Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential
Abstract
1. Introduction
1.1. Saliva
1.2. Trefoil Factor Family (TFF) Peptides
2. Potential Roles of Salivary TFF Peptides
2.1. Potential Role of Salivary TFF1
2.2. Potential Role of Salivary TFF2
2.3. Potential Role of Salivary TFF3
2.4. Summary
3. Therapeutic Potential and Clinical Perspectives
3.1. Saliva, Esophagus and Esophagogastric Junction
3.2. TFF Peptides and Their Use in Chemo- and Radiotherapy and in Artificial Saliva
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DMBT1 | Deleted in Malignant Brain Tumor 1 |
| DSS | Dextran sulfate sodium |
| FCGBP | IgG Fc binding protein |
| ROS | Reactive oxygen species |
| SRCR | Scavenger receptor cysteine-rich |
| TFF | Trefoil factor family |
References
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Amerongen, A.N.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Dodds, M.W.J.; Johnson, D.A.; Yeh, C.-K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Tabak, L.A. In defense of the oral cavity: The protective role of the salivary secretions. Pediatr. Dent. 2006, 28, 110–117. [Google Scholar]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Amado, F.M.L.; Ferreira, R.P.; Vitorino, R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin. Biochem. 2013, 46, 506–517. [Google Scholar] [CrossRef]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchigolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and biosensors: A focus on oral cancer detection through salivary biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Zallocco, L.; Giusti, L.; Ronci, M.; Mussini, A.; Trerotola, M.; Mazzoni, M.R.; Lucacchini, A.; Sebastiani, L. Salivary Proteome Changes in Response to Acute Psychological Stress Due to an Oral Exam Simulation in University Students: Effect of an Olfactory Stimulus. Int. J. Mol. Sci. 2021, 22, 4295. [Google Scholar] [CrossRef] [PubMed]
- Finamore, F.; Cecchettini, A.; Ceccherini, E.; Signore, G.; Ferro, F.; Rocchiccioli, S.; Baldini, C. Characterization of Extracellular Vesicle Cargo in Sjögren’s Syndrome through a SWATH-MS Proteomics Approach. Int. J. Mol. Sci. 2021, 22, 4864. [Google Scholar] [CrossRef]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins in host defense and disease prevention. J. Oral Microbiol. 2015, 7, 29759. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, A.J.; Veerman, E.C.; Nieuw Amerongen, A.V.; Mollenhauer, J. Salivary agglutinin/glycoprotein-340/DMBT1: A single molecule with variable composition and with different functions in infection, inflammation and cancer. Biol. Chem. 2007, 388, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, L.; Möschter, S.; Beileke, S.; Jäger, K.; Garreis, F.; Paulsen, F.P. Human parotid and submandibular glands express and secrete surfactant proteins A, B, C and D. Histochem. Cell Biol. 2009, 132, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, I.; Gerlach, K.L.; Zahl, C.; Hoffmann, W. Expression analysis of human salivary glands by laser microdissection: Differences between submandibular and labial glands. Cell. Physiol. Biochem. 2010, 26, 375–382. [Google Scholar] [CrossRef]
- Jagla, W.; Wiede, A.; Hinz, M.; Dietzmann, K.; Gülicher, D.; Gerlach, K.L.; Hoffmann, W. Secretion of TFF-peptides by human salivary glands. Cell Tissue Res. 1999, 298, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Storesund, T.; Schreurs, O.; Messelt, E.B.; Kolltveit, K.M.; Schenck, K. Trefoil factor family 3 expression in the oral cavity. Eur. J. Oral Sci. 2009, 117, 636–643. [Google Scholar] [CrossRef]
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. [Google Scholar] [CrossRef]
