Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Ethical Principals
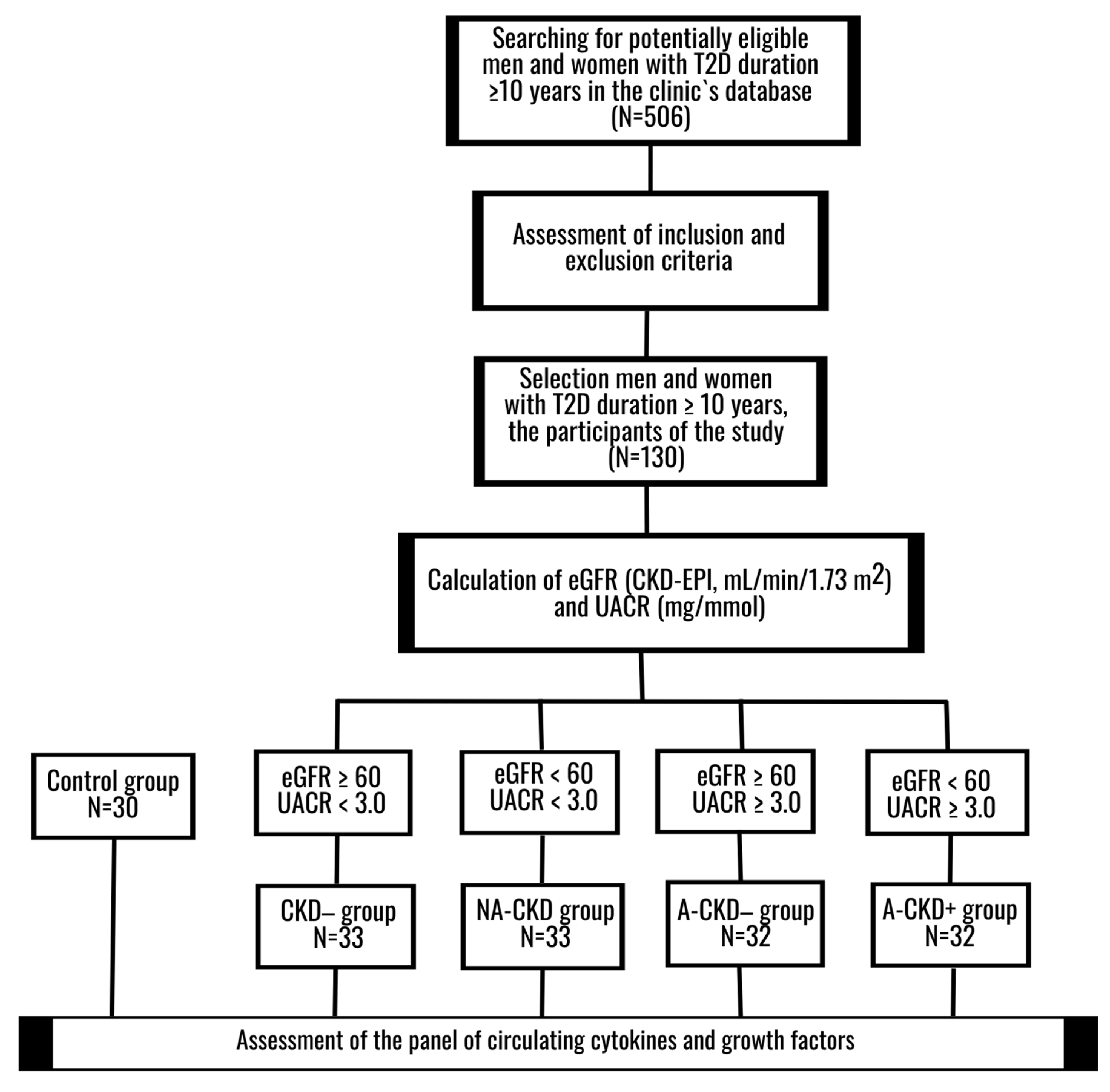
2.3. Participants
2.4. Methods
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of T2D Patients
3.2. IL-2, IL-4, IL-7, IL-9, IL-13, IL-15
3.3. IL-5, GM-CSF, G-CSF, IL-6, and IL-12
3.4. IL-10 and IFN-γ
3.5. IL-1β, IL-1Ra, TNF-α, and IL-17A
3.6. MCP-1, MIP-1α, MIP-1β, RANTES, Eotaxin, IP-10, and IL-8
3.7. bFGF, VEGF, and PGDF-BB
3.8. Correlation Analysis and Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameter | Discovery Cohort | Validation Cohort |
|---|---|---|
| N | 86 | 44 |
| Sex, M/F, n (%) | 29/57 (33.7%/66.3%) | 16/28 (36.4%/63.4%) |
| Age, years | 65 (58; 69) | 65.5 (58.5; 70) |
| Smokers, n (%) | 10 (11.6%) | 5 (11.4%) |
| BMI, kg/m2 | 33.4 (29.4; 37.4) | 33.2 (29.8; 38.9) |
| Diabetes duration, years | 13.5 (11; 17) | 14 (11; 18) |
| CKD−/NA-CKD/A-CKD−/A-CKD+, n | 22/22/21/21 | 11/11/11/11 |
Appendix B
| Family | Protein |
|---|---|
| Archetypical cytokines signaling through classical-cytokine receptors Type I helical Cytokine families signaling through Class I cytokine receptors (CRF1 family or Hematopoietin family) | |
| IL-2 Family, or Common Gamma Chain Receptor Family | IL-2, IL-4 subfamily(IL-4, IL-13), IL-9, IL-15, and IL-7 subfamily(IL-7) |
| Common Beta Chain Receptor Cytokine Family | IL-5, Colony Stimulating Factor 2/Granulocyte Monocyte-Stimulating Factor (CSF2/GM-CSF) |
| Prolactin family | Colony Stimulating Factor 3/Granulocyte-Stimulating Factor (CSF3/G-CSF) |
| IL-6 Family | IL-6 |
| IL-12 Family | IL-12 (p70) |
| Type II Cytokine families signaling through Class II cytokine receptors (CRF2 family or IL-10/IFN superfamily) | |
| IL-10 Family | IL-10 |
| Type II IFN | IFNγ |
| Cytokine families signaling through immunoglobulin (Ig) superfamily cytokine receptors non-Receptor tyrosine-kinase (RTK) | |
| IL-1 Family | IL-1β, IL-1Ra |
| Cytokine TNF family signaling through TNF receptor family | |
| Family A | TNFα (TNFSF2) |
| Chemokine superfamily signaling through chemokine receptors (seven-transmembrane heptahelical(serpentine) receptors associated with G-protein trimeric system) | |
| Chemokine CC Motif Ligand Family (CCL) | MCP1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5) and eotaxin(CCL11) |
| Chemokine CXC Motif Ligand Family (CXCL) | IP-10 (CXCL10), IL-8 (CXCL8) |
| Orphan and other cytokine family members | |
| IL-17 Family | IL-17A |
| Growth factors and signaling proteins | |
| Platelet-Derived Growth Factor Family | PDGF-BB (Platelet-Derived Growth Factor subunit B) |
| Vascular Endothelial Growth Factor Family | VEGF (Vascular Endothelial Growth Factor)/Vascular Permeability Factor (VPF) |
| Fibroblast Growth Factor Family | bFGF(FGF2/FGF-β) |
References
- Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2018. Available online: https://www.usrds.org/2018/view/Default.aspx (accessed on 16 July 2019).
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 27 April 2020).
