A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia
Abstract
:1. Introduction
2. Autonomic Regulation in Schizophrenia
2.1. Symptoms Related to Autonomic Abnormalities in Schizophrenia
2.2. Action Mechanisms of ANS Dysregulation
3. Kynurenine Pathway in Schizophrenia
4. The Interplay of Kynurenines and Autonomic Functions
5. The Potential Interaction of the ANS and KP in Schizophrenia, and Molecules Mutually Targeting KP and Autonomic Dysfunctions in Schizophrenia
5.1. Direct Modulators of the KP
5.2. Indirect Modulatory Options of the KP
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α7nAChR | α-7 nicotinic receptors acetylcholine |
| AHR | aryl hydrocarbon receptor |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor |
| ANS | autonomic nervous system |
| BBB | blood-brain barrier |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| GABA | γ-aminobutyric acid |
| GPR35 | G-protein-coupled receptor 35 |
| 3-HK | 3-hydroxykynurenine |
| HPA | hypothalamic–pituitary–adrenal |
| IDO | indoleamine 2,3-dioxygenase |
| icv | intracerebroventricular |
| KAR | kainite receptor |
| KAT | kynurenine aminotransferase |
| KMO | kynurenine 3-monooxygenase |
| KP | kynurenine pathway |
| KYNA | kynurenic acid |
| KYN | kynurenine |
| LC | locus coeruleus |
| NE | norepinephrine |
| NMDAR | N-methyl-d-aspartate receptor |
| NTS | nucleus tractus solitarii |
| QUIN | quinolinic acid |
| PCP | phencyclidine |
| PVN | paraventricular nucleus |
| TDO | tryptophan 2,3-dioxygenase |
| TRP | tryptophan |
| VNS | vagal nerve stimulation |
References
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [Green Version]
- Andlin-Sobocki, P.; Jönsson, B.; Wittchen, H.U.; Olesen, J. Cost of disorders of the brain in Europe. Eur. J. Neurol. 2005, 12 (Suppl. 1), 1–27. [Google Scholar] [CrossRef]
- Schultz, S.H.; North, S.W.; Shields, C.G. Schizophrenia: A Review. Am. Fam. Physician 2007, 75, 1821–1829. [Google Scholar] [PubMed]
- Penadés, R.; Franck, N.; González-Vallespí, L.; Dekerle, M. Neuroimaging Studies of Cognitive Function in Schizophrenia. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1118, pp. 117–134. [Google Scholar]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R.; Gallhofer, B. Cognitive function in schizophrenia. Int. Clin. Psychopharmacol. 1997, 12, S29. [Google Scholar] [CrossRef]
- Bär, K.J.; Koschke, M.; Boettger, M.K.; Berger, S.; Kabisch, A.; Sauer, H.; Voss, A.; Yeragani, V.K. Acute psychosis leads to increased QT variability in patients suffering from schizophrenia. Schizophr. Res. 2007, 95, 115–123. [Google Scholar] [CrossRef]
- Bär, K.J.; Boettger, M.K.; Schulz, S.; Harzendorf, C.; Agelink, M.W.; Yeragani, V.K.; Chokka, P.; Voss, A. The interaction between pupil function and cardiovascular regulation in patients with acute schizophrenia. Clin. Neurophysiol. 2008, 119, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.-J. Cardiac Autonomic Dysfunction in Patients with Schizophrenia and Their Healthy Relatives—A Small Review. Front. Neurol. 2015, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- Guccione, C.; Di Scalea, G.L.; Ambrosecchia, M.; Terrone, G.; Di Cesare, G.; Ducci, G.; Schimmenti, A.; Caretti, V. Early signs of schizophrenia and autonomic nervous system dysregulation: A literature review. Clin. Neuropsychiatry 2019, 16, 86–97. [Google Scholar]
- Montaquila, J.M.; Trachik, B.J.; Bedwell, J.S. Heart rate variability and vagal tone in schizophrenia: A review. J. Psychiatr. Res. 2015, 69, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.S. The Utilization of Pupillometry in the Differential Diagnosis and Treatment of Psychotic and Behavioral Disorders. In Pupillary Dynamics and Behavior; Springer: Boston, MA, USA, 1974; pp. 75–134. [Google Scholar]
- Schulz, S.; Bolz, M.; Bär, K.J.; Voss, A. Central-and autonomic nervous system coupling in schizophrenia. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150178. [Google Scholar] [CrossRef]
- Stogios, N.; Gdanski, A.; Gerretsen, P.; Chintoh, A.F.; Graff-Guerrero, A.; Rajji, T.K.; Remington, G.; Hahn, M.K.; Agarwal, S.M. Autonomic nervous system dysfunction in schizophrenia: Impact on cognitive and metabolic health. npj Schizophr. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Abi-Dargham, A. Alterations of Serotonin Transmission in Schizophrenia. Int. Rev. Neurobiol. 2007, 78, 133–164. [Google Scholar] [CrossRef]
- Kim, J.S.; Kornhuber, H.H.; Schmid-Burgk, W.; Holzmüller, B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980, 20, 379–382. [Google Scholar] [CrossRef]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2020, 118, 298–314. [Google Scholar] [CrossRef]
- Igbal, N.; van Praag, H.M. The role of serotonin in schizophrenia. Eur. Neuropsychopharmacol. 1995, 5, 11–23. [Google Scholar] [CrossRef]
- Yang, A.; Tsai, S.-J. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef]
- Chiappelli, J.; Rowland, L.M.; Notarangelo, F.M.; Wijtenburg, S.A.; Thomas, M.A.R.; Pocivavsek, A.; Jones, A.; Wisner, K.; Kochunov, P.; Schwarcz, R.; et al. Salivary kynurenic acid response to psychological stress: Inverse relationship to cortical glutamate in schizophrenia. Neuropsychopharmacology 2018, 43, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.K.; Linderholm, K.R.; Engberg, G.; Paulson, L.; Blennow, K.; Lindström, L.H.; Nordin, C.; Karanti, A.; Persson, P.; Erhardt, S. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr. Res. 2005, 80, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Koola, M.M. Kynurenine pathway and cognitive impairments in schizophrenia: Pharmacogenetics of galantamine and memantine. Schizophr. Res. Cogn. 2016, 4, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hashimoto, K. α7 nicotinic acetylcholine receptor as a potential therapeutic target for schizophrenia. Curr. Pharm. Des. 2011, 17, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Andiné, P.; Lehmann, A.; Ellrén, K.; Wennberg, E.; Kjellmer, I.; Nielsen, T.; Hagberg, H. The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci. Lett. 1988, 90, 208–212. [Google Scholar] [CrossRef]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425. [Google Scholar] [CrossRef]
- Zubcevic, J.; Richards, E.M.; Yang, T.; Kim, S.; Sumners, C.; Pepine, C.J.; Raizada, M.K. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ. Res. 2019, 125, 104–116. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef]
- Kiss, C.; Vécsei, L. Kynurenines in the Brain: Preclinical and Clinical Studies, Therapeutic Considerations. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: New York, NY, USA, 2009; pp. 91–105. [Google Scholar]
- Zádori, D.; Veres, G.; Szalárdy, L.; Klivényi, P.; Vécsei, L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J. Alzheimer’s Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef] [Green Version]
- Kraepelin, E. Psychiatrie: Ein Lehrbuch für Studirende und Aerzte. J. Ment. Sci. 1899, 45, 581–583. [Google Scholar]
- Clamor, A.; Lincoln, T.M.; Thayer, J.F.; Koenig, J. Resting vagal activity in schizophrenia: Meta-analysis of heart rate variability as a potential endophenotype. Br. J. Psychiatry 2016, 208, 9–16. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Kawanishi, C.; Kishida, I.; Furuno, T.; Fujibayashi, M.; Ishii, C.; Ishii, N.; Moritani, T.; Taguri, M.; Hirayasu, Y. Dose-dependent effect of antipsychotic drugs on autonomic nervous system activity in schizophrenia. BMC Psychiatry 2012, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Tzeng, N.S.; Yeh, C.B.; Kuo, T.B.J.; Huang, S.Y.; Chang, C.C.; Chang, H.A. Reduced cardiac autonomic response to deep breathing: A heritable vulnerability trait in patients with schizophrenia and their healthy first-degree relatives. Psychiatry Res. 2016, 243, 335–341. [Google Scholar] [CrossRef]
- Bär, K.J.; Boettger, M.K.; Berger, S.; Baier, V.; Sauer, H.; Yeragani, V.K.; Voss, A. Decreased baroreflex sensitivity in acute schizophrenia. J. Appl. Physiol. 2007, 102, 1051–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieda, M.; Miyaoka, T.; Wake, R.; Liaury, K.; Tsuchie, K.; Fukushima, M.; Araki, T.; Ezoe, S.; Inagaki, T.; Horiguchi, J. Evaluation of autonomic nervous system by salivary alpha-amylase level and heart rate variability in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 83–87. [Google Scholar] [CrossRef]
- Birkhofer, A.; Geissendoerfer, J.; Alger, P.; Mueller, A.; Rentrop, M.; Strubel, T.; Leucht, S.; Förstl, H.; Bär, K.J.; Schmidt, G. The deceleration capacity—A new measure of heart rate variability evaluated in patients with schizophrenia and antipsychotic treatment. Eur. Psychiatry 2013, 28, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.L.; Minassian, A.; Paulus, M.P.; Geyer, M.A.; Perry, W. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 2010, 44, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rechlin, T.; Claus, D.; Weis, M. Heart rate variability in schizophrenic patients and changes of autonomic heart rate parameters during treatment with clozapine. Biol. Psychiatry 1994, 35, 888–892. [Google Scholar] [CrossRef]
- Rachow, T.; Berger, S.; Boettger, M.K.; Schulz, S.; Guinjoan, S.; Yeragani, V.K.; Voss, A.; Bär, K.J. Nonlinear relationship between electrodermal activity and heart rate variability in patients with acute schizophrenia. Psychophysiology 2011, 48, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zaki, J.; Harvey, P.O.; Ochsner, K.; Green, M.F. Schizophrenia patients are impaired in empathic accuracy. Psychol. Med. 2011, 41, 2297–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.S.; Yoo, C.S.; Yi, S.H.; Hong, K.H.; Oh, H.S.; Hwang, J.Y.; Kim, S.G.; Ahn, Y.M.; Kim, Y.S. Differential pattern of heart rate variability in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 991–995. [Google Scholar] [CrossRef]
- Mujica-Parodi, L.R.; Yeragani, V.; Malaspina, D. Nonlinear complexity and spectral analyses of heart rate variability in medicated and unmedicated patients with schizophrenia. Neuropsychobiology 2005, 51, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.W.H.; Castle, D.J. Layer upon layer: Thermoregulation in schizophrenia. Schizophr. Res. 2004, 69, 149–157. [Google Scholar] [CrossRef]