- Samson, M.H.; Chaiyarit, P.; Nortvig, H.; Vestergaard, E.M.; Ernst, E.; Nexo, E. Trefoil factor family peptides in human saliva and cyclical cervical mucus. Method evaluation and results on healthy individuals. Clin. Chem. Lab. Med. 2011, 49, 861–868. [Google Scholar] [CrossRef]
- Devine, D.A.; High, A.S.; Owen, P.J.; Poulsom, R.; Bonass, W.A. Trefoil factor expression in normal and diseased human salivary glands. Hum. Pathol. 2000, 31, 509–515. [Google Scholar] [CrossRef]
- Kutta, H.; May, J.; Jaehne, M.; Münscher, A.; Paulsen, F.P. Antimicrobial defence mechanisms of the human parotid duct. J. Anat. 2006, 208, 609–619. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Utrawichian, A.; Leelayuwat, C.; Vatanasapt, P.; Chanchareonsook, N.; Samson, M.H.; Giraud, A.S. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin. Oral Investig. 2012, 16, 1549–1556. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Klanrit, P.; Phothipakdee, P.; Subarnbhesaj, A.; Thongprasom, K.; Giraud, A.S. Trefoil factor expression by immunohistochemistry in patients with oral lichen planus. Asian Biomed. 2014, 8, 743–749. [Google Scholar] [CrossRef]
- Siber-Hoogeboom, R.; Schicht, M.; Hoogeboom, S.; Paulsen, F.P.; Traxdorf, M. Obstructive sleep apnea and rhonchopathy are associated with downregulation of trefoil factor family peptide 3 (TFF3)—Implications of changes in oral mucus composition. PLoS ONE 2017, 12, e0185200. [Google Scholar]
- Chaiyarit, P.; Chayasadom, A.; Wara-Aswapati, N.; Hormdee, D.; Sittisomwong, S.; Nakaresisoon, S.; Samson, M.H.; Pitiphat, W.; Giraud, A.S. Trefoil factors in saliva and gingival tissues of patients with chronic periodontitis. J. Periodontol. 2012, 83, 1129–1138. [Google Scholar] [CrossRef]
- Verey, F.; Nexo, E.; Greenwood, R.; Berry, M.; Corfield, A.P. Trefoil factor family peptides are increased in the saliva of children with mucositis. Clin. Chem. Lab. Med. 2011, 49, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, P.; Klanrit, P.; Photipakdee, P.; Subarnbhesaj, A.; Giraud, A.S. Increased immunoexpression of trefoil factors in salivary gland tumors. Clin. Oral Investig. 2014, 18, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides. Encyclopedia 2021, 1, 974–987. [Google Scholar] [CrossRef]
- Choudhary, A.; Smitha, C.N.; Suresh, D.K. Trefoils: An unexplored natural protective shield of oral cavity. J. Oral Biol. Craniofac. Res. 2015, 5, 226–231. [Google Scholar] [CrossRef]
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. 1997, 53, 888–903. [Google Scholar] [CrossRef]
- Thim, L.; May, F.E.B. Structure of mammalian trefoil factors and functional insights. Cell. Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and their Different Roles in the Mucosal Innate Immune Defense and More: An Update. Curr. Med. Chem. 2021, 28, 7387–7399. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Braga Emidio, N.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The Tumor Suppressor TFF1 Occurs in Different Forms and Interacts with Multiple Partners in the Human Gastric Mucus Barrier: Indications for Diverse Protective Functions. Int. J. Mol. Sci. 2020, 21, 2508. [Google Scholar] [CrossRef]
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF Peptides xP1 and xP4 Appear in Distinctive Forms in the Xenopus laevis Gastric Mucosa: Indications for Different Protective Functions. Int. J. Mol. Sci. 2019, 20, 6052. [Google Scholar] [CrossRef]
- Allaoui, A.; Botteaux, A.; Dumont, J.E.; Hoste, C.; De Deken, X. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol. Med. 2009, 15, 571–579. [Google Scholar] [CrossRef]