- Viazzi, F.; Russo, G.T.; Ceriello, A.; Fioretto, P.; Giorda, C.; De Cosmo, S.; Pontremoli, R. Natural history and risk factors for diabetic kidney disease in patients with T2D: Lessons from the AMD-annals. J. Nephrol. 2019, 32, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Korbut, A.I.; Klimontov, V.V.; Vinogradov, I.V.; Romanov, V.V. Risk factors and urinary biomarkers of non-albuminuric and albuminuric chronic kidney disease in patients with type 2 diabetes. World J. Diabetes 2019, 10, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Nicolucci, A.; Penno, G.; RIACE Study Group. Chronic kidney disease in type 2 diabetes: Lessons from the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, A.; Bettini, S.; Russo, L.; Mazzocut, S.; Mauer, M.; Fioretto, P. Renal structure in type 2 diabetes: Facts and misconceptions [published online ahead of print, 2020 Jul 12]. J. Nephrol. 2020. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory cytokines in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 948417. [Google Scholar] [CrossRef]
- Pérez-Morales, R.E.; Del Pino, M.D.; Valdivielso, J.M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Inflammation in diabetic kidney disease. Nephron 2019, 143, 12–16. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Kelly, K.J.; Dominguez, J.H. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am. J. Nephrol. 2010, 32, 469–475. [Google Scholar] [CrossRef]
- Klessens, C.Q.F.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Rabelink, T.J.; Bajema, I.M.; IJpelaar, D.H.T. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2017, 32, 1322–1329. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhao, Y. Macrophage phenotype and its relationship with renal function in human diabetic nephropathy. PLoS ONE 2019, 14, e0221991. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, J.S.; Lu, K.C.; Chen, C.C.; Lin, S.H.; Chu, P.; Sytwu, H.K.; Lin, Y.F. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin. Chim. Acta 2010, 411, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.S.; Torquato, B.G.S.; da Silva, C.A.; Dos Reis Monteiro, M.L.G.; Dos Santos Martins, A.L.M.; da Silva, M.V.; Dos Reis, M.A.; Machado, J.R. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Salti, T.; Khazim, K.; Haddad, R.; Campisi-Pinto, S.; Bar-Sela, G.; Cohen, I. Glucose induces IL-1α-dependent inflammation and extracellular matrix proteins expression and deposition in renal tubular epithelial cells in diabetic kidney disease. Front. Immunol. 2020, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Stefan, G.; Stancu, S.; Zugravu, A.; Petre, N.; Mandache, E.; Mircescu, G. Histologic predictors of renal outcome in diabetic nephropathy: Beyond renal pathology society classification. Medicine 2019, 98, e16333. [Google Scholar] [CrossRef] [PubMed]
