A Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing
Abstract
:1. Introduction
2. Results
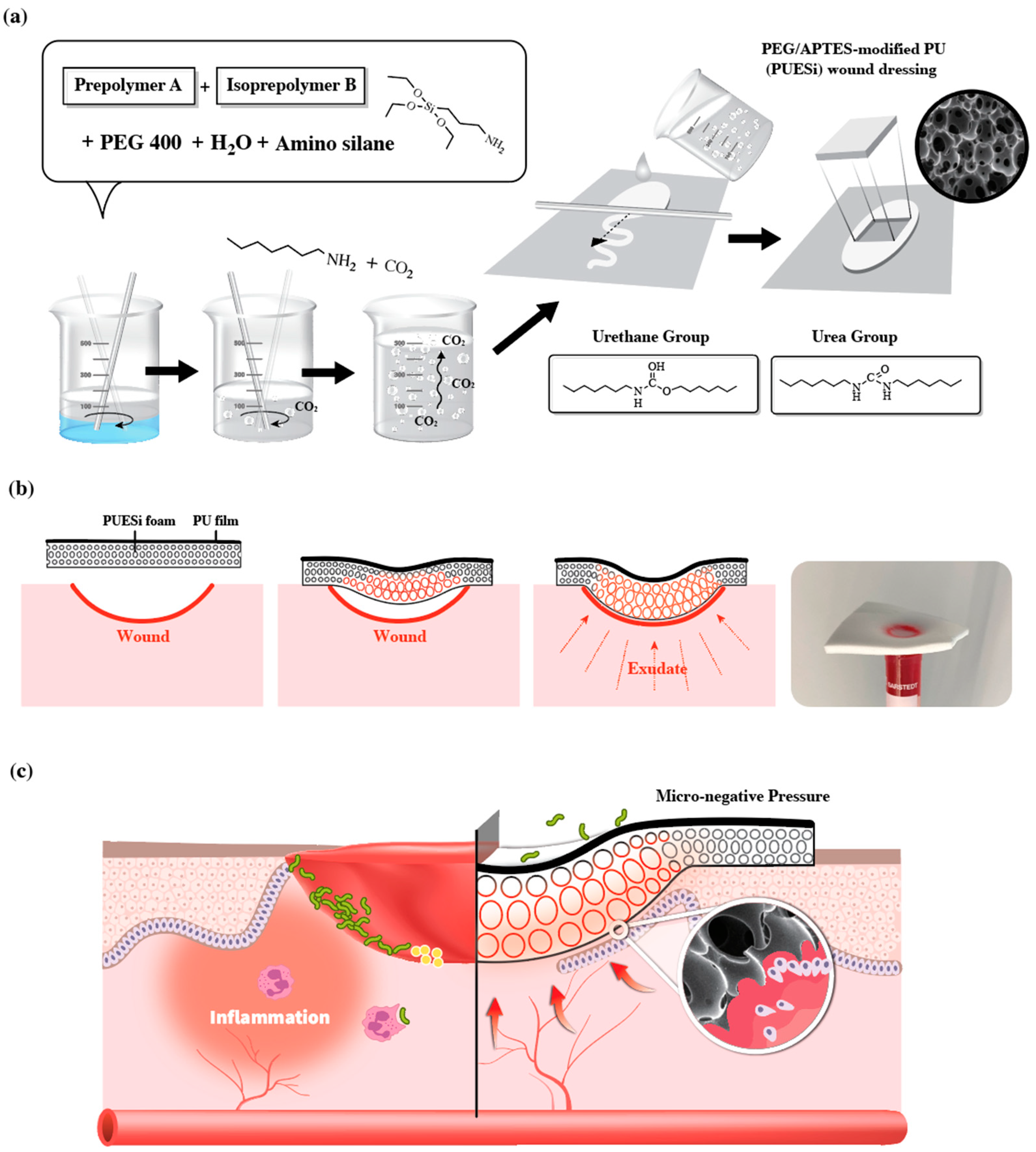
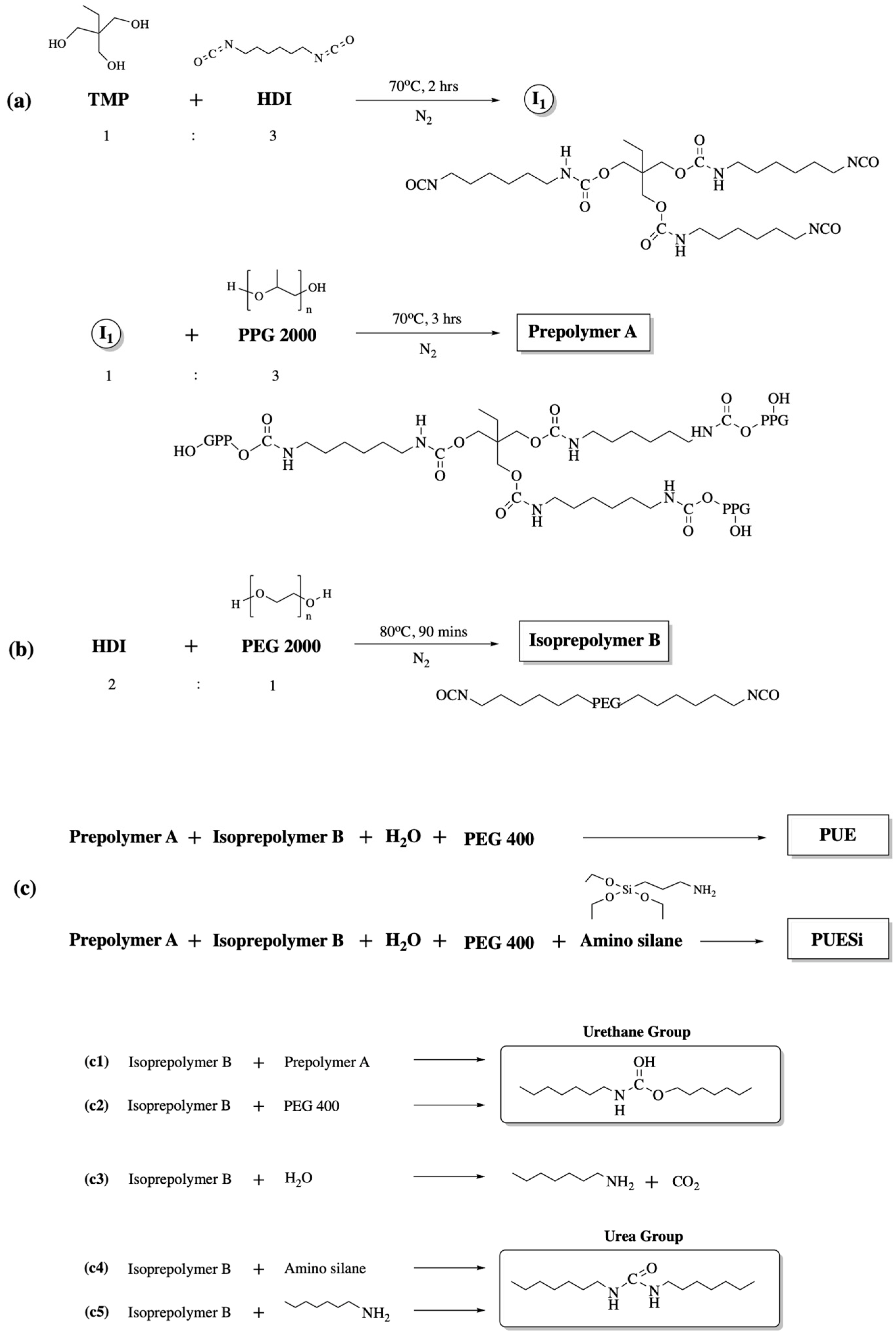
2.1. Synthesis and Preparation of PU Foams
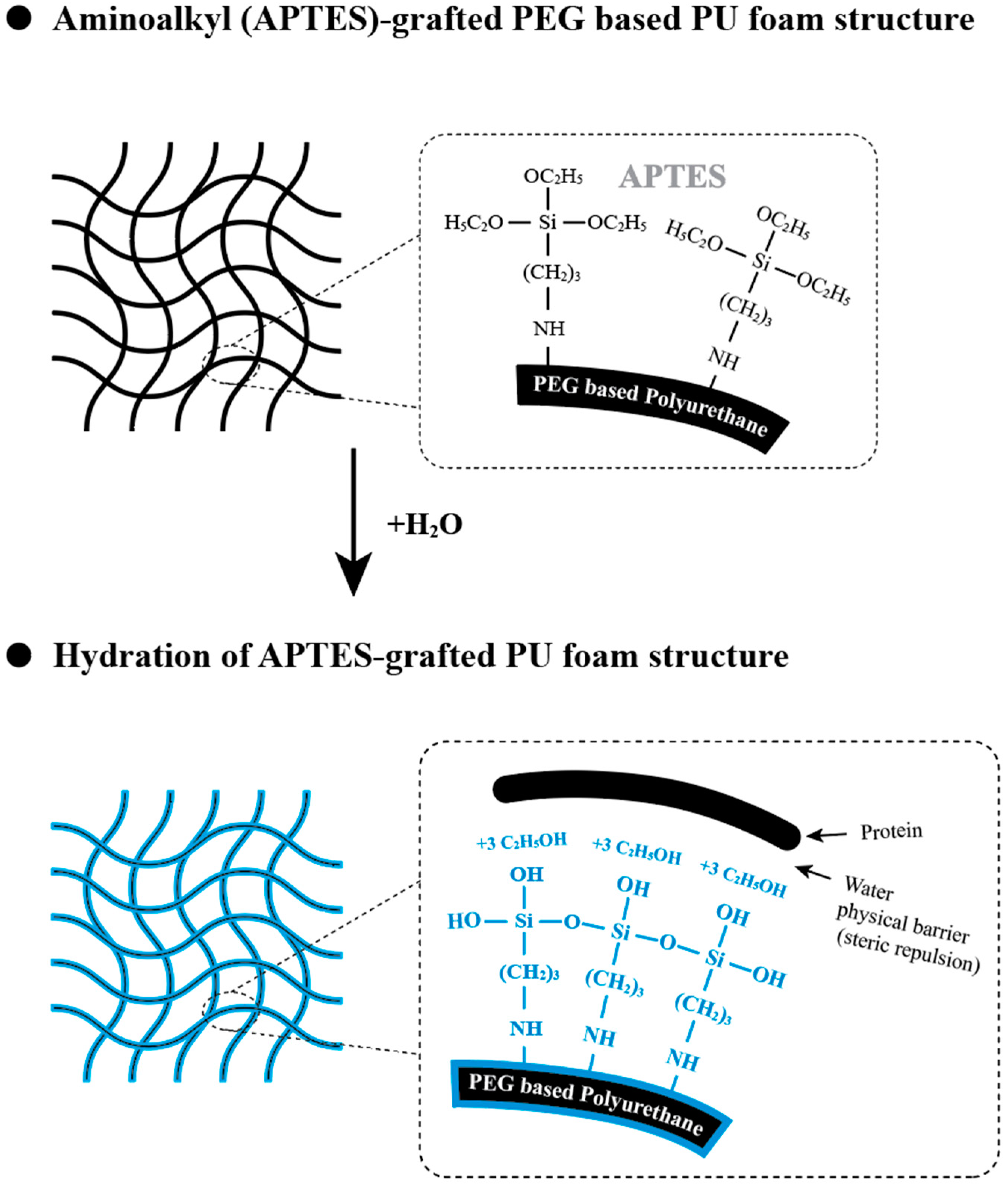
2.2. Chemical Synthesis Characterization and Morphological Observations
2.3. Mechanical Properties
2.4. Cytocompatibility
2.5. Water Uptake Ability
2.6. Moisture Vapor Transmission Rate (MVTR)
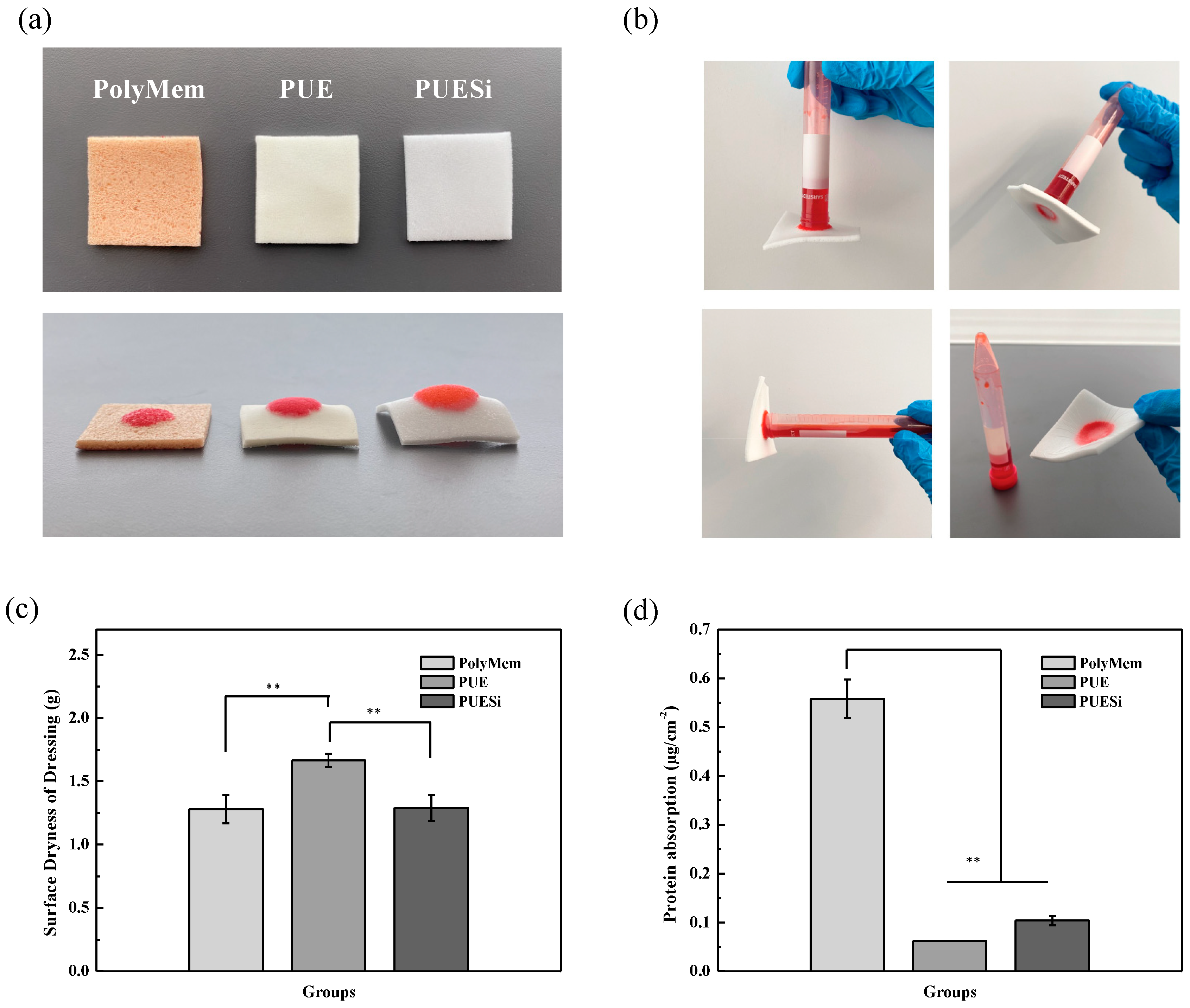
2.7. Swelling Characteristics and Micronegative-Pressure and Antiadhesion Properties
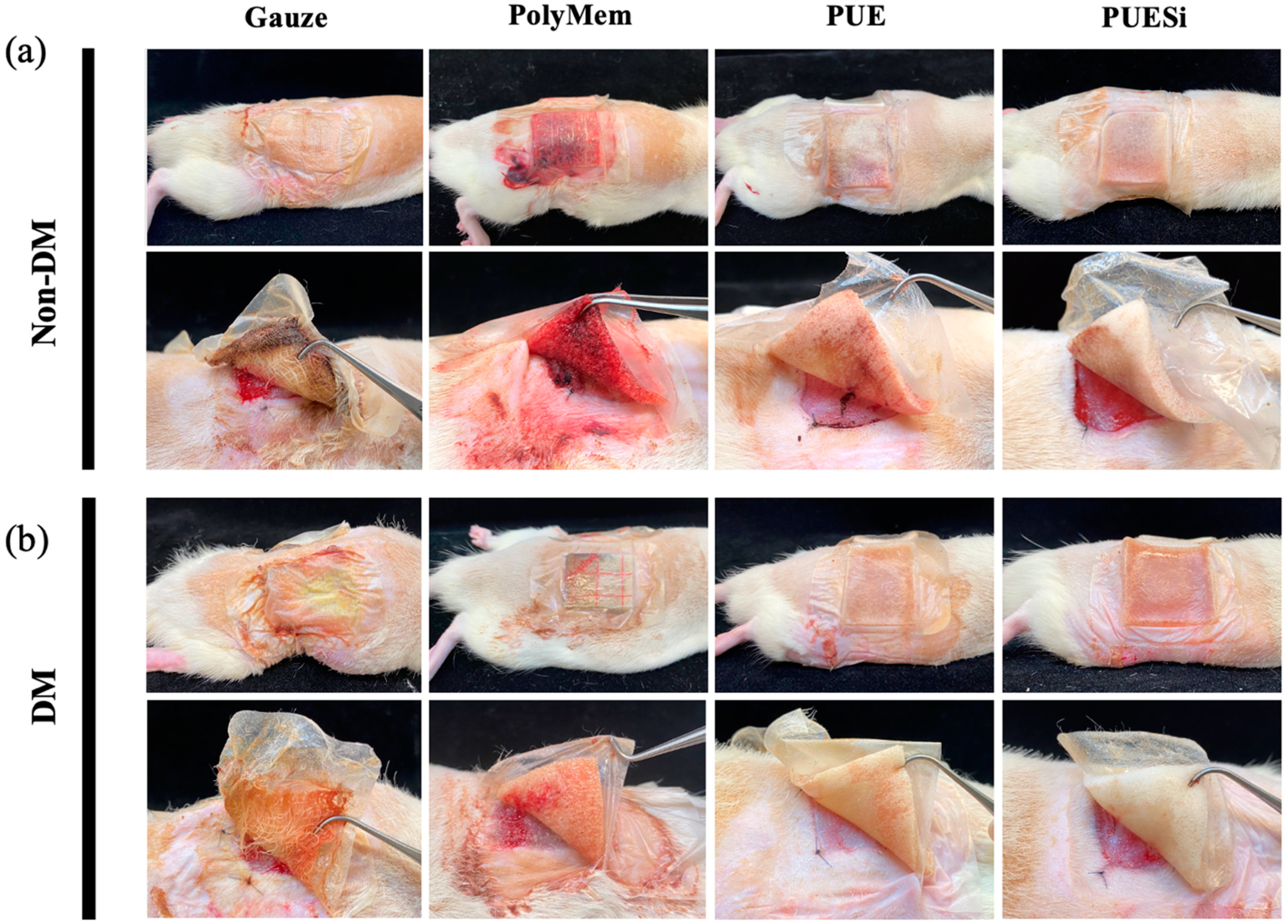
2.8. In Vivo Absorption and Anti-adhesion Tests
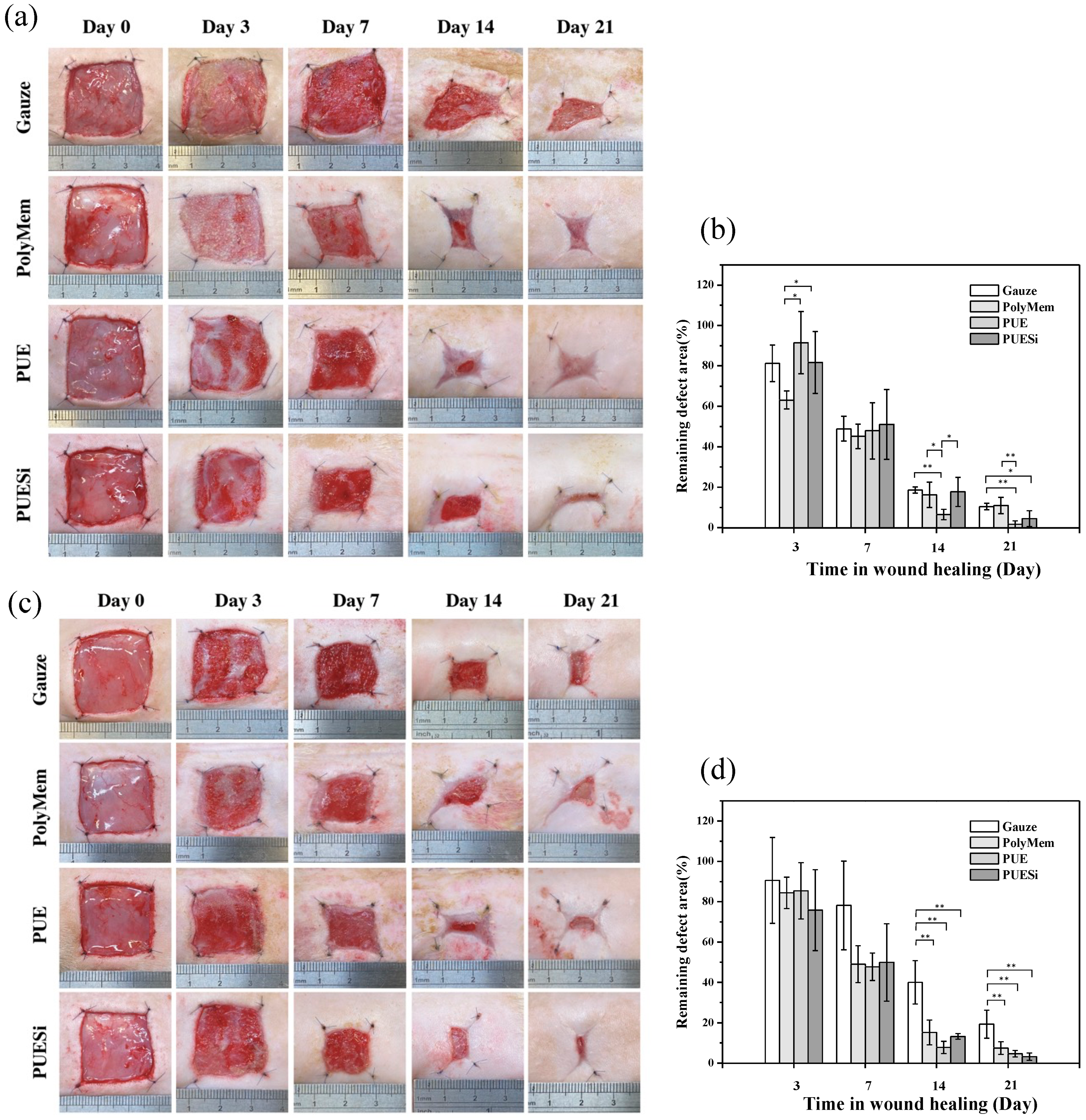
2.9. Wound Healing Evaluation
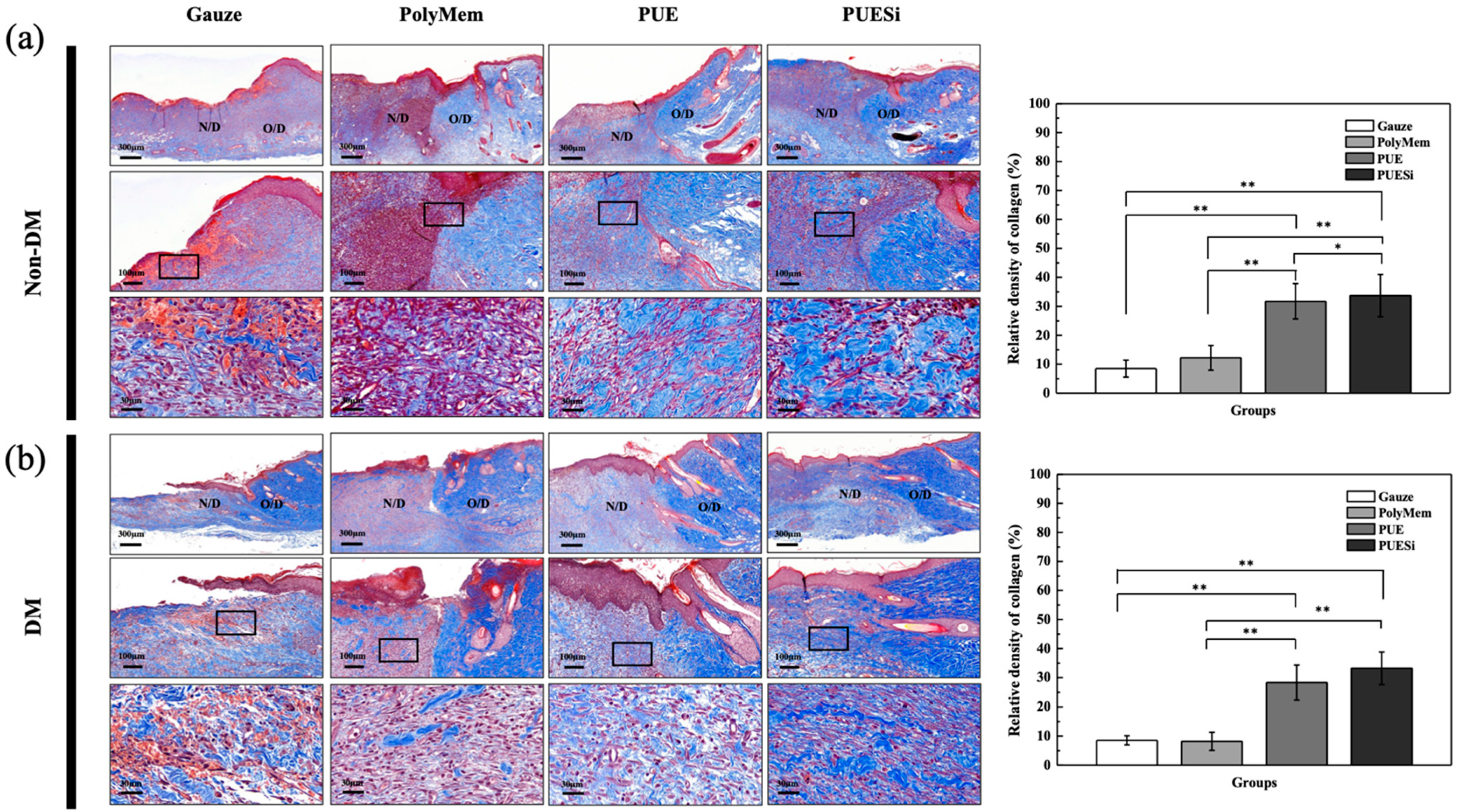
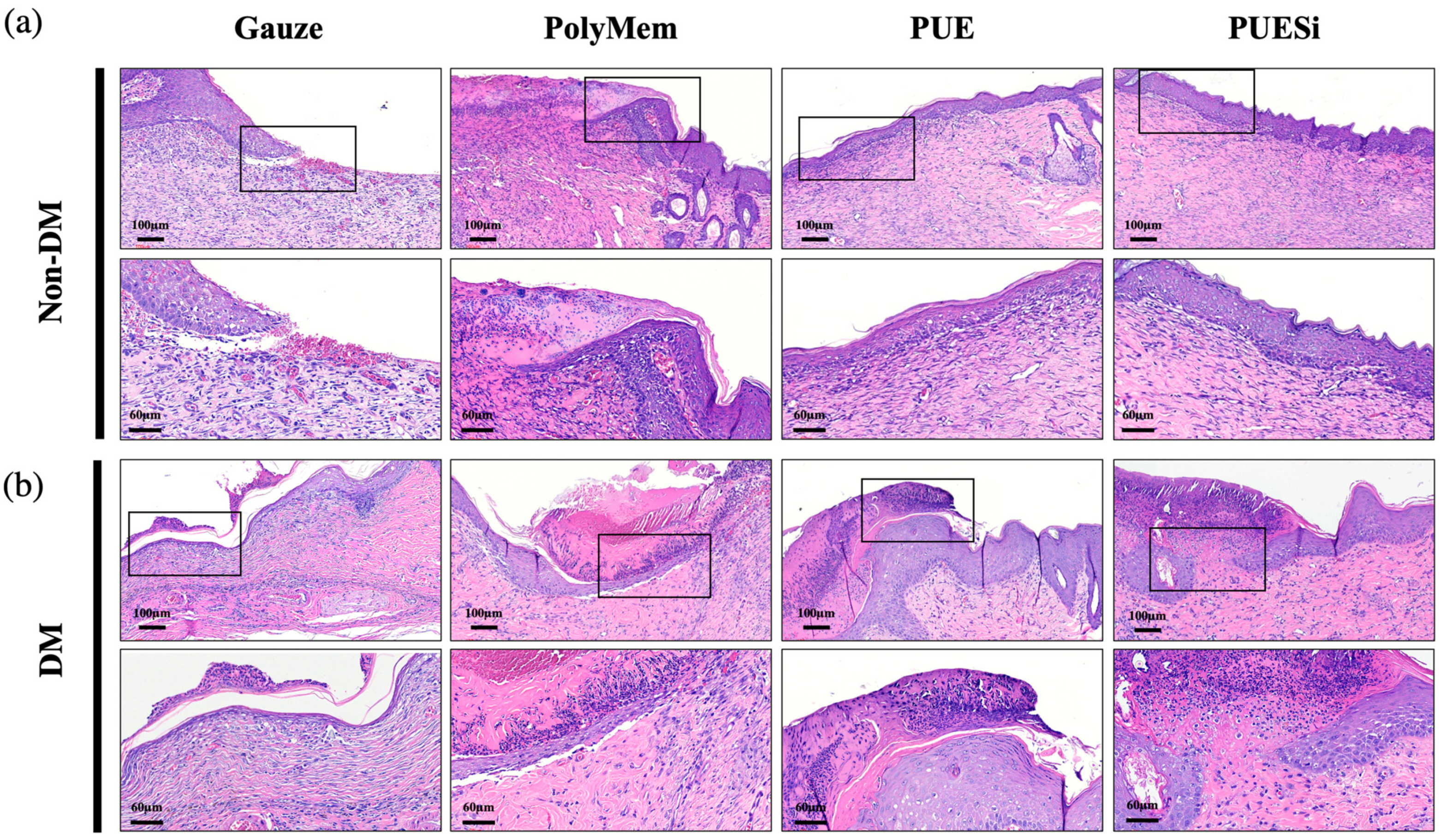
2.10. Histological Analysis
2.11. PUESi-Enhanced Epithelialization in Diabetic Rat Wounds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PEG/APTES-Modified PU Foam Dressing
4.3. Characterization
4.4. Mechanical Property Measurements
4.5. Water Absorption
4.6. Micronegative Pressure
4.7. Moisture Vapor Transmission Rate (MVTR)
4.8. Surface Dryness
4.9. Protein Absorbance
4.10. In Vitro Evaluation of Cytotoxicity
4.11. In Vivo Animal Tests
4.12. Streptozotocin (STZ)-Induced Diabetes
4.13. Experimental Design
4.14. In Vivo Anti-Adhesion Test
4.15. Wound Size Assessment
4.16. Histological Analysis and Immunohistochemistry
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameron, J. Exudate and care of the peri-wound skin. Nurs. Stand. 2004, 19, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Effectively managing wound exudate. Int. Wound J. 2015, 9 (Suppl. 2), 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef]