- Duncan, C.; Li, H.; Dykhuizen, R.; Frazer, R.; Johnston, P.; MacKnight, G.; Smith, L.; Lamza, K.; McKenzie, H.; Batt, L.; et al. Protection against oral and gastrointestinal diseases: Importance of dietary nitrate intake, oral nitrate reduction and enterosalivary nitrate circulation. Comp. Biochem. Physiol. 1997, 118A, 939–948. [Google Scholar] [CrossRef]
- McColl, K.E.L. When saliva meets acid: Chemical warfare at the oesophagogastric junction. Gut 2005, 54, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Clyne, M.; May, F.E.B. The interaction of Helicobacter pylori with TFF1 and its role in mediating the tropism of the bacteria within the stomach. Int. J. Mol. Sci. 2019, 20, 4400. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Karasawa, F.; Shiota, A.; Goso, Y.; Kobayashi, M.; Sato, Y.; Masumoto, J.; Fujiwara, M.; Yokosawa, S.; Muraki, T.; Miyagawa, S.; et al. Essential role of gastric gland mucin in preventing gastric cancer in mice. J. Clin. Investig. 2012, 122, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different forms of TFF2, a lectin of the human gastric mucus barrier: In vitro binding studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef]
- Schwarz, H.; Hoffmann, W. Subcellular Localization of the TFF Peptides xP1 and xP4 in the Xenopus laevis Gastric/Esophageal Mucosa: Different Secretion Modes Reflecting Diverse Protective Functions. Int. J. Mol. Sci. 2020, 21, 761. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Kalinski, T.; Peitz, U.; Mönkemüller, K.E.; Kalbacher, H.; Vieth, M.; Meyer, F.; Roessner, A.; Malfertheiner, P.; Lippert, H.; et al. Localization of TFF3 peptide in human esophageal submucosal glands and gastric cardia: Differentiation of two types of gastric pit cells along the rostro-caudal axis. Cell Tissue Res. 2007, 328, 365–374. [Google Scholar] [CrossRef]
- Khummuang, S.; Phanphrom, W.; Laopajon, W.; Kasinrerk, W.; Chaiyarit, P.; Pata, S. Production of Monoclonal Antibodies against Human Trefoil Factor 3 and Development of a Modified-Sandwich ELISA for Detection of Trefoil Factor 3 Homodimer in Saliva. Biol. Proced. Online 2017, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Neubert, H.; Gale, J.; Muirhead, D. Online high-flow peptide immunoaffinity enrichment and nanoflow LC-MS/MS: Assay development for total salivary pepsin/pepsinogen. Clin. Chem. 2010, 56, 1413–1423. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kume, E.; Igarashi, S.; Saito, N.; Narita, H. Role of salivary mucin in the protection of rat esophageal mucosa from acid and pepsin-induced injury. Am. J. Physiol. 1999, 277, G796–G800. [Google Scholar] [CrossRef]
- Wiede, A.; Jagla, W.; Welte, T.; Kohnlein, T.; Busk, H.; Hoffmann, W. Localization of TFF3, a new mucus-associated peptide of the human respiratory tract. Am. J. Respir. Crit. Care Med. 1999, 159, 1330–1335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wiede, A.; Hinz, M.; Canzler, E.; Franke, K.; Quednow, C.; Hoffmann, W. Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res. 2001, 303, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Klemke, R.L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000, 149, 223–236. [Google Scholar] [CrossRef]
- Storesund, T.; Hayashi, K.; Kolltveit, K.M.; Bryne, M.; Schenck, K. Salivary trefoil factor 3 enhances migration of oral keratinocytes. Eur. J. Oral Sci. 2008, 116, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Storesund, T.; Schenck, K.; Osmundsen, H.; Røed, A.; Helgeland, K.; Kolltveit, K.M. Signal transduction and gene transcription induced by TFF3 in oral keratinocytes. Eur. J. Oral Sci. 2009, 117, 511–517. [Google Scholar] [CrossRef]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 mediate TFF3-induced cell migration independently from the ERK1/2 signaling pathway. Investig. Ophthalmol. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef]