- Konenkov, V.I.; Klimontov, V.V.; Myakina, N.E.; Tyan, N.V.; Fazullina, O.N.; Romanov, V.V. Increased serum concentrations of inflammatory cytokines in type 2 diabetic patients with chronic kidney disease. Ther. Arch. 2015, 87, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.S.; da Silva, M.V.; da Silva, C.A.; Borges, M.F.; Palhares, H.M.D.C.; Rocha, L.P.; Corrêa, R.R.M.; Rodrigues Júnior, V.; Dos Reis, M.A.; Machado, J.R. Analysis of serum inflammatory mediators in type 2 diabetic patients and their influence on renal function. PLoS ONE 2020, 15, e0229765. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Sierra-Mondragon, E.; Molina-Jijon, E.; Namorado-Tonix, C.; Rodríguez-Muñoz, R.; Pedraza-Chaverri, J.; Reyes, J.L. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J. Nutr. Biochem. 2018, 60, 47–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Di, Y.P. Fast and efficient measurement of clinical and biological samples using immunoassay-based multiplexing systems. Methods Mol. Biol. 2020, 2102, 129–147. [Google Scholar] [CrossRef]
- Lioudaki, E.; Stylianou, K.G.; Petrakis, I.; Kokologiannakis, G.; Passam, A.; Mikhailidis, D.P.; Daphnis, E.K.; Ganotakis, E.S. Increased urinary excretion of podocyte markers in normoalbuminuric patients with diabetes. Nephron 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Abe, M.; Moritani, H.; Mitori, H.; Kondo, M.; Tanaka-Amino, K.; Eguchi, M.; Imasato, A.; Inoki, Y.; Kajiyama, H.; et al. Potential of urinary nephrin as a biomarker reflecting podocyte dysfunction in various kidney disease models. Exp. Biol. Med. 2016, 241, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.A.A.; Sallam, A.M.; El-Hefnawy, M.H.; El-Mesallamy, H.O. Epidermal growth factor receptor and podocin predict nephropathy progression in type 2 diabetic patients through interaction with the autophagy influencer ULK-1. J. Diabetes Complicat. 2019, 33, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, Y.; Cai, G.; Liang, J.; Yue, C.; Wang, F.; Song, J.; Wang, J.; Liu, M.; Luo, J.; et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget 2016, 7, 67748–67759. [Google Scholar] [CrossRef]
- Chen, P.; Yang, Q.; Li, X.; Qin, Y. Potential association between elevated serum human epididymis protein 4 and renal fibrosis: A systemic review and meta-analysis. Medicine 2017, 96, e7824. [Google Scholar] [CrossRef]
- García Morán, G.A.; Parra-Medina, R.; Cardona, A.G.; Cardona, A.G.; Quintero-Ronderos, P.; Rodríguez, É.G. Cytokines, chemokines and growth factors. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Eds.; El Rosario University Press: Bogota, Colombia, 2013; ISBN 9789587383768. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459450/ (accessed on 24 April 2020).
- Bafico, A.; Aaronson, S.A. Classification of growth factors and their receptors. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Eds.; BC Decker: Hamilton, ON, Canada, 2003; ISBN 101550092138. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12423/ (accessed on 24 April 2020).
- Sherbet, G.V.; Gajanan, V. Growth Factors and Their Receptors in Cell Differentiation, Cancer and Cancer Therapy; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2011; 347p. [Google Scholar]
- Pfister, I.B.; Zandi, S.; Gerhardt, C.; Spindler, J.; Reichen, N.; Garweg, J.G. Risks and Challenges in Interpreting Simultaneous Analyses of Multiple Cytokines. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- EUTOX Uremic Toxin Database. Available online: https://www.uremic-toxins.org/eutox-database/ (accessed on 27 April 2020).
