Personalized and Self-Management: Systematic Search and Evaluation Quality Factors and User Preference of Drug Reference Apps in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
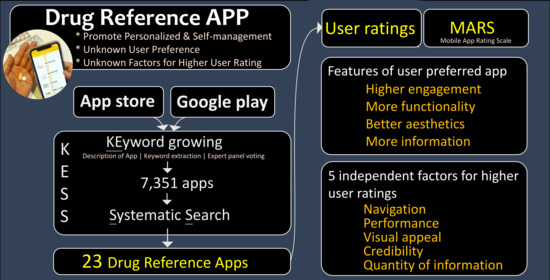
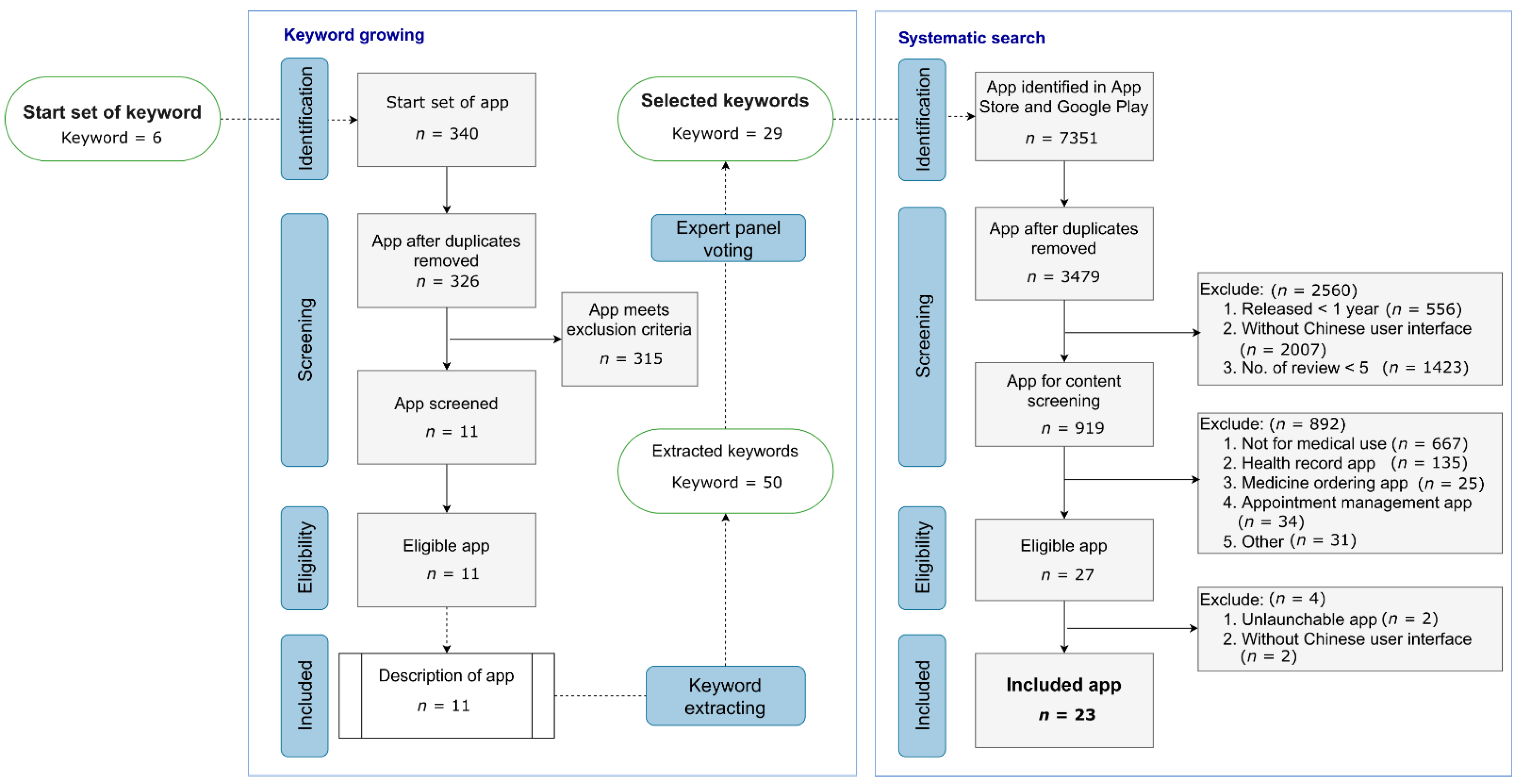
2.2. Searching Algorithm (KESS) for Drug Reference App: Keyword Growing and Systematic Search
2.2.1. Keyword Growing
2.2.2. Systematic Search
2.3. Quality Appraisal of Apps
2.4. User Ratings
2.5. Data and Statistical Analysis
3. Results
3.1. Drug Reference Apps in Taiwan
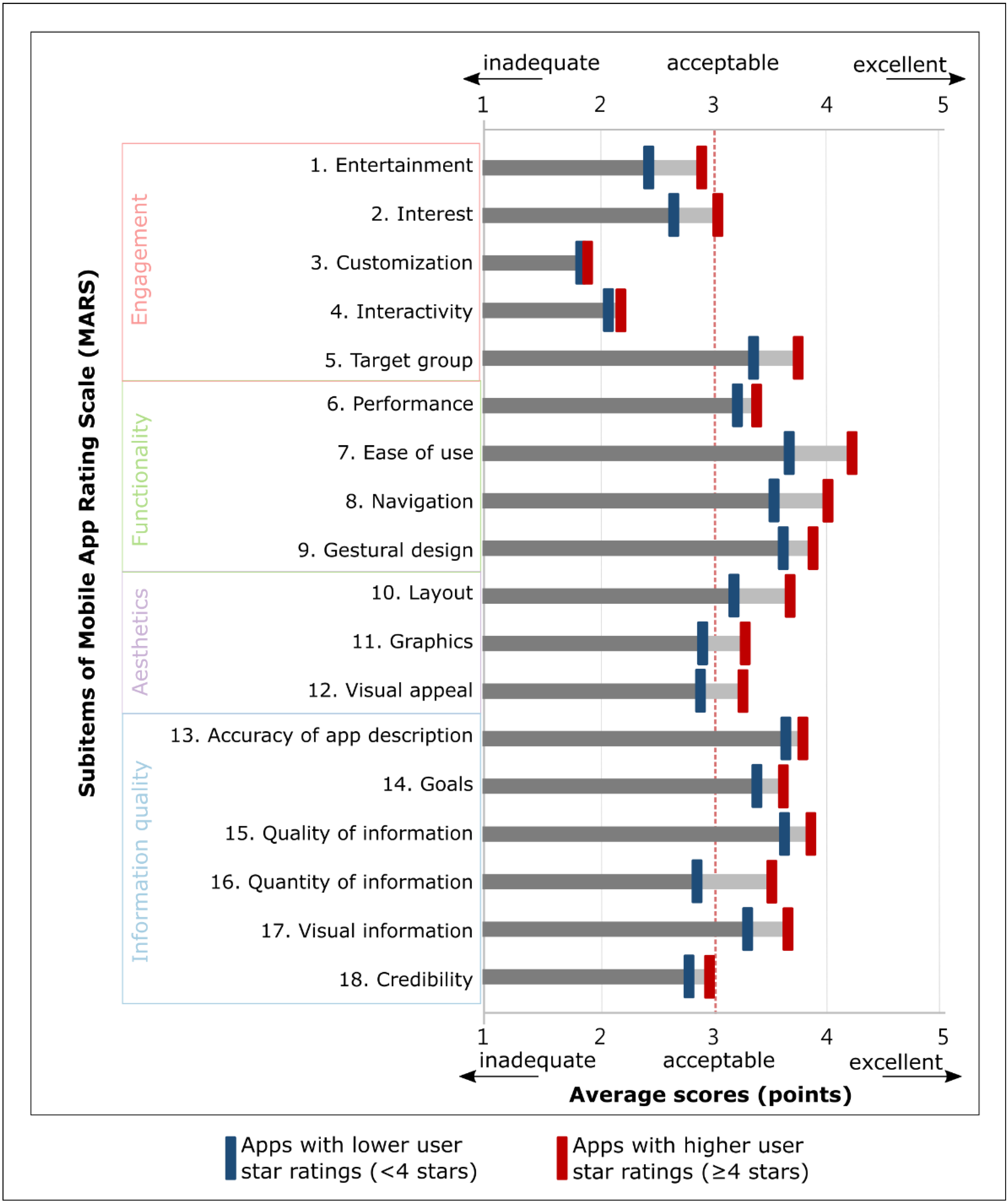
3.2. Apps with Higher User Ratings vs. Lower Ratings
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Details of Searching Algorithm (KESS) for Drug Reference App
Appendix A.1. Start Set of Keywords
Appendix A.2. Keyword Extracting
Appendix A.3. Expert Panel Voting
References
- Mosa, A.M.; Yoo, I.; Sheets, L. A Systematic Review of Healthcare Applications for Smartphones. BMC Med. Inform. Decis. Mak. 2012, 12, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Tabi, K.; Randhawa, A.S.; Choi, F.; Mithani, Z.; Albers, F.; Schnieder, M.; Nikoo, M.; Vigo, D.; Jang, K.; Demlova, R.; et al. Mobile Apps for Medication Management: Review and Analysis. JMIR Mhealth Uhealth 2019, 7, e13608. [Google Scholar] [CrossRef] [Green Version]
- Rowland, S.P.; Fitzgerald, J.E.; Holme, T.; Powell, J.; McGregor, A. What is the clinical value of mHealth for patients? NPJ Digit. Med. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Aungst, T.D.; Miranda, A.C.; Serag-Bolos, E.S. How mobile devices are changing pharmacy practice. Am. J. Health Syst. Pharm. 2015, 72, 494–500. [Google Scholar] [CrossRef]
- Apidi, N.A.; Murugiah, M.K.; Muthuveloo, R.; Soh, Y.C.; Caruso, V.; Patel, R.; Ming, L.C. Mobile Medical Applications for Dosage Recommendation, Drug Adverse Reaction, and Drug Interaction: Review and Comparison. Ther. Innov. Regul. Sci. 2017, 51, 480–485. [Google Scholar] [CrossRef]
- Morse, S.S.; Murugiah, M.K.; Soh, Y.C.; Wong, T.W.; Ming, L.C. Mobile Health Applications for Pediatric Care: Review and Comparison. Ther. Innov. Regul. Sci. 2018, 52, 383–391. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Andrews, T. Smartphones and mobile applications (apps) in clinical nursing education: A student perspective. Nurse Educ. Today 2018, 69, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.F.B.; Wharrad, H.; Watts, K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): A regional survey. BMC Med. Inform. Decis. Mak. 2012, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensing, H.T.; Vervloet, M.; van Dooren, A.A.; Bouvy, M.L.; Koster, E.S. Patient-pharmacist communication during a post-discharge pharmacist home visit. Int. J. Clin. Pharm. 