- Chinery, R.; Playford, R.J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin. Sci. 1995, 88, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Schnurra, I.; Thim, L.; Hoffmann, W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am. J. Respir. Cell Mol. Biol. 2004, 31, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Dürer, U.; Hartig, R.; Bang, S.; Thim, L.; Hoffmann, W. TFF3 and EGF induce different migration patterns of intestinal epithelial cells in vitro and trigger increased internalization of E-cadherin. Cell. Physiol. Biochem. 2007, 20, 329–346. [Google Scholar] [CrossRef]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef]
- Lang, T.; Klasson, S.; Larsson, E.; Johansson, M.E.; Hansson, G.C.; Samuelsson, T. Searching the Evolutionary Origin of Epithelial Mucus Protein Components-Mucins and FCGBP. Mol. Biol. Evol. 2016, 33, 1921–1936. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, H.; Zhang, Q.; Song, H.; Tang, J.; Fu, J.; Wang, X. FcGBP was upregulated by HPV infection and correlated to longer survival time of HNSCC patients. Oncotarget 2017, 8, 86503–86514. [Google Scholar] [CrossRef]
- Schwartz, J.L. Fcgbp—A Potential Viral Trap in RV144. Open AIDS J. 2014, 8, 21–24. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5, 20401. [Google Scholar] [CrossRef]
- Madsen, J.; Mollenhauer, J.; Holmskov, U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun. 2010, 16, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, M.P.; Holmskov, U.; Meri, S. SALSA—A dance on a slippery floor with changing partners. Mol. Immunol. 2017, 89, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornøe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar]
- Boks, M.A.; Gunput, S.T.; Kosten, I.; Gibbs, S.; van Vliet, S.J.; Ligtenberg, A.J.; van Kooyk, Y. The Human Glycoprotein Salivary Agglutinin Inhibits the Interaction of DC-SIGN and Langerin with Oral Micro-Organisms. J. Innate Immun. 2016, 8, 350–361. [Google Scholar] [CrossRef]
- Thim, L.; Mørtz, E. Isolation and characterization of putative trefoil peptide receptors. Regul. Pept. 2000, 90, 61–68. [Google Scholar] [CrossRef]
- Bastholm, S.K.; Samson, M.H.; Becher, N.; Hansen, L.K.; Stubbe, P.R.; Chronakis, I.S.; Nexo, E.; Uldbjerg, N. Trefoil factor peptide 3 is positively correlated with the viscoelastic properties of the cervical mucus plug. Acta Obstetr. Gynecol. Scand. 2017, 96, 47–52. [Google Scholar] [CrossRef]
- Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.-L. The cervicovaginal mucus barrier. Int. J. Mol. Sci. 2020, 21, 8266. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, H.; Wu, D.C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Peitz, U.; Kouznetsova, I.; Wex, T.; Gebert, I.; Vieth, M.; Roessner, A.; Hoffmann, W.; Malfertheiner, P. TFF3 expression at the esophagogastric junction is increased in gastro-esophageal reflux disease (GERD). Peptides 2004, 25, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Hershkovich, O.; Nagler, R.M. Saliva—A pivotal player in the pathogenesis of oropharyngeal cancer. Br. J. Cancer 2004, 91, 111–118. [Google Scholar] [CrossRef][Green Version]
- Jagla, W.; Wiede, A.; Kölle, S.; Hoffmann, W. Differential expression of the TFF-peptides xP1 and xP4 in the gastrointestinal tract of Xenopus laevis. Cell Tissue Res. 1998, 291, 13–18. [Google Scholar] [CrossRef]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–188. [Google Scholar]