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.B.; Ramos, A.M.; Sanchez-Niño, M.D.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni EstanTodos Los Que Son”. Toxins 2017, 9, 114. [Google Scholar] [CrossRef]
- Norlander, A.E.; Madhur, M.S. Inflammatory cytokines regulate renal sodium transporters: How, where, and why? Am. J. Physiol.-Ren. Physiol. 2017, 313, F141–F144. [Google Scholar] [CrossRef]
- Feigerlová, E.; Battaglia-Hsu, S.F. IL-6 signaling in diabetic nephropathy: From pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev. 2017, 37, 57–65. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Magno, A.L.; Heart, L.Y.; Carnagarin, R.; Schlaich, M.P.; Matthews, V.B. Current knowledge of IL-6 cytokine family members in acute and chronic kidney disease. Biomedicines 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jayakumar, C.; Chen, F.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J. Am. Soc. Nephrol. 2016, 27, 745–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, X.; Yin, J.; Wu, H.; Cai, X.; Wang, N.; Qian, Y.; Wang, F. IL-6 receptor blockade ameliorates diabetic nephropathy via inhibiting inflammasome in mice. Metabolism 2018, 83, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sangoi, M.B.; Carvalho, J.A.M.; Guarda, N.S.; Duarte, T.; Duarte, M.M.M.F.; Premaor, M.O.; Comim, F.V.; Moretto, M.B.; Moresco, R.N. Association between urinary levels of interleukin-6, interleukin-10 and tumor necrosis factor-alpha with glomerular and tubular damage indicators in patients with type 2 diabetes. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Cortvrindt, C.; Speeckaert, R.; Moerman, A.; Delanghe, J.R.; Speeckaert, M.M. The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology 2017, 49, 247–258. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.J.; Chen, X.; Kwan, T.; Chadban, S.J.; Wu, H. Interleukin 17A promotes diabetic kidney injury. Sci. Rep. 2019, 9, 2264. [Google Scholar] [CrossRef]
- Perlman, A.S.; Chevalier, J.M.; Wilkinson, P.; Liu, H.; Parker, T.; Levine, D.M.; Sloan, B.J.; Gong, A.; Sherman, R.; Farrell, F.X. Serum inflammatory and immune mediators are elevated in early stage diabetic nephropathy. Ann. Clin. Lab. Sci. 2015, 45, 256–263. [Google Scholar]
- Coto, E.; Gómez, J.; Suárez, B.; Tranche, S.; Díaz-Corte, C.; Ortiz, A.; Ruiz-Ortega, M.; Coto-Segura, P.; Batalla, A.; López-Larrea, C. Association between the IL17RA rs4819554 polymorphism and reduced renal filtration rate in the Spanish RENASTUR cohort. Hum. Immunol. 2015, 76, 75–78. [Google Scholar] [CrossRef]
- Kuo, H.L.; Huang, C.C.; Lin, T.Y.; Lin, C.Y. IL-17 and CD40 ligand synergistically stimulate the chronicity of diabetic nephropathy. Nephrol. Dial. Transplant. 2018, 33, 248–256. [Google Scholar] [CrossRef]
- Norlander, A.E.; Saleh, M.A.; Kamat, N.V.; Ko, B.; Gnecco, J.; Zhu, L.; Dale, B.L.; Iwakura, Y.; Hoover, R.S.; McDonough, A.A.; et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 2016, 68, 167–174. [Google Scholar] [CrossRef]
- Nishida, M.; Hamaoka, K. How does G-CSF act on the kidney during acute tubular injury? Nephron Exp. Nephrol. 2006, 104, e123–e128. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Ryu, J.H.; Piao, H.; Hwang, J.H.; Han, D.; Lee, S.K.; Jang, J.Y.; Lee, J.; Koo, T.Y.; Yang, J. Granulocyte colony-stimulating factor attenuates renal ischemia-reperfusion injury by inducing myeloid-derived suppressor cells. J. Am. Soc. Nephrol. 2020, 31, 731–746. [Google Scholar] [CrossRef] [PubMed]