- Pechnick, R.N.; George, R. Characterization of the effects of the acute and chronic administration of phencyclidine on body temperature in the rat: Lack of evidence for the involvement of opiate receptors. J. Pharmacol. Exp. Ther. 1989, 248, 900–906. [Google Scholar] [PubMed]
- Radonjić, N.V.; Petronijević, N.D.; Vučković, S.M.; Prostran, M.Š.; Nešić, Z.I.; Todorović, V.R.; Paunović, V.R. Baseline temperature in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Physiol. Behav. 2008, 93, 437–443. [Google Scholar] [CrossRef]
- Madjirova, N.P.; Petrova, N.S.; Delchev, N.K. Daily rhythmicity of temperature, pulse and blood pressure in schizophrenic patients. Schizophr. Res. 1995, 14, 183. [Google Scholar] [CrossRef]
- Buck, C.W.; Carscallen, H.B.; Hobbs, G.E. Temperature regulation in schizophrenia: I. comparison of schizophrenic and normal subjects ii. analysis by duration of psychosis. Arch. Neurol. Psychiatry 1950, 64, 828–842. [Google Scholar] [CrossRef]
- Bernstein, A.S.; Frith, C.D.; Gruzelier, J.H.; Patterson, T.; Straube, E.; Venables, P.H.; Zahn, T.P. An analysis of the skin conductance orienting response in samples of American, British, and German schizophrenics. Biol. Psychol. 1982, 14, 155–211. [Google Scholar] [CrossRef]
- Walker, E.; Shapiro, D.; Esterberg, M.; Trotman, H. Neurodevelopment and schizophrenia: Broadening the focus. Curr. Dir. Psychol. Sci. 2010, 19, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.F.; Trotman, H.D.; Goulding, S.M.; Holtzman, C.W.; Ryan, A.T.; McDonald, A.; Shapiro, D.I.; Brasfield, J.L. Developmental mechanisms in the prodrome to psychosis. Dev. Psychopathol. 2013, 25, 1585–1600. [Google Scholar] [CrossRef]
- Zahn, T.P. Sensitivity of measurement and electrodermal “nonresponding” in schizophrenic and normal subjects. Schizophr. Bull. 1978, 4, 153. [Google Scholar] [CrossRef] [Green Version]
- Zahn, T.P.; Carpenter, W.T.; McGlashan, T.H. Autonomic variables related to short-term outcome and clinical improvement in acute schizophrenia. Psychopharmacol. Bull. 1979, 15, 42–43. [Google Scholar] [PubMed]
- Zahn, T.P.; Jacobsen, L.K.; Gordon, C.T.; McKenna, K.; Frazier, J.A.; Rapoport, J.L. Autonomic nervous system markers of psychopathology in childhood-onset schizophrenia. Arch. Gen. Psychiatry 1997, 54, 904–912. [Google Scholar] [CrossRef]
- Morgan, R.; Cheadle, A.J. Circadian body temperature in chronic schizophrenia. Br. J. Psychiatry 1976, 129, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, R.; Weizman, A.; Epstein, Y.; Rosenberg, S.L.; Valevski, A.; Dorfman-Etrog, P.; Wiezer, N.; Katz, N.; Munitz, H.; Hermesh, H. Abnormal thermoregulation in drug-free male schizophrenia patients. Eur. Neuropsychopharmacol. 2001, 11, 285–288. [Google Scholar] [CrossRef]
- Horvath, G.; Kekesi, G.; Petrovszki, Z.; Benedek, G. Abnormal motor activity and thermoregulation in a schizophrenia rat model for translational science. PLoS ONE 2015, 10, e0143751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peupelmann, J.; Quick, C.; Berger, S.; Hocke, M.; Tancer, M.E.; Yeragani, V.K.; Bär, K.J. Linear and non-linear measures indicate gastric dysmotility in patients suffering from acute schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Kalmár, G.; Kekesi, G.; Benedek, G.; Nyúl, L.G.; Horvath, G. Impaired pupillary control in “schizophrenia-like” WISKET rats. Auton. Neurosci. Basic Clin. 2018, 213, 34–42. [Google Scholar] [CrossRef]
- Spohn, H.E.; Patterson, T. Recent studies of psychophysiology in schizophrenia. Schizophr. Bull. 1979, 5, 581–611. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; Hocke, M.; Bär, K.J. Gastric dysmotility in healthy first-degree relatives of patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1294–1299. [Google Scholar] [CrossRef]
- Inagaki, T.; Miyaoka, T.; Okazaki, S.; Yasuda, H.; Kawamukai, T.; Utani, E.; Wake, R.; Hayashida, M.; Horiguchi, J.; Tsuji, S. High salivary alpha-amylase levels in patients with schizophrenia: A pilot study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 688–691. [Google Scholar] [CrossRef]
- Varsamis, J.; Adamson, J.D. Somatic symptoms in schizophrenia. Can. Psychiatr. Assoc. J. 1976, 21, 1–6. [Google Scholar] [CrossRef]
- Kekesi, O.; Tuboly, G.; Szucs, M.; Birkas, E.; Morvay, Z.; Benedek, G.; Horvath, G. Long-lasting, distinct changes in central opioid receptor and urinary bladder functions in models of schizophrenia in rats. Eur. J. Pharmacol. 2011, 661, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Kim, M.; Mitchell, C.; Inskip, H. Twenty-five year mortality of a community cohort with schizophrenia. Br. J. Psychiatry 2010, 196, 116–121. [Google Scholar] [CrossRef]
- Chung, M.S.; Yang, A.C.; Lin, Y.C.; Lin, C.N.; Chang, F.R.; Shen, S.H.; Ouyang, W.C.; Loh, E.W.; Chiu, H.J. Association of altered cardiac autonomic function with psychopathology and metabolic profiles in schizophrenia. Psychiatry Res. 2013, 210, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, M.; Matsumoto, T.; Kishida, I.; Kimura, T.; Ishii, C.; Ishii, N.; Moritani, T. Autonomic nervous system activity and psychiatric severity in schizophrenia: Regular article. Psychiatry Clin. Neurosci. 2009, 63, 538–545. [Google Scholar] [CrossRef]
- Healy, D.; Le Noury, J.; Harris, M.; Butt, M.; Linden, S.; Whitaker, C.; Zou, L.; Roberts, A.P. Mortality in schizophrenia and related psychoses: Data from two cohorts, 1875–1924 and 1994–2010. BMJ Open 2012, 2, e001810. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Toichi, M.; Sakihama, M. Influences of an anticholinergic antiparkinsonian drug, parkinsonism, and psychotic symptoms on cardiac autonomic function in schizophrenia. J. Clin. Psychopharmacol. 2003, 23, 441–447. [Google Scholar] [CrossRef]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef] [Green Version]
- Toichi, M.; Kubota, Y.; Murai, T.; Kamio, Y.; Sakihama, M.; Toriuchi, T.; Inakuma, T.; Sengoku, A.; Miyoshi, K. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int. J. Psychophysiol. 1999, 31, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Boettger, S.; Hoyer, D.; Falkenhahn, K.; Kaatz, M.; Yeragani, V.K.; Bär, K.J. Altered diurnal autonomic variation and reduced vagal information flow in acute schizophrenia. Clin. Neurophysiol. 2006, 117, 2715–2722. [Google Scholar] [CrossRef]
- Kim, J.H.; Ann, J.H.; Lee, J. Relationship between heart rate variability and the severity of psychotic symptoms in schizophrenia. Acta Neuropsychiatr. 2011, 23, 161–166. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, S.H.; Yoo, C.S.; Yang, S.A.; Yoon, S.C.; Lee, K.Y.; Ahn, Y.M.; Kang, U.G.; Kim, Y.S. Heart rate dynamics and their relationship to psychotic symptom severity in clozapine-treated schizophrenic subjects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 371–378. [Google Scholar] [CrossRef]
- Hattori, S.; Suda, A.; Kishida, I.; Miyauchi, M.; Shiraishi, Y.; Fujibayashi, M.; Tsujita, N.; Ishii, C.; Ishii, N.; Moritani, T.; et al. Association between dysfunction of autonomic nervous system activity and mortality in schizophrenia. Compr. Psychiatry 2018, 86, 119–122. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D.; Casey, D.E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef]
- Malpas, S.C. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 2010, 90, 513–557. [Google Scholar] [CrossRef]
- Aysin, B.; Aysin, E. Effect of respiration in heart rate variability (HRV) analysis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, New Orleans, LA, USA, 10 April 2006; pp. 1776–1779. [Google Scholar]
- Low, P.A. Evaluation of autonomic function in the autonomic disorders. J. Auton. Nerv. Syst. 1993, 43, 27–29. [Google Scholar] [CrossRef]
- Przybylska-Felus, M.; Furgala, A.; Zwolinska-Wcislo, M.; Mazur, M.; Widera, A.; Thor, P.; Mach, T. Disturbances of autonomic nervous system activity and diminished response to stress in patients with celiac disease. J. Physiol. Pharmacol. 2014, 65, 833–841. [Google Scholar]
- Tai, Y.C.; Lin, S.-H.; Chen, K.C.; Lee, I.H.; Chen, P.S.; Lee, L.-T.; Tsai, H.C.; Yeh, T.L.; Yang, Y.K. Heart Rate Variability with Deep Breathing in Drug-Naïve Patients with Schizophrenia. Appl. Psychophysiol. Biofeedback 2020, 45, 275–282. [Google Scholar] [CrossRef]
- Chatterton, R.T.; Vogelsong, K.M.; Lu, Y.C.; Ellman, A.B.; Hudgens, G.A. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996, 16, 433–448. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef]
- Nater, U.M.; La Marca, R.; Florin, L.; Moses, A.; Langhans, W.; Koller, M.M.; Ehlert, U. Stress-induced changes in human salivary alpha-amylase activity—Associations with adrenergic activity. Psychoneuroendocrinology 2006, 31, 49–58. [Google Scholar] [CrossRef]
- Kanji, S.; Fonseka, T.M.; Marshe, V.S.; Sriretnakumar, V.; Hahn, M.K.; Müller, D.J. The microbiome-gut-brain axis: Implications for schizophrenia and antipsychotic induced weight gain. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 3–15. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Waise, T.M.Z.; Dranse, H.J.; Lam, T.K.T. The metabolic role of vagal afferent innervation. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 625–636. [Google Scholar] [CrossRef]
- Beissner, F.; Meissner, K.; Bär, K.J.; Napadow, V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013, 33, 10503–10511. [Google Scholar] [CrossRef] [Green Version]
- Lechan, R.M.; Toni, R. Functional Anatomy of the Hypothalamus and Pituitary. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bernstein, H.-G.; Keilhoff, G.; Steiner, J.; Dobrowolny, H.; Bogerts, B. The Hypothalamus in Schizophrenia Research: No Longer a Wallflower Existence. Open Neuroendocrinol. J. 2010, 3, 59–67. [Google Scholar] [CrossRef]
- Dougherty, P. Somatosensory Systems (Section 2, Chapter 2) Neuroscience Online: An Electronic Textbook for the Neurosciences | Department of Neurobiology and Anatomy—The University of Texas Medical School at Houston. Available online: https://nba.uth.tmc.edu/neuroscience/m/s4/chapter03.html (accessed on 21 June 2021).