2018, 40, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Linn, A.J.; van Weert, J.C.M.; van Dijk, L.; Horne, R.; Smit, E.G. The value of nurses’ tailored communication when discussing medicines: Exploring the relationship between satisfaction, beliefs and adherence. J. Health Psychol. 2016, 21, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Berauk, V.L.A.; Murugiah, M.K.; Soh, Y.C.; Sheng, Y.C.; Wong, T.W.; Ming, L.C. Mobile Health Applications for Caring of Older People: Review and Comparison. Ther. Innov. Regul. Sci. 2018, 52, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.C.; Hameed, M.A.; Lee, D.D.; Apidi, N.A.; Lai, P.S.M.; Hadi, M.A.; Al-Worafi, Y.M.A.; Khan, T.M. Use of Medical Mobile Applications Among Hospital Pharmacists in Malaysia. Ther. Innov. Regul. Sci. 2016, 50, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M.; Abebe, E.; Xiao, Y.; Brown, R.; Pena, I.M.; Gurses, A.P. Engineering care transitions: Clinician perceptions of barriers to safe medication management during transitions of patient care. Appl. Ergon. 2021, 91, 7. [Google Scholar] [CrossRef]
- Eggerth, A.; Hayn, D.; Schreier, G. Medication management needs information and communications technology-based approaches, including telehealth and artificial intelligence. Br. J. Clin. Pharmacol. 2020, 86, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jover, V.; Sala-Gonzalez, M.; Guilabert, M.; Joaquin Mira, J. Mobile Apps for Increasing Treatment Adherence: Systematic Review. J. Med. Internet Res. 2019, 21, e12505. [Google Scholar] [CrossRef] [Green Version]
- Schenk, A.; Eckardt-Felmberg, R.; Steinhagen-Thiessen, E.; Stegemann, S. Patient behaviour in medication management: Findings from a patient usability study that may impact clinical outcomes. Br. J. Clin. Pharmacol. 2020, 86, 1958–1968. [Google Scholar] [CrossRef] [Green Version]
- Joaquin Mira, J. Medication errors in the older people population. Expert Rev. Clin. Pharmacol. 2019, 12, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, B.; Bagala, M.; Creighton, A.; Leavey, T.; Nicholls, S.; Wood, C.; Longman, J.; Barker, J.; Pit, S. Mobile phone applications and their use in the self-management of Type 2 Diabetes Mellitus: A qualitative study among app users and non-app users. Diabetol. Metab. Syndr. 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Roux, P.; Santiago-Delefosse, M.; von Gunten, A.; Wernli, B.; Martins, M.M.; Verloo, H. Optimising medication management for polymedicated home-dwelling older adults with multiple chronic conditions: A mixed-methods study protocol. BMJ Open 2019, 9, e030030. [Google Scholar] [CrossRef]