- So, B.I.; Song, Y.S.; Fang, C.H.; Park, J.Y.; Lee, Y.; Shin, J.H.; Kim, H.; Kim, K.S. G-CSF prevents progression of diabetic nephropathy in rat. PLoS ONE 2013, 8, e77048. [Google Scholar] [CrossRef] [PubMed]
- Erbas, O.; Yapislar, H.; Oltulu, F.; Yavasoğlu, A.; Aktug, H.; Taskiran, D. Nephro-protective effect of granulocyte colony-stimulating factor in streptozotocin induced diabetic rats. Biotech. Histochem. 2014, 89, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ruster, C.; Wolf, G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front. Biosci. 2008, 13, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage inflammatory protein-1 alpha (MIP-1 alpha)/CCL3: As a biomarker. In General Methods in Biomarker Research and Their Applications; Preedy, V., Patel, V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 243–249. Print ISBN 978-94-007-7695-1, Online ISBN 978-94-007-7696-8. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, Y.; Mahajan, D.; Qin, X.; Wang, Y.; Wang, Y.; Alexander, S.I.; Harris, D.C. The role of tubulointerstitial inflammation. Kidney Int. 2005, 67 (Suppl. S94), S96–S100. [Google Scholar] [CrossRef][Green Version]
- Correa-Costa, M.; Braga, T.T.; Felizardo, R.J.; Andrade-Oliveira, V.; Perez, K.R.; Cuccovia, I.M.; Hiyane, M.I.; da Silva, J.S.; Câmara, N.O. Macrophage trafficking as key mediator of adenine-induced kidney injury. Mediat. Inflamm. 2014, 2014, 291024. [Google Scholar] [CrossRef]
- Strutz, F. The role of FGF-2 in renal fibrogenesis. Front. Biosci. 2009, 1, 125–131. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, C. Ablation of FGFR2 in fibroblasts ameliorates kidney fibrosis after ischemia/reperfusion injury in mice. Kidney Dis. 2017, 3, 160–170. [Google Scholar] [CrossRef]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-stage renal disease, inflammation and cardiovascular outcomes. In Expanded Hemodialysis—Innovative Clinical Approach in Dialysis. Contrib Nephrol.; Karger Publishers: Basel, Switzerland, 2017; Volume 191, pp. 32–43. [Google Scholar] [CrossRef]
- Wei, T.; Shu, Q.; Ning, J.; Wang, S.; Li, C.; Zhao, L.; Zheng, H.; Gao, H. The protective effect of basic fibroblast growth factor on diabetic nephropathy through remodeling metabolic phenotype and suppressing oxidative stress in mice. Front. Pharmacol. 2020, 11, 66. [Google Scholar] [CrossRef]
- Sheng, W.S.; Xu, H.L.; Zheng, L.; Zhuang, Y.D.; Jiao, L.Z.; Zhou, J.F.; ZhuGe, D.L.; Chi, T.T.; Zhao, Y.Z.; Lan, L. Intrarenal delivery of bFGF-loaded liposome under guiding of ultrasound-targeted microbubble destruction prevent diabetic nephropathy through inhibition of inflammation. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 373–385. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C.; Quimby, K.R.; Greenidge, A.R. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr. Pharm. Des. 2018, 24, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, R.; Xie, J.; Xiong, H.; He, J.C.; Chen, N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab. Investig. 2013, 93, 801–811. [Google Scholar] [CrossRef]
- Soranno, D.E.; Lu, H.D.; Weber, H.M.; Rai, R.; Burdick, J.A. Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J. Biomed. Mater. Res. Part A 2014, 102, 2173–2180. [Google Scholar] [CrossRef]
- Rodell, C.B.; Rai, R.; Faubel, S.; Burdick, J.A.; Soranno, D.E. Local immunotherapy via delivery of interleukin-10 and transforming growth factor β antagonist for treatment of chronic kidney disease. J. Control Release 2015, 206, 131–139. [Google Scholar] [CrossRef]