- Medina, A.; Scott, P.G.; Ghahary, A.; Tredget, E.E. Pathophysiology of chronic nonhealing wounds. J. Burn. Care Rehabil. 2005, 26, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Rehak, L.; Giurato, L.; Meloni, M.; Panunzi, A.; Manti, G.M.; Uccioli, L. The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration-A Narrative Review. J. Clin. Med. 2022, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, K.; Das, A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy 2019, 21, 1137–1150. [Google Scholar] [CrossRef]
- Ulrich, D.; Smeets, R.; Unglaub, F.; Woltje, M.; Pallua, N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J. Wound Ostomy Cont. Nurs. 2011, 38, 522–528. [Google Scholar] [CrossRef]
- Wysocki, A.B. Wound fluids and the Pathogenesis of Chronic Wounds. J. Wound Care 1996, 23, 283–290. [Google Scholar] [CrossRef]
- Xiang, Y. Highly efficient bacteria-infected diabetic wound healing employing a melanin-reinforced biopolymer hydrogel. Chem. Eng. J. 2023, 460, 451–462. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care (New Rochelle) 2021, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Tsao, C.T.; Chang, C.H.; Lai, Y.T.; Wu, M.F.; Chuang, C.N.; Chou, H.C.; Wang, C.K.; Hsieh, K.H. Assessment of reinforced poly(ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Tsao, C.-T.; Chang, C.-H.; Wu, Y.-M.; Liu, Z.-W.; Lin, C.-P.; Wang, C.-K.; Hsieh, K.-H. Synthesis and characterization of thermal-responsive chitin-based polyurethane copolymer as a smart material. Carbohydr. Polym. 2012, 88, 1483–1487. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsao, C.T.; Chang, K.Y.; Chen, S.H.; Han, J.L.; Hsieh, K.H. Effects of types and length of soft-segments on the physical properties and blood compatibility of polyurethanes. Biomed. Mater. Eng. 2012, 22, 373–382. [Google Scholar] [CrossRef]