- Pereira, F.; von Gunten, A.; Rosselet Amoussou, J.; De Giorgi Salamun, I.; Martins, M.M.; Verloo, H. Polypharmacy Among Home-Dwelling Older Adults: The Urgent Need for an Evidence-Based Medication Management Model. Patient Prefer. Adherence 2019, 13, 2137–2143. [Google Scholar] [CrossRef] [Green Version]
- Tobiano, G.; Chaboyer, W.; Teasdale, T.; Cussen, J.; Raleigh, R.; Manias, E. Older patient and family discharge medication communication: A mixed-methods study. J. Eval. Clin. Pract. 2021, 27, 898–906. [Google Scholar] [CrossRef]
- Lin, H.W.; Ku, C.H.; Li, J.F.; Tan, A.C.; Chou, C.H. A nationwide evaluation on electronic medication-related information provided by hospital websites. J. Eval. Clin. Pract. 2013, 19, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Nualdaisri, P.; Corlett, S.A.; Krska, J. Provision and Need for Medicine Information in Asia and Africa: A Scoping Review of the Literature. Drug Saf. 2021, 44, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Santo, K.; Richtering, S.S.; Chalmers, J.; Thiagalingam, A.; Chow, C.K.; Redfern, J. Mobile Phone Apps to Improve Medication Adherence: A Systematic Stepwise Process to Identify High-Quality Apps. JMIR Mhealth Uhealth 2016, 4, e6742. [Google Scholar] [CrossRef]
- Choi, S.K.; Yelton, B.; Ezeanya, V.K.; Kannaley, K.; Friedman, D.B. Review of the Content and Quality of Mobile Applications About Alzheimer’s Disease and Related Dementias. J. Appl. Gerontol. 2020, 39, 601–608. [Google Scholar] [CrossRef]
- Park, J.Y.E.; Li, J.; Howren, A.; Tsao, N.W.; De Vera, M. Mobile Phone Apps Targeting Medication Adherence: Quality Assessment and Content Analysis of User Reviews. JMIR Mhealth Uhealth 2019, 7, e11919. [Google Scholar] [CrossRef] [Green Version]
- Salgado, T.M.; Fedrigon, A.; Omichinski, D.R.; Meade, M.A.; Farris, K.B. Identifying Medication Management Smartphone App Features Suitable for Young Adults with Developmental Disabilities: Delphi Consensus Study. JMIR Mhealth Uhealth 2018, 6, e9527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A.M.; Smith, S.G.; Bailey, S.C.; Belter, L.T.; Pandit, A.U.; Hedlund, L.A.; Bojarski, E.A.; Rush, S.R.; Wolf, M.S. Older Adult Preferences of Mobile Application Functionality Supporting Medication Self-Management. J. Health Commun. 