- Gnudi, L.; Benedetti, S.; Woolf, A.S.; Long, D.A. Vascular growth factors play critical roles in kidney glomeruli. Clin. Sci. 2015, 129, 1225–1236. [Google Scholar] [CrossRef]
- Lin, S.; Teng, J.; Li, J.; Sun, F.; Yuan, D.; Chang, J. Association of chemerin and vascular endothelial growth factor (VEGF) with diabetic nephropathy. Med. Sci. Monit. 2016, 22, 3209–3214. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. The renal endothelium in diabetic nephropathy. Ren. Fail. 2013, 35, 592–599. [Google Scholar] [CrossRef]
- Majumder, S.; Advani, A. VEGF and the diabetic kidney: More than too much of a good thing. J. Diabetes Complicat. 2017, 31, 273–279. [Google Scholar] [CrossRef]
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004, 65, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J. Eotaxin-1 (CCL11). Front. Immunol. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Rothenberg, M.E. The regulatory function of eosinophils. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Gauckler, P.; Shin, J.I.; Mayer, G.; Kronbichler, A. Eosinophilia and kidney disease: More than just an incidental finding? J. Clin. Med. 2018, 7, 529. [Google Scholar] [CrossRef]
- Laurentius, T.; Raffetseder, U.; Fellner, C.; Kob, R.; Nourbakhsh, M.; Floege, J.; Bertsch, T.; Bollheimer, L.C.; Ostendorf, T. High-fat diet-induced obesity causes an inflammatory microenvironment in the kidneys of aging Long-Evans rats. J. Inflamm. 2019, 16, 14. [Google Scholar] [CrossRef]
- Mansouri, L.; Paulsson, J.M.; Moshfegh, A.; Jacobson, S.H.; Lundahl, J. Leukocyte proliferation and immune modulator production in patients with chronic kidney disease. PLoS ONE 2013, 8, e73141. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Tyan, N.V.; Fazullina, O.N.; Myakina, N.E.; Lykov, A.P.; Konenkov, V.I. Clinical and metabolic factors associated with chronic low-grade inflammation in type 2 diabetic patients. Diabetes Mellit. 2016, 19, 295–302. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Korbut, A.I. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 474–479. [Google Scholar] [CrossRef]
- Fioretto, P.; Caramori, M.L.; Mauer, M. The kidney in diabetes: Dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia 2008, 51, 1347–1355. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Robles-Osorio, M.L.; Sabath, E. Tubular dysfunction and non-albuminuric renal disease in subjects with type 2 diabetes mellitus. Rev. Investig. Clin. 2014, 66, 234–239. [Google Scholar]
- Kosmas, C.E.; Silverio, D.; Tsomidou, C.; Salcedo, M.D.; Montan, P.D.; Guzman, E. The impact of insulin resistance and chronic kidney disease on inflammation and cardiovascular disease. Clin. Med. Insights Endocrinol. Diabetes 2018, 11, 1179551418792257. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, J.B.; Li, S.; Ton, T.G.N.; Peng, Y.; Hansen, M.K.; Neslusan, C.; Riley, R.; Liu, J.; Gilbertson, D.T. Association of diabetes-related kidney disease with cardiovascular and non-cardiovascular outcomes: A retrospective cohort study. BMC Endocr. Disord. 2019, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z.I. The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr. Rev. 2020, 41, 202–231. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, S.; Wang, M.; Lu, W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-κB signaling pathway. Mol. Med. Rep. 2018, 18, 3625–3630. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Butler, A.E.; Atkin, S.L.; Katsiki, N.; Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: Possible molecular pathways. J. Cell. Physiol. 2018, 234, 223–230. [Google Scholar] [CrossRef]