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness, V.S.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypothalamic Abnormalities in Schizophrenia: Sex Effects and Genetic Vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef]
- Klomp, A.; Koolschijn, P.C.M.P.; Hulshoff Pol, H.E.; Kahn, R.S.; Van Haren, N.E.M. Hypothalamus and pituitary volume in schizophrenia: A structural MRI study. Int. J. Neuropsychopharmacol. 2012, 15, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolschijn, P.C.M.P.; van Haren, N.E.M.; Hulshoff Pol, H.E.; Kahn, R.S. Hypothalamus volume in twin pairs discordant for schizophrenia. Eur. Neuropsychopharmacol. 2008, 18, 312–315. [Google Scholar] [CrossRef]
- Tognin, S.; Rambaldelli, G.; Perlini, C.; Bellani, M.; Marinelli, V.; Zoccatelli, G.; Alessandrini, F.; Pizzini, F.B.; Beltramello, A.; Terlevic, R.; et al. Enlarged hypothalamic volumes in schizophrenia. Psychiatry Res.—Neuroimaging 2012, 204, 75–81. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Stanarius, A.; Baumann, B.; Henning, H.; Krell, D.; Danos, P.; Falkai, P.; Bogerts, B. Nitric oxide synthase-containing neurons in the human hypothalamus: Reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 1998, 83, 867–875. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Krause, S.; Krell, D.; Dobrowolny, H.; Wolter, M.; Stauch, R.; Ranft, K.; Danos, P.; Jirikowski, G.F.; Bogerts, B. Strongly reduced number of parvalbumin-immunoreactive projection neurons in the mammillary bodies in schizophrenia: Further evidence for limbic neuropathology. Ann. N. Y. Acad. Sci. 2007, 1096, 120–127. [Google Scholar] [CrossRef]
- Briess, D.; Cotter, D.; Doshi, R.; Everail, L. Mamillary body abnormalities in schizophrenia. Lancet 1998, 352, 789–790. [Google Scholar] [CrossRef]
- Weinshenker, D.; Holmes, P.V. Regulation of neurological and neuropsychiatric phenotypes by locus coeruleus-derived galanin. Brain Res. 2016, 1641, 320–337. [Google Scholar] [CrossRef] [Green Version]
- Szabadi, E. Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 2013, 27, 659–693. [Google Scholar] [CrossRef]
- Yamamoto, K.I.; Shinba, T.; Yoshii, M. Psychiatric symptoms of noradrenergic dysfunction: A pathophysiological view. Psychiatry Clin. Neurosci. 2014, 68, 1–20. [Google Scholar] [CrossRef]
- Samuels, E.; Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr. Neuropharmacol. 2008, 6, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Bondy, B.; Ackenheil, M.; Birzle, W.; Elbers, R.; Fröhler, M. Catecholamines and their receptors in blood: Evidence for alterations in schizophrenia. Biol. Psychiatry 1984, 19, 1377–1393. [Google Scholar] [PubMed]
- Kemali, D.; Del Vecchio, M.; Maj, M. Increased noradrenaline levels in CSF and plasma of schizophrenic patients. Biol. Psychiatry 1982, 17, 711–717. [Google Scholar]
- Gay, N.; Cottraux, J.A.; Denoroy, L.; Tommasi, M.; Kopp, N. Possible increase of dopamine-beta-hydroxylase activity in the locus ceruleus of paranoid schizophrenic patients: A preliminary post-mortem study. Psychiatry Res. 1989, 27, 31–38. [Google Scholar] [CrossRef]
- Wise, C.D.; Stein, L. Dopamine-β-hydroxylase deficits in the brains of schizophrenic patients. Science (80-) 1973, 181, 344–347. [Google Scholar] [CrossRef]
- Benros, M.E.; Eaton, W.W.; Mortensen, P.B. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol. Psychiatry 2014, 75, 300–306. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Khandaker, G.M.; Zimbron, J.; Dalman, C.; Lewis, G.; Jones, P.B. Childhood infection and adult schizophrenia: A meta-analysis of population-based studies. Schizophr. Res. 2012, 139, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Zádor, F.; Nagy-Grócz, G.; Kekesi, G.; Dvorácskó, S.; Szucs, E.; Tömböly, C.; Horvath, G.; Benyhe, S.; Vécsei, L. Kynurenines and the endocannabinoid system in schizophrenia: Common points and potential interactions. Molecules 2019, 24, 3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–20. [Google Scholar]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [Green Version]
- Pedraz-Petrozzi, B.; Elyamany, O.; Rummel, C.; Mulert, C. Effects of inflammation on the kynurenine pathway in schizophrenia—A systematic review. J. Neuroinflamm. 2020, 17, 1–17. [Google Scholar] [CrossRef]
- Erhardt, S.; Olsson, S.K.; Engberg, G. Pharmacological manipulation of kynurenic acid: Potential in the treatment of psychiatric disorders. CNS Drugs 2009, 23, 91–101. [Google Scholar] [CrossRef]
- Kegel, M.E.; Bhat, M.; Skogh, E.; Samuelsson, M.; Lundberg, K.; Dahl, M.L.; Sellgren, C.; Schwieler, L.; Engberg, G.; Schuppe-Koistinen, I.; et al. Imbalanced Kynurenine Pathway in Schizophrenia. Int. J. Tryptophan Res. 2014, 7, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in Bipolar Depression. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Price, J.B.; Bronars, C.; Erhardt, S.; Cullen, K.R.; Schwieler, L.; Berk, M.; Walder, K.; McGee, S.L.; Frye, M.A.; Tye, S.J. Bioenergetics and synaptic plasticity as potential targets for individualizing treatment for depression. Neurosci. Biobehav. Rev. 2018, 90, 212–220. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Boros, F.A.; Vécsei, L. Immunomodulatory Effects of Genetic Alterations Affecting the Kynurenine Pathway. Front. Immunol. 2019, 10, 2570. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; Prendergast, G.C.; Mellor, A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer 2019, 19, 162–175. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef] [Green Version]
- Golimbet, V.E.; Korovaitseva, G.I.; Gabaeva, M.V.; Velikaya, N.V.; Snegireva, A.A.; Kasparov, S.V.; Kolesina, N.Y.; Ganisheva, T.K.; Savel’eva, T.M. A study of IL-1β and IDO gene polymorphisms in patients with schizophrenia. Zhurnal Nevrol. Psihiatr. Im. SS Korsakova 2014, 2014, 46–49. [Google Scholar]
- Réus, G.Z.; Becker, I.R.T.; Scaini, G.; Petronilho, F.; Oses, J.P.; Kaddurah-Daouk, R.; Ceretta, L.B.; Zugno, A.I.; Dal-Pizzol, F.; Quevedo, J.; et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 55–63. [Google Scholar] [CrossRef]
- Muller, N.; Schwarz, M.J. The Role of Immune System in Schizophrenia. Curr. Immunol. Rev. 2010, 6, 213–220. [Google Scholar] [CrossRef]
- Babcock, T.A.; Carlin, J.M. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor α in interferon-treated epithelial cells. Cytokine 2000, 12, 588–594. [Google Scholar] [CrossRef]