- Kjellev, S. The trefoil factor family—Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Braga Emidio, N.; Hoffmann, W.; Brierley, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.L.; Wong, J.F.; Li, Y.; Swaminathan, S.; Xavier, R.J.; Devaney, K.L.; Podolsky, D.K. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 2004, 126, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Barker, N.P.; Akhmadullina, L.I.; Rodionova, I.; Sherman, N.Z.; Davidenko, I.S.; Rakovskaya, G.N.; Gotovkin, E.A.; Shinkarev, S.A.; Kopp, M.V.; et al. Phase II, randomized, double-blind, placebo-controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil-based chemotherapy. J. Clin. Oncol. 2009, 27, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, K.; Hans, W.; Van Huysse, J.; Neirynck, S.; Demetter, P.; Remaut, E.; Rottiers, P.; Steidler, L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 2004, 127, 502–513. [Google Scholar] [CrossRef]
- Caluwaerts, S.; Vandenbroucke, K.; Steidler, L.; Neirynck, S.; Vanhoenacker, P.; Corveleyn, S.; Watkins, B.; Sonis, S.; Coulie, B.; Rottiers, P. AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol. 2010, 46, 564–570. [Google Scholar] [CrossRef]
- Limaye, S.A.; Haddad, R.I.; Cilli, F.; Sonis, S.T.; Colevas, A.D.; Brennan, M.T.; Hu, K.S.; Murphy, B.A. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 2013, 119, 4268–4276. [Google Scholar] [CrossRef]
- Chen, R.-M.; Chiou, Y.-S.; Chong, Q.-Y.; Poh, H.-M.; Tan, T.-Z.; Zhang, M.-Y.; Ma, L.; Zhu, T.; Pandey, V.; Kumar, A.P.; et al. Pharmacological Inhibition of TFF3 Enhances Sensitivity of CMS4 Colorectal Carcinoma to 5-Fluorouracil through Inhibition of p44/42 MAPK. Int. J. Mol. Sci. 2019, 20, 6215. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.; de Bont, E.S.; Tissing, W.J. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial saliva: Challenges and future perspectives for the treatment of xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Tang, Y.L.; Pang, X.; Zheng, M.; Tang, Y.J.; Liang, X.H. The maintenance of an oral epithelial barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: In vitro binding studies. ChemBioChem 2018, 19, 2598–2608. [Google Scholar] [CrossRef]
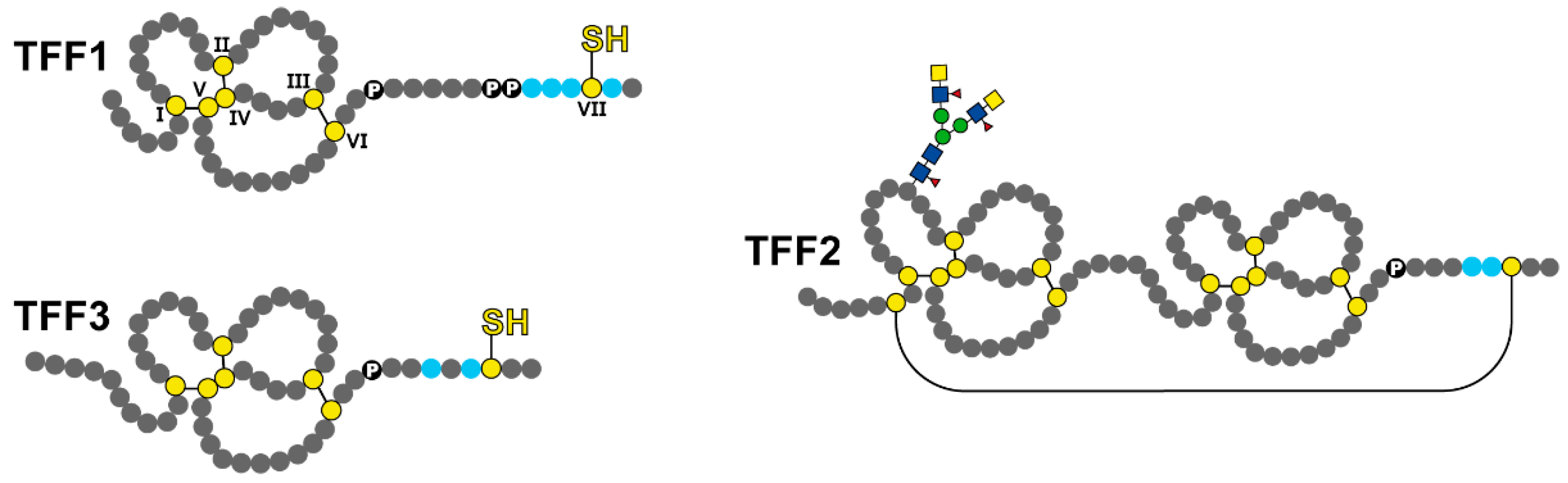
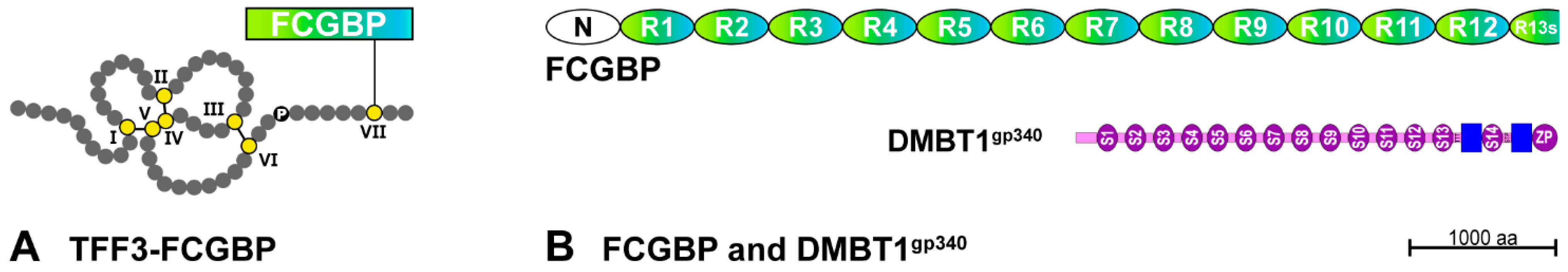
| TFF Peptides | Possible Role of Salivary TFF Peptides | References |
|---|---|---|
| TFF1 monomer | Scavenger for ROS and RNS | [27,34,35,36] |
| Intracellular chaperone (ER) | [27,32] | |
| TFF1 homodimer | Interaction with H. pylori | [40] |
| Binding to MUC6 (esophagogastric junction) | [34] | |
| TFF2 | Weak motogenic activity | [27,28,32,33] |
| Anti-inflammatory effect | [28,32] | |
| Binding to MUC6 (esophagogastric junction) | [27,33,41] | |
| TFF3 homodimer | Weak motogenic activity | [27,28,32,33,53,54] |
| Binding to DMBT1gp340 (at least in vitro) | [33,69] | |
| TFF3-FCGBP | Regulation of pathogen attachment and clearing of microorganisms | [27,33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, W. Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 12221. https://doi.org/10.3390/ijms222212221
Hoffmann W. Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential. International Journal of Molecular Sciences. 2021; 22(22):12221. https://doi.org/10.3390/ijms222212221
Chicago/Turabian StyleHoffmann, Werner. 2021. "Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential" International Journal of Molecular Sciences 22, no. 22: 12221. https://doi.org/10.3390/ijms222212221
APA StyleHoffmann, W. (2021). Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential. International Journal of Molecular Sciences, 22(22), 12221. https://doi.org/10.3390/ijms222212221