2018, 23, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Terhorst, Y.; Philippi, P.; Sander, L.B.; Schultchen, D.; Paganini, S.; Bardus, M.; Santo, K.; Knitza, J.; Machado, G.C.; Schoeppe, S.; et al. Validation of the Mobile Application Rating Scale (MARS). PLoS ONE 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile app rating scale: A new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015, 3, e27. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Booth, A.; Varley-Campbell, J.; Britten, N.; Garside, R. Defining the process to literature searching in systematic reviews: A literature review of guidance and supporting studies. BMC Med. Res. Methodol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portenoy, J.; West, J.D. Constructing and evaluating automated literature review systems. Scientometrics 2020, 125, 3233–3251. [Google Scholar] [CrossRef]
- Choong, M.K.; Galgani, F.; Dunn, A.G.; Tsafnat, G. Automatic Evidence Retrieval for Systematic Reviews. J. Med. Internet Res. 2014, 16, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Grp, P.S. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Anderson, K.; Burford, O.; Emmerton, L. Mobile Health Apps to Facilitate Self-Care: A Qualitative Study of User Experiences. PLoS ONE 2016, 11, e0156164. [Google Scholar] [CrossRef] [Green Version]
- Kubler, R.; Pauwels, K.; Yildirim, G.; Fandrich, T. App Popularity: Where in the World Are Consumers Most Sensitive to Price and User Ratings? J. Mark. 2018, 82, 20–44. [Google Scholar] [CrossRef]
- Huh, J.H. Big Data Analysis for Personalized Health Activities: Machine Learning Processing for Automatic Keyword Extraction Approach. Symmetry 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Tsafnat, G.; Glasziou, P.; Choong, M.K.; Dunn, A.; Galgani, F.; Coiera, E. Systematic review automation technologies. Syst. Rev. 2014, 3, 74. [Google Scholar] [CrossRef] [Green Version]
- Aakre, C.A.; Maggio, L.A.; Del Fiol, G.; Cook, D.A. Barriers and facilitators to clinical information seeking: A systematic review. J. Am. Med. Inf. Assoc. 2019, 26, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Horsky, J.; Drucker, E.A.; Ramelson, H.Z. Higher accuracy of complex medication reconciliation through improved design of electronic tools. J. Am. Med. Inf. Assoc. 2018, 25, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Laera, E.; Gutzman, K.; Spencer, A.; Beyer, C.; Bolore, S.; Gallagher, J.; Pidgeon, S.; Rodriguez, R. Why are they not accessing it? User barriers to clinical information access. J. Med. Libr. Assoc. 2021, 109, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, E.; Torres-Robles, A.; Cutler, R.L.; Benrimoj, S.I.; Garcia-Cardenas, V. Impact of a Multicomponent Digital Therapeutic Mobile App on Medication Adherence in Patients with Chronic Conditions: Retrospective Analysis. J. Med. Internet Res. 2020, 22, 11. [Google Scholar] [CrossRef]