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neurosci. Lett. 2008, 441, 29–34. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Andre, C.; Wang, Y.; Lawson, M.A.; Szegedi, S.S.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Interferon- and Tumor Necrosis Factor- Mediate the Upregulation of Indoleamine 2,3-Dioxygenase and the Induction of Depressive-Like Behavior in Mice in Response to Bacillus Calmette-Guerin. J. Neurosci. 2009, 29, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012, 37, 939–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batabyal, D.; Yeh, S.R. Human tryptophan dioxygenase: A comparison to indoleamine 2,3-dioxygenase. J. Am. Chem. Soc. 2007, 129, 15690–15701. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Llenos, I.C.; Dulay, J.R.; Barillo, M.M.; Yolken, R.H.; Weis, S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004, 15, 618–629. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Cullen, K.M.; Lim, C.K.; Smythe, G.A.; Garner, B.; Kapoor, V.; Takikawa, O.; Brew, B.J. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007, 27, 12884–12892. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Li, J.; Li, J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur. J. Biochem. 2004, 271, 4804–4814. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Dounay, A.B.; Tuttle, J.B.; Verhoest, P.R. Challenges and Opportunities in the Discovery of New Therapeutics Targeting the Kynurenine Pathway. J. Med. Chem. 2015, 58, 8762–8782. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of Indoleamine 2,3-Dioxygenase and Production of Quinolinic Acid by Human Microglia, Astrocytes, and Neurons. Wiley Online Libr. 2004, 49, 15–23. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Myint, A.M.; Schwarz, M.J.; Verkerk, R.; Mueller, H.H.; Zach, J.; Scharpé, S.; Steinbusch, H.W.M.; Leonard, B.E.; Kim, Y.K. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain. Behav. Immun. 2011, 25, 1576–1581. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur. J. Neurosci. 2007, 25, 2656–2665. [Google Scholar] [CrossRef]
- Zeppillo, T.; Schulmann, A.; Macciardi, F.; Hjelm, B.E.; Föcking, M.; Sequeira, P.A.; Guella, I.; Cotter, D.; Bunney, W.E.; Limon, A.; et al. Functional impairment of cortical AMPA receptors in schizophrenia. Schizophr. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Beneyto, M.; Kristiansen, L.V.; Oni-Orisan, A.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007, 32, 1888–1902. [Google Scholar] [CrossRef]
- Meador-Woodruff, J.H.; Daniel, J.H. Glutamate receptor expression in schizophrenic brain. Brain Res. Rev. 2000, 31, 288–294. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.R.; Eisenberg, H.M.; Kajii, Y.; Schwarcz, R.; Albuquerque, E.X. Age dependency of inhibition of α7 nicotinic receptors and tonically active N-methyl-D-aspartate receptors by endogenously produced kynurenic acid in the brain. J. Pharmacol. Exp. Ther. 2011, 337, 572–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, C.; Pereira, E.F.R.; Wu, H.Q.; Purushottamachar, P.; Njar, V.; Schwarcz, R.; Albuquerque, E.X. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at α7* nicotinic receptors. J. Pharmacol. Exp. Ther. 2007, 322, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Q.; Pereira, E.F.R.; Bruno, J.P.; Pellicciari, R.; Albuquerque, E.X.; Schwarcz, R. The Astrocyte-Derived α7 Nicotinic Receptor Antagonist Kynurenic Acid Controls Extracellular Glutamate Levels in the Prefrontal Cortex. J. Mol. Neurosci. 2010, 40, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.W. Relationships and Interactions between Ionotropic Glutamate Receptors and Nicotinic Receptors in the CNS. Neuroscience 2021, 468, 321–365. [Google Scholar] [CrossRef]
- Schwieler, L.; Erhardt, S. Inhibitory action of clozapine on rat ventral tegmental area dopamine neurons following increased levels of endogenous kynurenic acid. Neuropsychopharmacology 2003, 28, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- Shepard, P.D.; Joy, B.; Clerkin, L.; Schwarcz, R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology 2003, 28, 1454–1462. [Google Scholar] [CrossRef]
- Schubert, K.O.; Föcking, M.; Cotter, D.R. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: Potential roles in GABAergic interneuron pathology. Schizophr. Res. 2015, 167, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Varga, D.P.; Menyhárt, Á.; Puskás, T.; Bari, F.; Farkas, E.; Kis, Z.; Vécsei, L.; Toldi, J.; Gellért, L. Systemic administration of L-kynurenine sulfate induces cerebral hypoperfusion transients in adult C57Bl/6 mice. Microvasc. Res. 2017, 114, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaccarino, H.F.; Suckow, R.F.; Xie, S.; Bucci, D.J. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia. Schizophr. Res. 2013, 150, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Olsson, S.; Larsson, M.; Erhardt, S. Subchronic elevation of brain kynurenic acid augments amphetamine-induced locomotor response in mice. J. Neural Transm. 2012, 119, 155–163. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.-Q.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur. J. Neurosci. 2012, 35, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trecartin, K.V.; Bucci, D.J. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr. Res. 2011, 133, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, R. Kynurenines and Glutamate. Multiple Links and Therapeutic Implications. Adv. Pharmacol. 2016, 76, 13–37. [Google Scholar]
- Akagbosu, C.O.; Evans, G.C.; Gulick, D.; Suckow, R.F.; Bucci, D.J. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr. Bull. 2012, 38, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Alexander, K.S.; Pocivavsek, A.; Wu, H.Q.; Pershing, M.L.; Schwarcz, R.; Bruno, J.P. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: Reversal with galantamine. Neuroscience 2013, 238, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Chess, A.C.; Bucci, D.J. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav. Brain Res. 2006, 170, 326–332. [Google Scholar] [CrossRef]
- Deangeli, N.E.; Todd, T.P.; Chang, S.E.; Yeh, H.H.; Yeh, P.W.; Bucci, D.J. Acid during adolescence increases sign-tracking and impairs long-term potentiation in adulthood. Front. Behav. Neurosci. 2015, 8, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erhardt, S.; Schwieler, L.; Emanuelsson, C.; Geyer, M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatry 2004, 56, 255–260. [Google Scholar] [CrossRef]
- Forrest, C.M.; McNair, K.; Pisar, M.; Khalil, O.S.; Darlington, L.G.; Stone, T.W. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience 2015, 310, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Juricek, L.; Coumoul, X. The aryl hydrocarbon receptor and the nervous system. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linderholm, K.R.; Skogh, E.; Olsson, S.K.; Dahl, M.L.; Holtze, M.; Engberg, G.; Samuelsson, M.; Erhardt, S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012, 38, 426–432. [Google Scholar] [CrossRef]
- Olsson, S.K.; Samuelsson, M.; Saetre, P.; Lindström, L.; Jönsson, E.G.; Nordin, C.; Engberg, G.; Erhardt, S.; Landén, M. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatry Neurosci. 2010, 35, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Roberts, R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 2001, 50, 521–530. [Google Scholar] [CrossRef]
- Koola, M.M.; Buchanan, R.W.; Pillai, A.; Aitchison, K.J.; Weinberger, D.R.; Aaronson, S.T.; Dickerson, F.B. Potential role of the combination of galantamine and memantine to improve cognition in schizophrenia. Schizophr. Res. 2014, 157, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, F.; Cioni, R.M.; Callovini, T.; Cavaleri, D.; Crocamo, C.; Carrà, G. The kynurenine pathway in schizophrenia and other mental disorders: Insight from meta-analyses on the peripheral blood levels of tryptophan and related metabolites. Schizophr. Res. 2021, 232, 61–62. [Google Scholar] [CrossRef]
- Morrens, M.; De Picker, L.; Kampen, J.K.; Coppens, V. Blood-based kynurenine pathway alterations in schizophrenia spectrum disorders: A meta-analysis. Schizophr. Res. 2020, 223, 43–52. [Google Scholar] [CrossRef]