- Dos Santos, M.R.; Alcaraz-Espinoza, J.J.; da Costa, M.M.; de Oliveira, H.P. Usnic acid-loaded polyaniline/polyurethane foam wound dressing: Preparation and bactericidal activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 33–40. [Google Scholar] [CrossRef]
- Lih, E.; Oh, S.H.; Joung, Y.K.; Lee, J.H.; Han, D.K. Polymers for cell/tissue anti-adhesion. Prog. Polym. Sci. 2015, 44, 28–61. [Google Scholar] [CrossRef]
- Francolini, I.; Silvestro, I.; Di Lisio, V.; Martinelli, A.; Piozzi, A. Synthesis, Characterization, and Bacterial Fouling-Resistance Properties of Polyethylene Glycol-Grafted Polyurethane Elastomers. Int. J. Mol. Sci. 2019, 20, 1001. [Google Scholar] [CrossRef] [Green Version]
- Lundin, J.G.; Daniels, G.C.; McGann, C.L.; Stanbro, J.; Watters, C.; Stockelman, M.; Wynne, J.H. Multi-Functional Polyurethane Hydrogel Foams with Tunable Mechanical Properties for Wound Dressing Applications. Macromol. Mater. Eng. 2016, 302, 1600375. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory Microenvironment of Skin Wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110–118. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Zheng, Y.; Qiao, S.; Xie, Y.; Sun, Y.; Qiao, K.; Feng, Z.; Wang, X.; Wang, J. Preparation of aminoalkyl-grafted bacterial cellulose membranes with improved antimicrobial properties for biomedical applications. J. Biomed. Mater. Res. A 2020, 108, 1086–1098. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Wanag, A.; Sienkiewicz, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of APTES modified TiO(2) on antioxidant enzymes activity secreted by Escherichia coli and Staphylococcus epidermidis. Biochem. Biophys. Res. Commun. 2021, 534, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, Q.; Zhu, J.; Liu, H.; Chen, L.; Yu, L. Anchor and bridge functions of APTES layer on interface between hydrophilic starch films and hydrophobic soyabean oil coating. Carbohydr. Polym. 2021, 272, 118450. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.C.; Fu, T.F.; Wang, H.J.; Lin, C.W.; Lee, G.H.; Wu, S.C.; Wang, C.K. Aspartic acid-based modified PLGA-PEG nanoparticles for bone targeting: In vitro and in vivo evaluation. Acta Biomater. 2014, 10, 4583–4596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Lee, T.M.; Lui, T.S. Enhanced osteoblastic cell response on zirconia by bio-inspired surface modification. Colloids Surf. B Biointerfaces 2013, 106, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Salekdeh, S.S.; Daemi, H.; Zare-Gachi, M.; Rajabi, S.; Bazgir, F.; Aghdami, N.; Nourbakhsh, M.S.; Baharvand, H. Assessment of the Efficacy of Tributylammonium Alginate Surface-Modified Polyurethane as an Antibacterial Elastomeric Wound Dressing for both Noninfected and Infected Full-Thickness Wounds. ACS Appl. Mater. Interfaces 2020, 12, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Shams, E.; Yeganeh, H.; Naderi-Manesh, H.; Gharibi, R.; Mohammad Hassan, Z. Polyurethane/siloxane membranes containing graphene oxide nanoplatelets as antimicrobial wound dressings: In vitro and in vivo evaluations. J. Mater. Sci. Mater. Med. 2017, 28, 75–89. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Chen, K.C.; Chen, S. Rapid hemostatic and mild polyurethane-urea foam wound dressing for promoting wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 289–297. [Google Scholar] [CrossRef]
- Fu, Y.C.; Chen, C.H.; Wang, C.Z.; Wang, Y.H.; Chang, J.K.; Wang, G.J.; Ho, M.L.; Wang, C.K. Preparation of porous bioceramics using reverse thermo-responsive hydrogels in combination with rhBMP-2 carriers: In vitro and in vivo evaluation. J. Mech. Behav. Biomed. Mater. 2013, 27, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Ansell, D.M.; Marsh, C.; Walker, L.; Hardman, M.J.; Holden, K. Evaluating STZ-Induced Impaired Wound Healing in Rats. J. Invest. Dermatol. 2018, 138, 994–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.R.; Wang, C.T.; Wang, F.S.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair. Regen. 2009, 17, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Wang, C.T.; Cheng, J.T.; Kao, G.S.; Chiang, Y.C.; Wang, C.J. Adipose-Derived Stem Cells Accelerate Diabetic Wound Healing Through the Induction of Autocrine and Paracrine Effects. Cell Transpl. 2016, 25, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kong, S.C.; Chen, C.H.; Lin, Y.C.; Lee, K.T.; Wang, Y.H. The Effects of Photobiomodulation on Bone Defect Repairing in a Diabetic Rat Model. Int. J. Mol. Sci. 2021, 22, 11026. [Google Scholar] [CrossRef]
- Kuo, Y.R.; Wang, C.T.; Wang, F.S.; Yang, K.D.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 2009, 17, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Wang, C.T.; Cheng, J.T.; Wang, F.S.; Chiang, Y.C.; Wang, C.J. Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast. Reconstr. Surg. 2011, 128, 872–880. [Google Scholar] [CrossRef]