- Abraham, O.; Lemay, S.; Bittner, S.; Thakur, T.; Stafford, H.; Brown, R. Investigating Serious Games That Incorporate Medication Use for Patients: Systematic Literature Review. JMIR Serious Games 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Sabate, L.R.; Diego, L. Are we offering patients the right medicines information? A retrospective evaluation of readability and quality in online patient drug information. Eur. J. Hosp. Pharm. 2021, 28, 144–148. [Google Scholar] [CrossRef]
- Raynor, D.K.; Blenkinsopp, A.; Knapp, P.; Grime, J.; Nicolson, D.J.; Pollock, K.; Dorer, G.; Gilbody, S.; Dickinson, D.; Maule, A.J.; et al. A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines. Health Technol. Assess. 2007, 11, 1–117. [Google Scholar] [CrossRef]
- Kusch, M.K.P.; Haefeli, W.E.; Seidling, H.M. How to meet patients’ individual needs for drug information—A scoping review. Patient Prefer. Adherence 2018, 12, 2339–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal-Cadavid, J.; Amariles, P.; Pino-Marin, D.; Granados, J.; Giraldo, N. Design and development of a mobile app of drug information for people with visual impairment. Res. Soc. Adm. Pharm. 2020, 16, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Schubbe, D.; Scalia, P.; Yen, R.W.; Saunders, C.H.; Cohen, S.; Elwyn, G.; van den Muijsenbergh, M.; Durand, M.A. Using pictures to convey health information: A systematic review and meta-analysis of the effects on patient and consumer health behaviors and outcomes. Patient Educ. Couns. 2020, 103, 1935–1960. [Google Scholar] [CrossRef]
- Merks, P.; Swieczkowski, D.; Balcerzak, M.; Drelich, E.; Bialoszewska, K.; Cwalina, N.; Krysinski, J.; Jaguszewski, M.; Pouliot, A.; Vaillancourt, R. The evaluation of pharmaceutical pictograms among elderly patients in community pharmacy settings—A multicenter pilot study. Patient Prefer. Adherence 2018, 12, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Niemand, T.; Mai, R.; Kraus, S. The zero-price effect in freemium business models: The moderating effects of free mentality and price-quality inference. Psychol. Mark. 2019, 36, 773–790. [Google Scholar] [CrossRef]
- Chinese Knowledge and Information Processing. Available online: https://ckip.iis.sinica.edu.tw/service/corenlp/ (accessed on 15 March 2021).