- Miller, C.L.; Llenos, I.C.; Dulay, J.R.; Weis, S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006, 1073–1074, 25–37. [Google Scholar] [CrossRef]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Yao, J.K.; Dougherty, G.G.; Reddy, R.D.; Keshavan, M.S.; Montrose, D.M.; Matson, W.R.; Rozen, S.; Krishnan, R.R.; McEvoy, J.; Kaddurah-Daouk, R. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol. Psychiatry 2010, 15, 938–953. [Google Scholar] [CrossRef] [Green Version]
- Condray, R.; Dougherty, G.G.; Keshavan, M.S.; Reddy, R.D.; Haas, G.L.; Montrose, D.M.; Matson, W.R.; McEvoy, J.; Kaddurah-Daouk, R.; Yao, J.K. 3-Hydroxykynurenine and clinical symptoms in first-episode neuroleptic-naive patients with schizophrenia. Int. J. Neuropsychopharmacol. 2011, 14, 756–767. [Google Scholar] [CrossRef]
- Oxenkrug, G.; van der Hart, M.; Roeser, J.; Summergrad, P. Anthranilic Acid: A Potential Biomarker and Treatment Target for Schizophrenia. Ann. Psychiatry Ment. Heal. 2016, 4, 1059. [Google Scholar]
- Holtze, M.; Saetre, P.; Engberg, G.; Schwieler, L.; Werge, T.; Andreassen, O.A.; Hall, H.; Terenius, L.; Agartz, I.; Jönsson, E.G.; et al. Kynurenine 3-monooxygenase polymorphisms: Relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 2012, 37, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Lavebratt, C.; Olsson, S.; Backlund, L.; Frisé, L.; Sellgren, C.; Priebe, L.; Nikamo, P.; Träskman-Bendz, L.; Cichon, S.; Vawter, M.P.; et al. The KMO allele encoding Arg 452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol. Psychiatry 2013, 19, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonodi, I.; Stine, O.C.; Sathyasaikumar, K.V.; Roberts, R.C.; Mitchell, B.D.; Hong, L.E.; Kajii, Y.; Thaker, G.K.; Schwarcz, R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry 2011, 68, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Rothermundt, M.; Ponath, G.; Arolt, V. S100B in schizophrenic psychosis. Int. Rev. Neurobiol. 2004, 59, 445–470. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; O’Connor, J.C. Kynurenine 3-Monooxygenase: An Influential Mediator of Neuropathology. Front. Psychiatry 2015, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H. Conceptual Confluence: The Kynurenine Pathway as a Common Target for Ketamine and the Convergence of the Inflammation and Glutamate Hypotheses of Depression. Neuropsychopharmacology 2013, 38, 1607–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 2017, 112, 307–323. [Google Scholar] [CrossRef] [Green Version]
- Owe-Young, R.; Webster, N.L.; Mukhtar, M.; Pomerantz, R.J.; Smythe, G.; Walker, D.; Armati, P.J.; Crowe, S.M.; Brew, B.J. Kynurenine pathway metabolism in human blood-brain-barrier cells: Implications for immune tolerance & neurotoxicity. J. Neurochem. 2008, 105, 1346–1357. [Google Scholar] [CrossRef]
- Walker, A.K.; Wing, E.E.; Banks, W.A.; Dantzer, R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry 2019, 24, 1523–1532. [Google Scholar] [CrossRef]
- Chopra, K.; Baveja, A.; Kuhad, A. MMPs: A novel drug target for schizophrenia. Expert Opin. Ther. Targets 2015, 19, 77–85. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.A.; Mizoguchi, Y.; Horikawa, H.; Seki, Y.; Kasai, M.; Yamauchi, Y.; Yamada, S.; Kanba, S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 42, 115–121. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef]
- Steiner, J.; Bogerts, B.; Sarnyai, Z.; Walter, M.; Gos, T.; Bernstein, H.-G.; Myint, A.-M. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood–brain barrier integrity. World J. Biol. Psychiatry 2012, 13, 482–492. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, J.; Pocivavsek, A.; Nugent, K.L.; Notarangelo, F.M.; Kochunov, P.; Rowland, L.M.; Schwarcz, R.; Hong, L.E. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry 2014, 71, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, J.-T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J. Neurol. Sci. 2012, 323, 1–8. [Google Scholar] [CrossRef]
- Lechner, S.M.; Curtis, A.L.; Brons, R.; Valentino, R.J. Locus coeruleus activation by colon distention: Role of corticotropin- releasing factor and excitatory amino acids. Brain Res. 1997, 756, 114–124. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, L. Improvement in detrusor-sphincter dyssynergia by bladder-wall injection of replication-defective herpes simplex virus vector-mediated gene delivery of kynurenine aminotransferase II in spinal cord injury rats. Nat. Publ. Gr. 2017, 55, 155–161. [Google Scholar] [CrossRef]
- Manjunath, R.; Ramasarma, T. Stimulation of liver tryptophan pyrrolase during heat exposure. Biochem. J. 1985, 226, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Shirokawa, T.; Isobe, K. Changes in brain tryptophan metabolism elicited by ageing, social environment, and psychological stress in mice. Stress 2008, 11, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Nomura, J. Effects of stress and psychotropic drugs on rat liver tryptophan. Endocrinology 1965, 76, 1190–1194. [Google Scholar] [CrossRef]
- Pawlak, D.; Takada, Y.; Urano, T.; Takada, A. Serotonergic and kynurenic pathways in rats exposed to foot shock. Brain Res. Bull. 2000, 52, 197–205. [Google Scholar] [CrossRef]
- Salter, M.; Pogson, C.I. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells: Effects of glucocorticoids and experimental diabetes. Biochem. J. 1985, 229, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Savtchenko, L.P.; Korogod, S.M.; Rusakov, D.A. Nicotine-induced excitation of locus coeruleus neurons is blocked by elevated levels of endogenous kynurenic acid. Synapse 2000, 37, 104–108. [Google Scholar] [CrossRef]
- Zakrocka, I.; Targowska-Duda, K.M.; Wnorowski, A.; Kocki, T.; Jóźwiak, K.; Turski, W.A. Angiotensin II Type 1 Receptor Blockers Inhibit KAT II Activity in the Brain—Its Possible Clinical Applications. Neurotox. Res. 2017, 32, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.; Minson, J.; Drolet, G.; Chalmers, J. Effect of intrathecal amino acid receptor antagonists on basal blood pressure and pressor responses to brainstem stimulation in normotensive and hypertensive rats. J. Cardiovasc. Pharmacol. 1990, 15, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Mastelari, R.B.; de Abreu, S.B.; de Aguiar Corrêa, F.M.; de Souza, H.C.D.; Martins-Pinge, M.C. Glutamatergic neurotransmission in the hypothalamus PVN on heart rate variability in exercise trained rats. Auton. Neurosci. Basic Clin. 2012, 170, 42–47. [Google Scholar] [CrossRef]
- Kapoor, R.; Okuno, E.; Kido, R.; Kapoor, V. Immuno-localization of kynurenine aminotransferase (KAT) in the rat medulla and spinal cord. Neuroreport 1997, 8, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Komatsu, K.; Tsukamoto, K.; Sved, A.F. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 2000, 35, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Willette, R.N.; Punnen-Grandy, S.; Krieger, A.J.; Sapru, H.N. Differential regulation of regional vascular resistance by the rostral and caudal ventrolateral medulla in the rat. J. Auton. Nerv. Syst. 1987, 18, 143–151. [Google Scholar] [CrossRef]
- Kapoor, V.; Kapoor, R.; Chalmers, J. Kynurenic acid, an endogenous glutamate antagonist, in SHR and WKY rats: Possible role in central blood pressure regulation. Clin. Exp. Pharmacol. Physiol. 1994, 21, 891–896. [Google Scholar] [CrossRef]