- Solmaz, A.; Bahadir, E.; Gulcicek, O.B.; Yigitbas, H.; Celik, A.; Karagoz, A.; Ozsavci, D.; Sirvanci, S.; Yegen, B.C. Nesfatin-1 improves oxidative skin injury in normoglycemic or hyperglycemic rats. Peptides 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Ram, M.; Singh, V.; Kumawat, S.; Kant, V.; Tandan, S.K.; Kumar, D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. Int. Immunopharmacol. 2016, 30, 137–149. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem. Cell Res. Ther. 2020, 11, 259–274. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids. Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Invest. Dermatol. 2018, 138, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, L.Y.; Chen, C.H.; Chuang, S.C.; Cheng, T.L.; Lin, Y.H.; Chou, H.C.; Fu, Y.C.; Wang, Y.H.; Wang, C.Z. Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38. Int. J. Mol. Sci. 2020, 21, 7210. [Google Scholar] [CrossRef]
- Song, E.H.; Jeong, S.H.; Park, J.U.; Kim, S.; Kim, H.E.; Song, J. Polyurethane-silica hybrid foams from a one-step foaming reaction, coupled with a sol-gel process, for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Casey, G. Wound repair: Advanced dressing materials. Nurs. Stand. 2002, 17, 49–53. [Google Scholar] [CrossRef]
- Jones, M.L. A short history of thedevelopment of woundcare dressings. Br. J. Healthc. Assist. 2015, 9, 482–485. [Google Scholar] [CrossRef]
- Atkin, L. Chronic wounds: The challenges of appropriate management. Br. J. Community Nurs. 2019, 24, S26–S32. [Google Scholar] [CrossRef]