- Yin, W.; Xu, S.; Wang, Z.; Liu, H.; Peng, L.; Fang, Q.; Deng, T.; Zhang, W.; Lou, J. Recombinant human GLP-1 (rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats. Biochem. Biophys. Res. Commun. 2018, 495, 793–800. [Google Scholar] [CrossRef]
- Chang, J.T.; Liang, Y.J.; Hsu, C.Y.; Chen, C.Y.; Chen, P.J.; Yang, Y.F.; Chen, Y.L.; Pei, D.; Chang, J.B.; Leu, J.G. Glucagon-like peptide receptor agonists attenuate advanced glycation end products-induced inflammation in rat mesangial cells. BMC Pharmacol. Toxicol. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020, 241, 117152. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]






| Parameter | CKD− | NA-CKD | A-CKD− | A-CKD+ |
|---|---|---|---|---|
| N | 33 | 33 | 32 | 32 |
| Sex, M/F, n | 12/21 | 12/21 | 12/20 | 12/20 |
| Age, years | 64 (58; 69) | 65 (58; 67) | 63 (57; 67) | 65 (58; 69.5) |
| Smokers, n (%) | 3 (9.1%) | 5 (15.1%) | 4 (12.5%) | 3 (9.4%) |
| BMI, kg/m2 | 34.4 (28.1; 36.9) | 34.7 (30.1; 38.9) | 33.8 (31.2; 40.0) | 32.9 (30.3; 36.7) |
| WHR | 0.97 (0.93; 1.1) | 1.02 (0.9; 1.1) | 1.00 (0.93; 1.1) | 1.00 (0.91; 1.07) |
| Diabetes duration, years | 13 (11; 16) | 14 (12; 19) | 13.5 (11; 17) | 13.5 (11; 17.5) |
| Diabetic complications and associated diseases | ||||
| Obesity, n (%) | 21 (63.6%) | 24 (72.7%) | 25 (78.2%) | 26 (81.3%) |
| Diabetic retinopathy, n (%) | 17 (51.5%) | 17 (51.5%) | 20 (62.5%) | 21 (65.6%) |
| Arterial hypertension, n (%) | 31 (93.9%) | 33 (100%) | 31 (96.9%) | 32 (100%) |
| Coronary artery disease, n (%) | 13 (39.4%) | 16 (48.5%) | 16 (50.0%) | 18 (56.2%) |
| Myocardial infarction in anamnesis, n (%) | 4 (12.1) | 7 (21.2%) | 5 (15.6%) | 10 (31.3%) |
| Cerebrovascular event in anamnesis, n (%) | 1 (3.0%) | 6 (18.2%) | 3 (9.4%) | 2 (6.3%) |
| Peripheral artery disease, n (%) | 17 (51.5%) | 22 (66.7%) | 20 (62.5%) | 23 (71.9%) |
| Treatment | ||||
| Metformin, n (%) | 26 (78.8%) | 20 (60.6%) | 23 (71.9%) | 20 (62.5%) |
| Sulfonylurea, n (%) | 8 (24.2%) | 15 (45.5%) | 12 (37.5%) | 9 (28.1%) |
| Insulin, n (%) | 21 (63.6%) | 22 (66.7%) | 21 (65.6%) | 26 (81.3%) |
| RAS blockers, n (%) | 33 (100%) | 27 (81.8%) | 26 (81.3%) | 27 (84.4%) |
| Diuretics, n (%) | 12 (36.4%) | 17 (51.5%) | 16 (50.0%) | 17 (53.1%) |
| Calcium channel blockers, n (%) | 8 (24.2%) | 16 (48.5%) | 10 (31.3%) | 14 (43.8%) |
| Antiplatelet agents, n (%) | 21 (63.6%) | 27 (81.8%) | 19 (59.4%) | 24 (75.0%) |
| Statins, n (%) | 13 (39.4%) | 23 (69.7%) | 7 (21.9%) ### | 17 (53.1%) |
| Parameter | CKD− | NA-CKD | A-CKD− | A-CKD+ | |
|---|---|---|---|---|---|
| N | 33 | 33 | 32 | 32 | |
| Renal tests | |||||
| Serum creatinine, μmol/L | 76 (68; 87) | 111 (102; 124) *** | 87 (79; 97) §§§ ### | 117 (98; 144) *** | |
| eGFR, mL/min/1.73 m2 | 84 (73; 94) | 51 (45; 55) *** | 71 (65; 77) §§§ ### | 51 (43; 55) *** | |
| UACR, mg/mmol | 0.6 (0.3; 0.9) | 0.6 (0.3; 0.9) | 13.5 (6.4; 38.4) *** ### | 14.1 (7.2; 82.5) *** ### | |
| Urinary nephrin excretion, ng/mmol | 12.1 (4.7; 21.1) | 7.8 (4.1; 16.3) | 22.6 (16.6; 36.2) *** ### | 22.2 (17.1; 32.9) *** ### | |
| Urinary podocin excretion, ng/mmol | 133 (86; 222) | 105 (65.4; 193.9) | 250 (144; 421) *** ### | 268 (134; 411) *** ### | |
| Urinary excretion of WFDC2, ng/mmol | M | 442 (310; 941) +++ | 723 (406; 811) +++ | N/D | 628 (410; 1277) +++ |
| F | 5.6 (0; 81) | 129 (0; 330) ** | N/D | 231 (110; 619) ** | |
| Other biochemical parameters | |||||
| HbA1C | % | 8.48 (7.5; 9.8) | 9.06 (7.9; 10.26) | 9.36 (8.25; 11.58) | 8.81 (7.64; 10.1) |
| mmol/mol | 69 (58; 84) | 76 (63; 89) | 79 (67; 103) | 73 (60; 87) | |