- Kwok, J.B.J.; Kapoor, R.; Gotoda, T.; Iwamoto, Y.; Iizuka, Y.; Yamada, N.; Isaacs, K.E.; Kushwaha, V.V.; Bret Church, W.; Schofield, P.R.; et al. A missense mutation in kynurenine aminotransferase-1 in spontaneously hypertensive rats. J. Biol. Chem. 2002, 277, 35779–35782. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.-P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haibara, A.S.; Bonagamba, L.G.H.; Machado, B.H. Sympathoexcitatory neurotransmission of the chemoreflex in the NTS of awake rats. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 1999, 276, R69–R80. [Google Scholar] [CrossRef]
- Kaszaki, J.; Palásthy, Z.; Érczes, D.; Rácz, A.; Torday, C.; Varga, G.; Vécsei, L.; Boros, M. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol. Motil. 2008, 20, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Knyihar-Csillik, E.; Mihaly, A.; Krisztin-Peva, B.; Robotka, H.; Szatmari, I.; Fulop, F.; Toldi, J.; Csillik, B.; Vécsei, L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: Comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008, 61, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Varga, G.; Érces, D.; Fazekas, B.; Fülöp, M.; Kovács, T.; Kaszaki, J.; Fülöp, F.; Vécsei, L.; Boros, M. N-Methyl-d-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol. Motil. 2010, 22, 217.e68. [Google Scholar] [CrossRef] [PubMed]
- Page, M.E.; Akaoka, H.; Aston-Jones, G.; Valentino, R.J. Bladder distention activates noradrenergic locus coeruleus neurons by an excitatory amino acid mechanism. Neuroscience 1992, 51, 555–563. [Google Scholar] [CrossRef]
- Iwabuchi, N. Sacral glutamatergic transmission in the descending limb of the micturition reflex in the cat. Fukuoka Igaku Zasshi 1997, 88, 30–38. [Google Scholar]
- Braga, V.A.; Machado, B.H. Chemoreflex sympathoexcitation was not altered by the antagonism of glutamate receptors in the commissural nucleus tractus solitarii in the working heart-brainstem preparation of rats. Exp. Physiol. 2006, 91, 551–559. [Google Scholar] [CrossRef]
- Furness, J.B.; Kunze, W.A.A.; Bertrand, P.P.; Clerc, N.; Bornstein, J.C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 1998, 54, 1–18. [Google Scholar] [CrossRef]
- Kaszaki, J.; Érces, D.; Varga, G.; Szabó, A.; Vécsei, L.; Boros, M. Kynurenines and intestinal neurotransmission: The role of N-methyl-d-aspartate receptors. J. Neural Transm. 2012, 119, 211–223. [Google Scholar] [CrossRef]
- Liu, M.T.; Rothstein, J.D.; Gershon, M.D.; Kirchgessner, A.L. Glutamatergic enteric neurons. J. Neurosci. 1997, 17, 4764–4784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroni, F.; Luzzi, S.; Franchi-Micheli, S.; Zilletti, L. The presence of N-methyl-d-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci. Lett. 1986, 68, 57–62. [Google Scholar] [CrossRef]
- Shannon, H.E.; Sawyer, B.D. Glutamate receptors of the N-methyl-D-aspartate subtype in the myenteric plexus of the guinea pig ileum. J. Pharmacol. Exp. Ther. 1989, 251, 518–523. [Google Scholar] [PubMed]
- Wiley, J.W.; Lu, Y.X.; Owyang, C. Evidence for a glutamatergic neural pathway in the myenteric plexus. Am. J. Physiol. Liver Physiol. 1991, 261, G693–G700. [Google Scholar] [CrossRef]
- Giaroni, C.; Zanetti, E.; Chiaravalli, A.M.; Albarello, L.; Dominioni, L.; Capella, C.; Lecchini, S.; Frigo, G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur. J. Pharmacol. 2003, 476, 63–69. [Google Scholar] [CrossRef]
- Kohjitani, A.; Funahashi, M.; Miyawaki, T.; Hanazaki, M.; Matsuo, R.; Shimada, M. Peripheral N-methyl-D-aspartate receptors modulate nonadrenergic noncholinergic lower esophageal sphincter relaxation in rabbits. Anesth. Analg. 2005, 101, 1681–1688. [Google Scholar] [CrossRef]
- Milusheva, E.A.; Kuneva, V.I.; Itzev, D.E.; Kortezova, N.I.; Sperlagh, B.; Mizhorkova, Z.N. Glutamate stimulation of acetylcholine release from myenteric plexus is mediated by endogenous nitric oxide. Brain Res. Bull. 2005, 66, 229–234. [Google Scholar] [CrossRef]
- Cohen, H.; Loewenthal, U.; Matar, M.; Kotler, M. Association of autonomic dysfunction and clozapine: Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br. J. Psychiatry 2001, 179, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, J.S.; Olson, D.; Crilly, J.F.; Olivares, T.; Williams, G.C.; Tu, X.; Tang, W.; Wiener, K.; Dvorin, S.; Dietz, M.B. Prevalence of the metabolic syndrome among patients receiving clozapine. Am. J. Psychiatry 2006, 163, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Kishida, I.; Suda, A.; Miyauchi, M.; Shiraishi, Y.; Fujibayashi, M.; Tsujita, N.; Ishii, C.; Ishii, N.; Moritani, T.; et al. Effects of four atypical antipsychotics on autonomic nervous system activity in schizophrenia. Schizophr. Res. 2018, 193, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Chang, L.R.; Kuo, T.B.J.; Lin, Y.H.; Chen, Y.Z.; Yang, C.C.H. Impact of antipsychotics and anticholinergics on autonomic modulation in patients with schizophrenia. J. Clin. Psychopharmacol. 2013, 33, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.W.Y.; Kim, D.D.; Procyshyn, R.M.; White, R.F.; Honer, W.G.; Barr, A.M. Clozapine-induced cardiovascular side effects and autonomic dysfunction: A systematic review. Front. Neurosci. 2018, 12, 203. [Google Scholar] [CrossRef] [Green Version]
- Kopin, I.J. Catecholamine metabolism: Basic aspects and clinical significance. Pharmacol. Rev. 1985, 37, 333–364. [Google Scholar]
- Brown, A.S.; Gewirtz, G.; Harkavy-Friedman, J.; Cooper, T.; Brébion, G.; Amador, X.F.; Malaspina, D.; Gorman, J.M. Effects of clozapine on plasma catecholamines and relation to treatment response in schizophrenia: A within-subject comparison with haloperidol. Neuropsychopharmacology 1997, 17, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Corsi-Zuelli, F.M.d.G.; Brognara, F.; Quirino, G.F.d.S.; Hiroki, C.H.; Fais, R.S.; Del-Ben, C.M.; Ulloa, L.; Salgado, H.C.; Kanashiro, A.; Loureiro, C.M. Neuroimmune Interactions in Schizophrenia: Focus on Vagus Nerve Stimulation and Activation of the Alpha-7 Nicotinic Acetylcholine Receptor. Front. Immunol. 2017, 8, 618. [Google Scholar] [CrossRef] [Green Version]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Konsman, J.P.; Parnet, P.; Dantzer, R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002, 25, 154–159. [Google Scholar] [CrossRef]
- Tu, H.; Rady, P.L.; Juelich, T.; Smith, E.M.; Tyring, S.K.; Hughes, T.K. Cytokine Regulation of Tryptophan Metabolism in the Hypothalamic-Pituitary-Adrenal (HPA) Axis: Implications for Protective and Toxic Consequences in Neuroendocrine Regulation. Cell. Mol. Neurobiol. 2005, 25, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lian, Y.-J.; Dong, X.; Peng, W.; Liu, L.-L.; Su, W.-J.; Gong, H.; Zhang, T.; Jiang, C.-L.; Li, J.-S.; et al. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behav. Brain Res. 2018, 353, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Danesch, U.; Hashimoto, S.; Renkawitz, R.; Schutz, G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J. Biol. Chem. 1983, 258, 4750–4753. [Google Scholar] [CrossRef]
- Danesch, U.; Gloss, B.; Schmid, W.; Schütz, G.; Schüle, R.; Renkawitz, R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987, 6, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Pan, Z.Z.; Luo, D.Y. TDO as a therapeutic target in brain diseases. Metab. Brain Dis. 2016, 31, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shinno, H.; Ichihara, A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J. Biol. Chem. 1980, 255, 7533–7535. [Google Scholar] [CrossRef]