- Liu, M.; Liu, T.; Chen, X.; Yang, J.; Deng, J.; He, W.; Zhang, X.; Lei, Q.; Hu, X.; Luo, G.; et al. Nano-silver-incorporated biomimetic polydopamine coating on a thermoplastic polyurethane porous nanocomposite as an efficient antibacterial wound dressing. J. Nanobiotechnol. 2018, 16, 89–108. [Google Scholar] [CrossRef]
- Forni, C.; Gazineo, D.; Allegrini, E.; Bolgeo, T.; Brugnolli, A.; Canzan, F.; Chiari, P.; Evangelista, A.; Grugnetti, A.M.; Grugnetti, G.; et al. Effectiveness of a multi-layer silicone-adhesive polyurethane foam dressing as prevention for sacral pressure ulcers in at-risk in-patients: Randomized controlled trial. Int. J. Nurs. Stud. 2022, 127, 104172. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Haryanto, H.; Arisandi, D.; Suriadi, S.; Imran, I.; Ogai, K.; Sanada, H.; Okuwa, M.; Sugama, J. Relationship between maceration and wound healing on diabetic foot ulcers in Indonesia: A prospective study. Int. Wound J. 2017, 14, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Razatos, A. Force Measurements between Bacteria and Poly(ethylene glycol)-Coated Surfaces. Am. Chem. Soc. 2000, 16, 9155–9158. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein-Surface Interactions in the Presence of Polyethylene Oxide. J. Colloid Interface Sci. 1990, 142, 149–158. [Google Scholar] [CrossRef]
- Younan, G.J.; Heit, Y.I.; Dastouri, P.; Kekhia, H.; Xing, W.; Gurish, M.F.; Orgill, D.P. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast. Reconstr. Surg. 2011, 128, 649–658. [Google Scholar] [CrossRef]