| Characteristics | n = 23 | (%) |
|---|---|---|
| App store | ||
| Google Play Store | 15 | (65.2) |
| Apple App Store | 8 | (34.8) |
| Primary category/genre | ||
| Medical | 16 | (69.6) |
| Health and fitness | 4 | (17.4) |
| Tools/Utilities | 3 | (13.0) |
| Developer type | ||
| Hospital | 13 | (56.5) |
| Private group | 8 | (34.8) |
| University | 2 | (8.7) |
| User feedbacks | ||
| User star rating (star) | ||
| Average (SD 2) | 3.65 | (0.95) |
| ≤2 | 5 | (21.7) |
| 3 | 8 | (34.8) |
| 4–5 | 10 | (43.5) |
| No. of reviews | ||
| <50 | 14 | (60.9) |
| 50–100 | 6 | (26.1) |
| >100 | 3 | (13.0) |
| No. of downloads 1 | ||
| 500–5000 | 5 | (33.3) |
| 5000–10,000 | 2 | (13.3) |
| 10,000–50,000 | 5 | (33.3) |
| >50,000 | 3 | (20.0) |
| MARS score 3 | ||
| ≥4: good or above | 1 | (4.3) |
| 3: acceptable | 18 | (78.3) |
| ≤2: poor | 4 | (17.4) |
| App with Higher User Star Rating (≥4 Stars) | App with Lower User Star Rating (<4 Stars) | |||||
|---|---|---|---|---|---|---|
| n = 10 | (%) | n = 13 | (%) | p Values | Sig. 1 | |
| Developer type | 0.241 | |||||
| Hospital | 5 | (50.0) | 8 | (61.5) | ||
| Private group | 3 | (30.0) | 5 | (38.5) | ||
| University | 2 | (20.0) | ||||
| Primary category/genre | 0.276 | |||||
| Medical | 6 | (60.0) | 10 | (76.9) | ||
| Health and fitness | 2 | (20.0) | 1 | (7.7) | ||
| Tools/Utilities | 2 | (20.0) | 2 | (15.4) | ||
| Quality of app | ||||||
| MARS 2 score (average, SD 3) | 3.38 | (0.64) | 3.05 | (0.64) | <0.001 | *** |
| Engagement | 2.70 | (0.60) | 2.50 | (0.60) | 0.005 | ** |
| Functionality | 3.85 | (0.78) | 3.49 | (0.78) | 0.003 | ** |
| Aesthetics | 3.39 | (0.77) | 2.98 | (0.83) | <0.001 | *** |
| Information quality | 3.55 | (0.82) | 3.25 | (0.86) | 0.005 | ** |
| Adjusted Odds Ratio (aOR) for Higher User Ratings | Average Marginal Effects (AME) for Higher User Ratings | |||||
|---|---|---|---|---|---|---|
| Items of MARS | aOR | (95% CI 2) | p-Value | AME (%) | (95% CI 2) | p-Value |
| Navigation | 2.18 | (1.23–3.86) | 0.008 | 13.15 | (3.32–22.98) | 0.009 |
| Performance | 2.07 | (1.25–3.44) | 0.005 | 11.03 | (2.50–19.56) | 0.005 |
| Visual appeal | 1.83 | (1.11–3.04) | 0.018 | 10.87 | (1.57–20.18) | 0.022 |
| Credibility | 1.79 | (1.04–3.08) | 0.035 | 10.67 | (1.30–20.04) | 0.015 |
| Quantity of information | 1.77 | (1.25–2.52) | 0.001 | 10.42 | (4.41–16.43) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Liao, W.-W.; Su, M.-C.; Lin, Y.-H. Personalized and Self-Management: Systematic Search and Evaluation Quality Factors and User Preference of Drug Reference Apps in Taiwan. J. Pers. Med. 2021, 11, 790. https://doi.org/10.3390/jpm11080790
Chen Y-C, Liao W-W, Su M-C, Lin Y-H. Personalized and Self-Management: Systematic Search and Evaluation Quality Factors and User Preference of Drug Reference Apps in Taiwan. Journal of Personalized Medicine. 2021; 11(8):790. https://doi.org/10.3390/jpm11080790
Chicago/Turabian StyleChen, Yu-Chun, Wei-Wei Liao, Mei-Chin Su, and Yen-Hsi Lin. 2021. "Personalized and Self-Management: Systematic Search and Evaluation Quality Factors and User Preference of Drug Reference Apps in Taiwan" Journal of Personalized Medicine 11, no. 8: 790. https://doi.org/10.3390/jpm11080790
APA StyleChen, Y.-C., Liao, W.-W., Su, M.-C., & Lin, Y.-H. (2021). Personalized and Self-Management: Systematic Search and Evaluation Quality Factors and User Preference of Drug Reference Apps in Taiwan. Journal of Personalized Medicine, 11(8), 790. https://doi.org/10.3390/jpm11080790