- Braidman, I.P.; Rose, P. Effects of sex hormones on three glucocorticoid-inducible enzymes concerned with amino acid metabolism in rat liver. Endocrinology 1971, 89, 1250–1255. [Google Scholar] [CrossRef]
- Schutz, G.; Killewich, L.; Chen, G.; Feigelson, P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc. Natl. Acad. Sci. USA 1975, 72, 1017–1020. [Google Scholar] [CrossRef] [Green Version]
- Wolf, H.; Brown, R.R. Studies on tryptophan metabolism in male subjects treated with hydrocortisone. J. Clin. Endocrinol. Metab. 1971, 33, 838–843. [Google Scholar] [CrossRef]
- Altman, K.; Greengard, O. Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J. Clin. Invest. 1966, 45, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Knox, W.E.; Mehler, A.H. The adaptive increase of the tryptophan peroxidase-oxidase system of liver. Science (80-) 1951, 113, 237–238. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, N.; Zhang, X.; Han, X.; Zhai, X.; Lu, Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab. Brain Dis. 2018, 33, 1787–1800. [Google Scholar] [CrossRef]
- Baran, H.; Kepplinger, B. D-cycloserine lowers kynurenic acid formation-New mechanism of action. Eur. Neuropsychopharmacol. 2014, 24, 639–644. [Google Scholar] [CrossRef]
- Gottlieb, J.D.; Cather, C.; Shanahan, M.; Creedon, T.; Macklin, E.A.; Goff, D.C. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr. Res. 2011, 131, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Koshy Cherian, A.; Gritton, H.; Johnson, D.E.; Young, D.; Kozak, R.; Sarter, M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology 2014, 82, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culman, J.; Von Heyer, C.; Piepenburg, B.; Rascher, W.; Unger, T. Effects of systemic treatment with irbesartan and losartan on central responses to angiotensin II in conscious, normotensive rats. Eur. J. Pharmacol. 1999, 367, 255–265. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y.; Sunagawa, K. Sympathoinhibition caused by orally administered telmisartan through inhibition of the AT1 receptor in the rostral ventrolateral medulla of hypertensive rats. Hypertens. Res. 2012, 35, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, M.; Gebhart, G.F. Effects of neonatal capsaicin treatment on descending modulation of spinal nociception from the rostral, medial medulla in adult rat. Brain Res. 1994, 645, 164–178. [Google Scholar] [CrossRef]
- Cozzi, A.; Carpenedo, R.; Moroni, F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: Studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2YL]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects. JAMA Psychiatry 2014, 71, 1381. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Mayberg, H.S.; Krahl, S.E.; McNamara, J.; Frazer, A.; Henry, T.R.; George, M.S.; Charney, D.S.; Brannan, S.K. VNS Therapy in Treatment-Resistant Depression: Clinical Evidence and Putative Neurobiological Mechanisms. Neuropsychopharmacology 2006, 31, 1345–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhondzadeh, S.; Shasavand, E.; Jamilian, H.R.; Shabestari, O.; Kamalipour, A. Dipyridamole in the treatment of schizophrenia: Adenosine-dopamine receptor interactions. J. Clin. Pharm. Ther. 2000, 25, 131–137. [Google Scholar] [CrossRef]
- Nematollahi, A.; Sun, G.; Jayawickrama, G.S.; Church, W.B. Kynurenine Aminotransferase Isozyme Inhibitors: A Review. Int. J. Mol. Sci. 2016, 17, 946. [Google Scholar] [CrossRef]
- Bortz, D.M.; Wu, H.Q.; Schwarcz, R.; Bruno, J.P. Oral administration of a specific kynurenic acid synthesis (KAT II) inhibitor attenuates evoked glutamate release in rat prefrontal cortex. Neuropharmacology 2017, 121, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Dounay, A.B.; Anderson, M.; Bechle, B.M.; Campbell, B.M.; Claffey, M.M.; Evdokimov, A.; Evrard, E.; Fonseca, K.R.; Gan, X.; Ghosh, S.; et al. Discovery of Brain-Penetrant, Irreversible Kynurenine Aminotransferase II Inhibitors for Schizophrenia. ACS Med. Chem. Lett. 2012, 3, 187–192. [Google Scholar] [CrossRef]
- Dounay, A.B.; Anderson, M.; Bechle, B.M.; Evrard, E.; Gan, X.; Kim, J.Y.; McAllister, L.A.; Pandit, J.; Rong, S.; Salafia, M.A.; et al. PF-04859989 as a template for structure-based drug design: Identification of new pyrazole series of irreversible KAT II inhibitors with improved lipophilic efficiency. Bioorganic Med. Chem. Lett. 2013, 23, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.R.; Castellano-González, G.; Guillemin, G.J.; Lovejoy, D.B. Major Developments in the Design of Inhibitors along the Kynurenine Pathway. Curr. Med. Chem. 2017, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Jayawickrama, G.S.; Nematollahi, A.; Sun, G.; Church, W.B. Improvement of kynurenine aminotransferase-II inhibitors guided by mimicking sulfate esters. PLoS ONE 2018, 13, e0196404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawickrama, G.S.; Nematollahi, A.; Sun, G.; Gorrell, M.D.; Church, W.B. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci. Rep. 2017, 7, 17559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocivavsek, A.; Elmer, G.I.; Schwarcz, R. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus 2019, 29, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Okuyama, M.; Kajii, Y.; Pocivavsek, A.; Bruno, J.P.; Schwarcz, R. Targeting kynurenine aminotransferase II in psychiatric diseases: Promising effects of an orally active enzyme inhibitor. Schizophr. Bull. 2014, 40, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 388. [Google Scholar] [CrossRef] [Green Version]
- Giorgini, F.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Thomas, M.A.R.; Tararina, M.; Wu, H.Q.; Schwarcz, R.; Muchowski, P.J. Targeted deletion of kynurenine 3-monooxygenase in mice: A new tool for studying kynurenine pathway metabolism in periphery and brain. J. Biol. Chem. 2013, 288, 36554–36566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, M.; Levy, C.; Heyes, D.J.; Lafite, P.; Outeiro, T.F.; Giorgini, F.; Leys, D.; Scrutton, N.S. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 2013, 496, 382–385. [Google Scholar] [CrossRef]
- Chiarugi, A.; Carpenedo, R.; Molina, M.T.; Mattoli, L.; Pellicciari, R.; Moroni, F. Comparison of the Neurochemical and Behavioral Effects Resulting from the Inhibition of Kynurenine Hydroxylase and/or Kynureninase. J. Neurochem. 1995, 65, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, L.; Rassoulpour, A.; Guidetti, P.; Samadi, P.; Bédard, P.J.; Izzo, E.; Schwarcz, R.; Di Paolo, T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 2008, 186, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [Green Version]
- Cimpianu, C.-L.; Strube, W.; Falkai, P.; Palm, U.; Hasan, A. Vagus nerve stimulation in psychiatry: A systematic review of the available evidence. J. Neural Transm. 2017, 124, 145–158. [Google Scholar] [CrossRef]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and Brain Function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef] [PubMed]
- Biaggioni, I. Adenosine Receptors and Autonomic Regulation. In Primer on the Autonomic Nervous System; Elsevier: Amsterdam, The Netherlands, 2012; pp. 95–97. ISBN 9780123865250. [Google Scholar]