| Total cholesterol, mmol/L | 4.92 (4.32; 5.51) | 5.14 (4.11; 6.07) | 4.89 (4.06; 5.89) | 5.5 (4.19; 6.25) | |
| LDL-cholesterol, mmol/L | 3.02 (2.7; 3.59) | 3.21 (2.51; 3.92) | 3.19 (2.6; 3.95) | 3.26 (2.54; 4.18) | |
| HDL-cholesterol, mmol/L | 1.13 (1; 1.31) | 1.22 (1.09; 1.41) | 1.07 (0.93; 1.25) # | 1.12 (1; 1.41) | |
| Triglycerides, mmol/L | 1.76 (1.15; 2.47) | 1.9 (1.5; 2.6) | 2.2 (1.64; 3.3) | 1.93 (1.54; 3.08) | |
| Uric acid, μmol/L | 298 (243; 352) | 341 (315; 392) | 322 (281; 383) | 366 (272; 418) | |
| Serum hs-CRP, mg/L | 4.1 (2.3; 8.2) | 6.2 (2.3; 8.4) | 4.4 (1.4; 7.3) | 6.7 (2.2; 13.1) | |
| Hematology and coagulation tests | |||||
| Hemoglobin, g/L | 139 (131; 148) | 141 (128; 151) | 141 (127; 152) | 141 (126; 153) | |
| RBC, × 1012/L | 4.79 (4.5; 4.9) | 4.72 (4.44; 5.06) | 4.85 (4.6; 5.13) | 4.7 (4.26; 4.87) | |
| WBC, × 109/L | 6.18 (5.93; 7.65) | 7.44 (5.61; 8.34) | 7.52 (6.03; 8.85) | 7.31 (5.53; 8.15) | |
| Platelets, × 109/L | 244 (218; 269) | 253 (207; 282) | 247 (212; 278) | 206 (181; 275) | |
| ESR, mm/h | 15 (9; 22) | 19 (12; 26.5) | 22.5 (15.5; 29) | 23.5 (18; 30) ** | |
| Fibrinogen, g/L | 4.15 (3.3; 5.3) | 3.9 (3.2; 4.45) | 4.4 (3.7; 5.5) | 4.6 (4; 5.3) | |
| SFMCs, mg/dL | 4 (3.5; 13.5) | 14 (3.5; 21) | 10.5 (6; 21.5) | 12 (8; 19) | |
| D-dimer, ng/mL | 255 (215; 309) | 251 (231; 385) | 268 (231; 297) | 277 (238; 327) | |
| Parameter | Crude OR (95% CI), p-Value | Adjusted OR (95% CI), p-Value |
|---|---|---|
| eGFR <60 mL/min × 1.73 m21 | ||
| IL-17A, pg/mL | 1.04(1.01–1.09), p = 0.004 | 1.03(1.01–1.09), p = 0.01 |
| MIP-1α, pg/mL | 1.30(1.06–1.49), p = 0.02 | 1.15(1.02–1.50), p = 0.03 |
| Serum hs-CRP, mg/L | 1.01(0.96–1.05), p = 0.10 | 1.20(1.02–1.30), p = 0.02 |
| Age, years | 1.06 (0.98–1.15), p = 0.16 | 1.04(0.99–1.14), p = 0.08 |
| UACR ≥3.0 mg/mmol2 | ||
| Eotaxin, 10 pg/mL | 0.98(0.94–1.00), p = 0.09 | 0.95(0.90–1.00), p = 0.03 |
| IL-15, 10 pg/mL | 1.03(0.98–1.06), p = 0.20 | 1.04(0.98–1.07), p = 0.09 |
| Parameter | Crude OR (95% CI), p-Value | Adjusted OR (95% CI), p-Value |
|---|---|---|
| NA-CKD1 | ||
| IL-17A, pg/mL | 1.08 (1.04–1.18), p = 0.001 | 1.06 (1.02–1.12), p = 0.004 |
| MIP-1α, pg/mL | 1.70 (1.20–2.30), p = 0.008 | 1.45 (1.02–2.06), p = 0.03 |
| A-CKD−2 | ||
| IL-13, pg/mL | 1.20 (0.96–1.50), p = 0.09 | 1.24 (1.01–1.54), p = 0.04 |
| HbA1C, % | 1.15 (0.94–1.52), p = 0.12 | 1.30 (0.98–1.62), p = 0.06 |
| A-CKD+3 | ||
| IL-6, pg/mL | 1.27 (1.02–1.64), p = 0.02 | 1.37 (1.08–1.69), p = 0.009 |
| Serum hs-CRP, mg/L | 1.06 (0.92–1.32), p = 0.21 | 1.18 (1.01–1.36), p = 0.04 |
| Age, years | 1.03 (0.90; 1.12), p = 0.26 | 1.09 (0.98–1.20), p = 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimontov, V.V.; Korbut, A.I.; Orlov, N.B.; Dashkin, M.V.; Konenkov, V.I. Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 3006. https://doi.org/10.3390/jcm9093006
Klimontov VV, Korbut AI, Orlov NB, Dashkin MV, Konenkov VI. Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease. Journal of Clinical Medicine. 2020; 9(9):3006. https://doi.org/10.3390/jcm9093006
Chicago/Turabian StyleKlimontov, Vadim V., Anton I. Korbut, Nikolai B. Orlov, Maksim V. Dashkin, and Vladimir I. Konenkov. 2020. "Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease" Journal of Clinical Medicine 9, no. 9: 3006. https://doi.org/10.3390/jcm9093006
APA StyleKlimontov, V. V., Korbut, A. I., Orlov, N. B., Dashkin, M. V., & Konenkov, V. I. (2020). Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease. Journal of Clinical Medicine, 9(9), 3006. https://doi.org/10.3390/jcm9093006






