 2 | 60.0 | |
| Nasal | 1 | 2 | 33.3 | |
| Site of progression after ICI therapy | ||||
| Locoregional | 7 | 7 | 50.0 | 0.38 |
| Distant | 1 | 3 | 25.0 | |
| Type of ICI | ||||
| Nivo | 7 | 7 | 50.0 | 0.38 |
| Pemb | 1 | 3 | 25.0 | |
| Best response to ICI | ||||
| CR, PR | 3 | 2 | 60.0 | 0.41 |
| SD, PD | 5 | 8 | 38.5 | |
| Cetuximab-containing chemotherapy before ICI therapy | ||||
| Yes | 2 | 5 | 28.6 | 0.28 |
| No | 6 | 5 | 54.5 | |
| All Grades | Grade 3 | |
|---|---|---|
| Patients, n (%) | Patients, n (%) | |
| Hematologic | ||
| Neutropenia | 14 (77.8) | 3 (16.7) |
| Anemia | 13 (72.2) | 1 (5.6) |
| Thrombocytopenia | 9 (50.0) | 0 |
| Non-hematologic | ||
| Hypomagnesemia | 4 (22.2) | 0 |
| Acne-like rash | 15 (83.3) | 0 |
| Paronychia | 3 (16.7) | 1 (5.6) |
| Asthenia | 1 (5.6) | 1 (5.6) |
| Peripheral neuropathy | 3 (16.7) | 1 (5.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, S.; Toyoma, S.; Kawasaki, Y.; Koizumi, K.; Iikawa, N.; Shiina, K.; Endo, T.; Abe, T.; Kouga, T.; Yamada, T. Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Medicina 2021, 57, 1151. https://doi.org/10.3390/medicina57111151
Suzuki S, Toyoma S, Kawasaki Y, Koizumi K, Iikawa N, Shiina K, Endo T, Abe T, Kouga T, Yamada T. Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Medicina. 2021; 57(11):1151. https://doi.org/10.3390/medicina57111151
Chicago/Turabian StyleSuzuki, Shinsuke, Satoshi Toyoma, Yohei Kawasaki, Koh Koizumi, Nobuko Iikawa, Kazuhiro Shiina, Tentaro Endo, Tomoe Abe, Teppei Kouga, and Takechiyo Yamada. 2021. "Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma" Medicina 57, no. 11: 1151. https://doi.org/10.3390/medicina57111151
APA StyleSuzuki, S., Toyoma, S., Kawasaki, Y., Koizumi, K., Iikawa, N., Shiina, K., Endo, T., Abe, T., Kouga, T., & Yamada, T. (2021). Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Medicina, 57(11), 1151. https://doi.org/10.3390/medicina57111151