- Bassetto, F.; Lancerotto, L.; Salmaso, R.; Pandis, L.; Pajardi, G.; Schiavon, M.; Tiengo, C.; Vindigni, V. Histological evolution of chronic wounds under negative pressure therapy. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 91–99. [Google Scholar] [CrossRef]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Group | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|
| PolyMem | 0.024 ± 0.0014 A | 91.49 ± 0.2051 A |
| PUE | 0.104 ± 0.0072 B | 220.16 ± 7.1540 B |
| PUESi | 0.109 ± 0.0029 B | 227.92 ± 2.7862 B |
| Physiological Saline Uptake (%) | MVTR (g/m2·24 h) | |
|---|---|---|
| PolyMem | 745 ± 20.00 A | 1296.33 ± 22.89 A |
| PUE | 1435 ± 35.00 B | 1943.66 ± 6.65 B |
| PUESi | 1488 ± 98.14 B | 1951.33 ± 16.16 B |
| Relative Collagen Density (%) | Treatment Groups | |||
|---|---|---|---|---|
| Gauze | PolyMem | PUE | PUESi | |
| Non-DM group | 8.87 ± 2.91 A | 12.19 ± 4.23 A | 31.70 ± 6.11 B | 33.69 ± 7.29 BC |
| DM group | 8.51 ± 1.57 A | 8.18 ± 3.09 A | 28.30 ± 6.03 B | 33.19 ± 5.60 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-F.; Chen, S.-H.; Chen, R.-F.; Liu, K.-F.; Kuo, Y.-R.; Wang, C.-K.; Lee, T.-M.; Wang, Y.-H. A Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing. Int. J. Mol. Sci. 2023, 24, 12506. https://doi.org/10.3390/ijms241512506
Chen C-F, Chen S-H, Chen R-F, Liu K-F, Kuo Y-R, Wang C-K, Lee T-M, Wang Y-H. A Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing. International Journal of Molecular Sciences. 2023; 24(15):12506. https://doi.org/10.3390/ijms241512506
Chicago/Turabian StyleChen, Chiu-Fang, Szu-Hsien Chen, Rong-Fu Chen, Keng-Fan Liu, Yur-Ren Kuo, Chih-Kuang Wang, Tzer-Min Lee, and Yan-Hsiung Wang. 2023. "A Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing" International Journal of Molecular Sciences 24, no. 15: 12506. https://doi.org/10.3390/ijms241512506