- Biaggioni, I. The pharmacology of autonomic failure: From hypotension to hypertension. Pharmacol. Rev. 2017, 69, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Zuccarini, M.; Cassano, T.; Borroto-Escuela, D.O.; Di Iorio, P.; Schwarcz, R.; Fuxe, K.; Ferraro, L. Adenosine and Kynurenic Acid Interactions: Possible Relevance for Schizophrenia Treatment? Front. Pharmacol. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Cheffer, A.; Castillo, A.R.G.; Corrêa-Velloso, J.; Gonçalves, M.C.B.; Naaldijk, Y.; Nascimento, I.C.; Burnstock, G.; Ulrich, H. Purinergic system in psychiatric diseases. Mol. Psychiatry 2018, 23, 94–106. [Google Scholar] [CrossRef]
- Krügel, U. Purinergic receptors in psychiatric disorders. Neuropharmacology 2016, 104, 212–225. [Google Scholar] [CrossRef]
- Lara, D.R.; Souza, D.O. Schizophrenia: A purinergic hypothesis. Med. Hypotheses 2000, 54, 157–166. [Google Scholar] [CrossRef]
- Boison, D.; Singer, P.; Shen, H.Y.; Feldon, J.; Yee, B.K. Adenosine hypothesis of schizophrenia—Opportunities for pharmacotherapy. Neuropharmacology 2012, 62, 1527–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, T.; Kishi, T. Adenosine hypothesis in schizophrenia and bipolar disorder: A systematic review and meta-analysis of randomized controlled trial of adjuvant purinergic modulators. Schizophr. Res. 2013, 149, 88–95. [Google Scholar] [CrossRef]
- Lara, D.R.; Dall’Igna, O.P.; Ghisolfi, E.S.; Brunstein, M.G. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 617–629. [Google Scholar] [CrossRef]
- Rial, D.; Lara, D.R.; Cunha, R.A. The Adenosine Neuromodulation System in Schizophrenia. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 119, pp. 395–449. [Google Scholar]
- Boison, D.; Aronica, E. Comorbidities in Neurology: Is adenosine the common link? Neuropharmacology 2015, 97, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Borycz, J.; Pereira, M.F.; Melani, A.; Rodrigues, R.J.; Köfalvi, A.; Panlilio, L.; Pedata, F.; Goldberg, S.R.; Cunha, R.A.; Ferré, S. Differential glutamate-dependent and glutamate-independent adenosine A 1 receptor-mediated modulation of dopamine release in different striatal compartments. J. Neurochem. 2007, 101, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more D2 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tost, H.; Alam, T.; Meyer-Lindenberg, A. Dopamine and psychosis: Theory, pathomechanisms and intermediate phenotypes. Neurosci. Biobehav. Rev. 2010, 34, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Agnati, L.F.; Jacobsen, K.; Hillion, J.; Canals, M.; Torvinen, M.; Tinner-Staines, B.; Staines, W.; Rosin, D.; Terasmaa, A.; et al. Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson’s disease. Neurology 2003, 61, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Marcellino, D.; Leo, G.; Agnati, L.F. Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis. Curr. Opin. Pharmacol. 2010, 10, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Ferré, S.; Genedani, S.; Franco, R.; Agnati, L.F. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007, 92, 210–217. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Genedani, S.; Agnati, L. Adenosine A 2A receptors, dopamine D 2 receptors and their interactions in Parkinson’s disease. Mov. Disord. 2007, 22, 1990–2017. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Haydon, P.G. Astrocytic adenosine: From synapses to psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azdad, K.; Gall, D.; Woods, A.S.; Ledent, C.; Ferré, S.; Schiffmann, S.N. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D 2 receptor heteromerization. Neuropsychopharmacology 2009, 34, 972–986. [Google Scholar] [CrossRef] [Green Version]
- Phillis, J.W.; Scislo, T.J.; O’Leary, D.S. Purines and the nucleus tractus solitarius: Effects on cardiovascular and respiratory function. Clin. Exp. Pharmacol. Physiol. 1997, 24, 738–742. [Google Scholar] [CrossRef]
- Campbell, N.G.; Zhu, C.-B.; Lindler, K.M.; Yaspan, B.L.; Kistner-Griffin, E.; Hewlett, W.A.; Tate, C.G.; Blakely, R.D.; Sutcliffe, J.S. Rare coding variants of the adenosine A3 receptor are increased in autism: On the trail of the serotonin transporter regulome. Mol. Autism 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, S.H.; Jaafari, N.; Cimarosti, H.; Hanley, J.G.; Henley, J.M.; Mellor, J.R. Oxygen/glucose deprivation induces a reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptors. J. Neurosci. 2011, 31, 11941–11952. [Google Scholar] [CrossRef] [PubMed]
- Brunstein, M.G.; Silveira, E.M.; Chaves, L.S.; Machado, H.; Schenkel, O.; Belmonte-de-Abreu, P.; Souza, D.O.; Lara, D.R. Increased serum adenosine deaminase activity in schizophrenic receiving antipsychotic treatment. Neurosci. Lett. 2007, 414, 61–64. [Google Scholar] [CrossRef]
- Dutra, G.P.; Ottoni, G.L.; Lara, D.R.; Bogo, M.R. Lower frequency of the low activity adenosine deaminase allelic variant (ADA1*2) in schizophrenic patients. Rev. Bras. Psiquiatr. 2010, 32, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Cunha, R.; Ferre, S.; Vaugeois, J.-M.; Chen, J.-F. Potential Therapeutic Interest of Adenosine A2A Receptors in Psychiatric Disorders. Curr. Pharm. Des. 2008, 14, 1512–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Fuxe, K. Adenosine heteroreceptor complexes in the basal ganglia are implicated in Parkinson’s disease and its treatment. J. Neural Transm. 2019, 126, 455–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrich, J.; Schmitz, T.; Saft, C.; Postert, T.; Kraus, P.; Epplen, J.T.; Przuntek, H.; Agelink, M.W. Autonomic nervous system function in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Eren, O.E.; Ruscheweyh, R.; Schankin, C.; Schöberl, F.; Straube, A. The cold pressor test in interictal migraine patients—Different parasympathetic pupillary response indicates dysbalance of the cranial autonomic nervous system. BMC Neurol. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 8, 278. [Google Scholar] [CrossRef]
- Pintér, A.; Cseh, D.; Sárközi, A.; Illigens, B.; Siepmann, T. Autonomic Dysregulation in Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 16920–16952. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease—An update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Kim, Y.-K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryn, V.; Verkerk, R.; Skjeldal, O.H.; Saugstad, O.D.; Ormstad, H. Kynurenine Pathway in Autism Spectrum Disorders in Children. Neuropsychobiology 2017, 76, 82–88. [Google Scholar] [CrossRef] [PubMed]
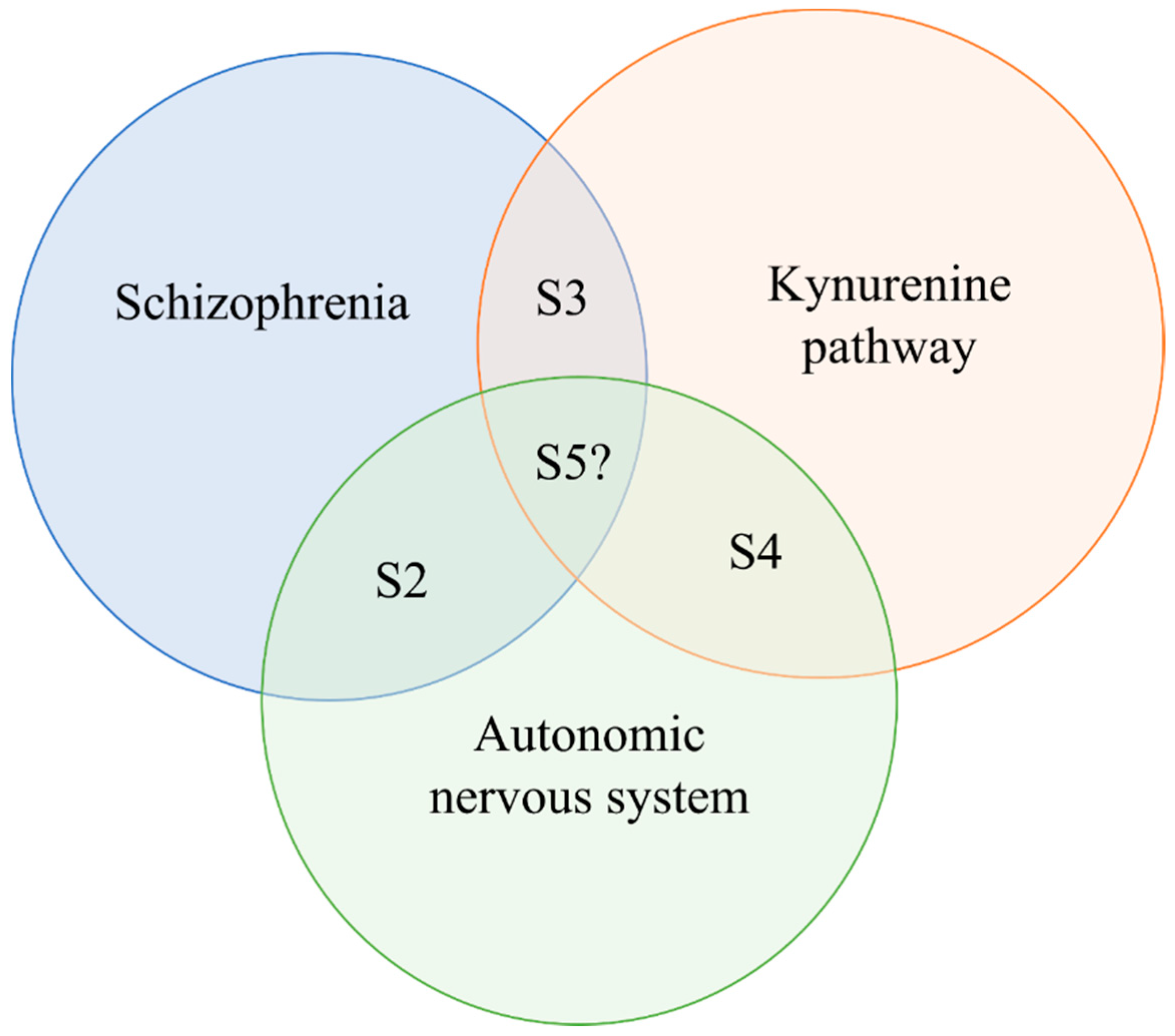
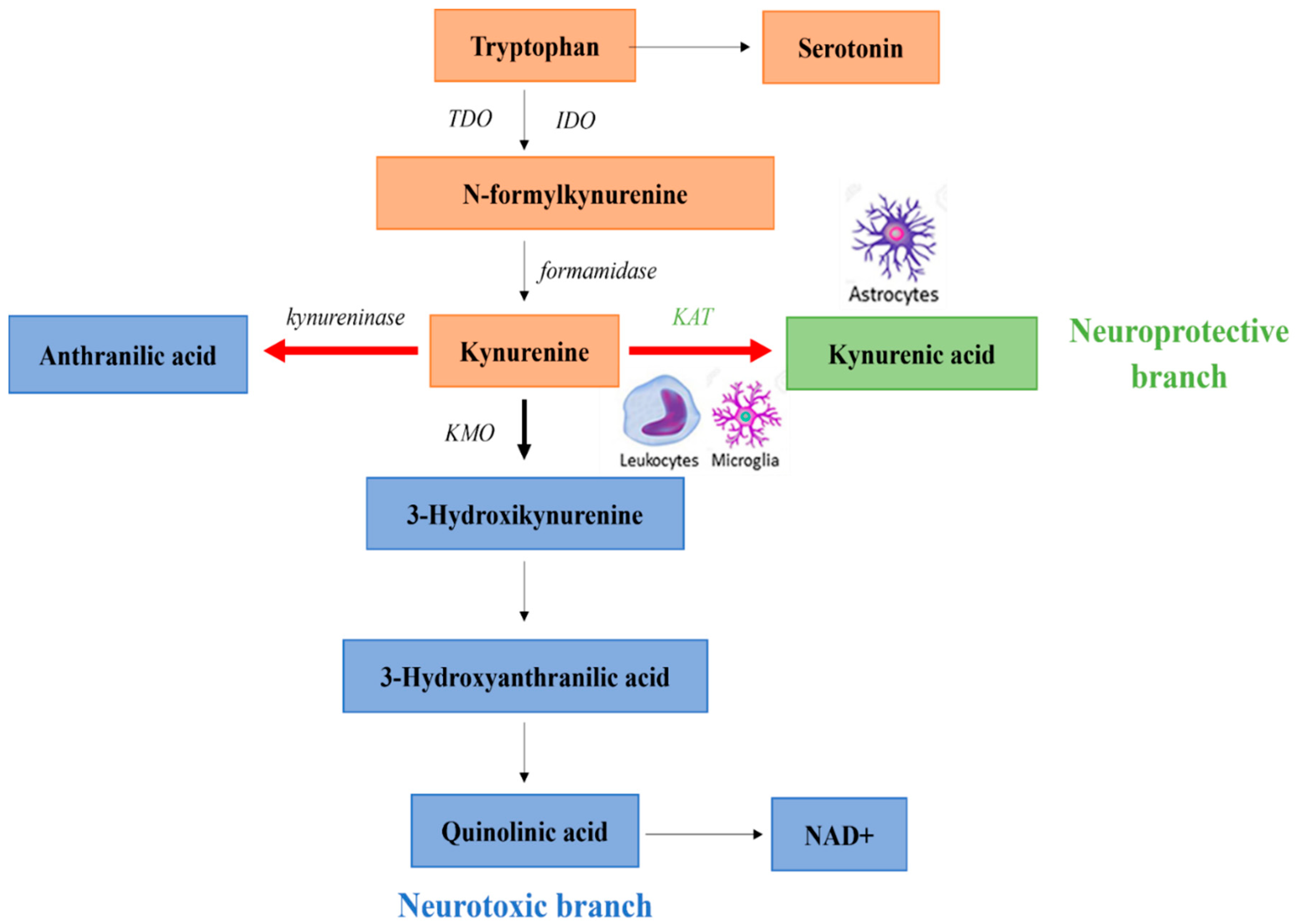
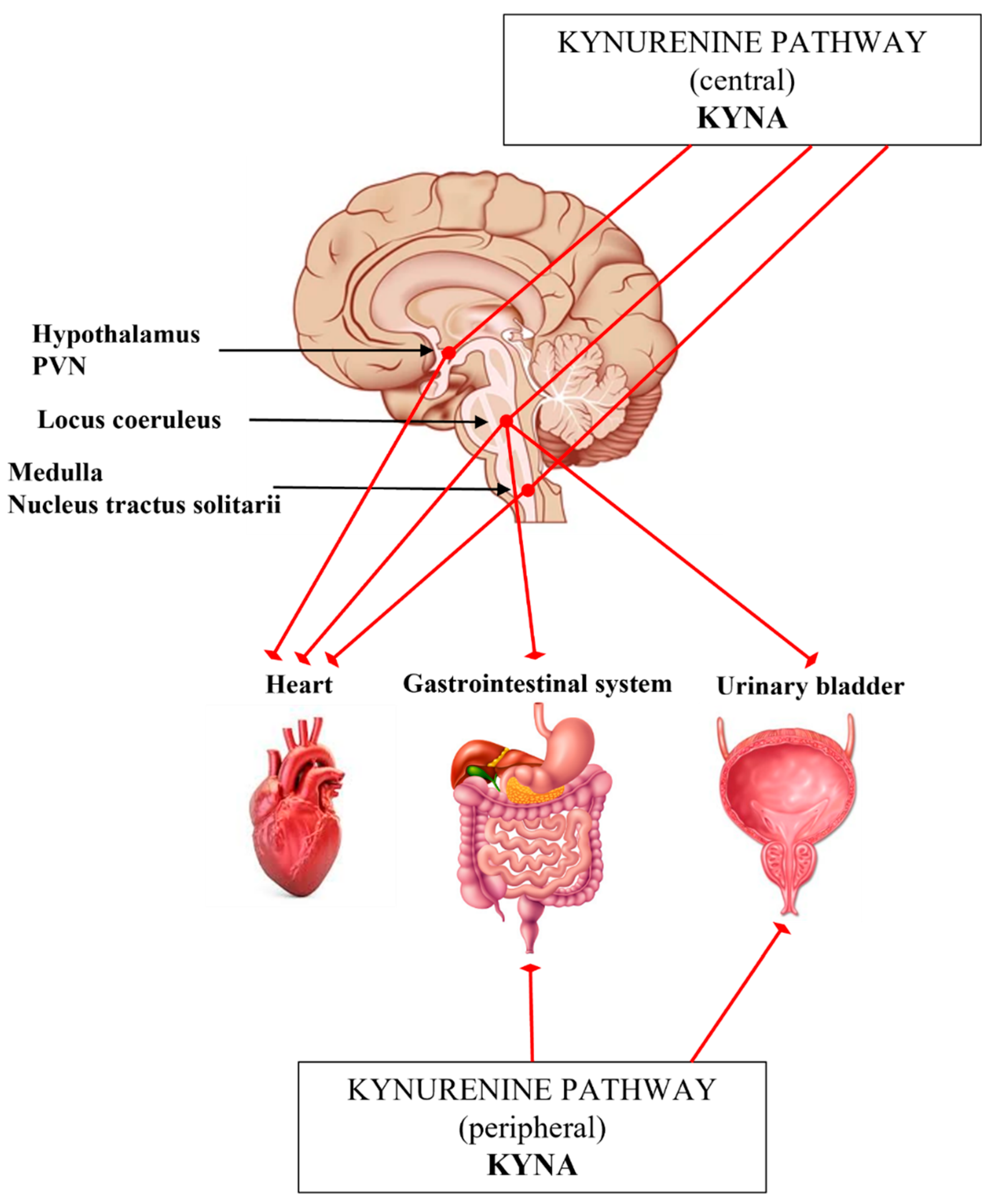
| ANS Assessment | Clinical Findings | References | Preclinical Findings | References |
|---|---|---|---|---|
| Blood pressure | Moderately higher systolic and diastolic blood pressure | [39] | ||
| Blood pressure variability | No changes | [39] | ||
| Heart rate variability | Reduced complexity | [7,46,47] | ||
| Thermoregulation | Abnormal diurnal variation of body temperature | [48] | PCP rat model: Acute PCP: hypothermia Chronic PCP: hyperthermia Perinatal PCP: long-lasting effect on the baseline temperature | [49,50] |
| Impaired ability to heat stress adaptation Higher skin conductance Disturbed sympathetic skin responses | [44,48,51,52,53,54,55,56,57,58,59,60] | Wisket rat model: Higher body temperature, Higher alteration rate | [61] | |
| Higher rate of sweating | [62] | |||
| Pupil function | Increased resting pupil diameter | [8] | Wisket rat model: Sedation—several parameters disturbed Anesthesia—prolonged constriction and redilation processes, blunted differences | [63] |
| A “sluggish” parasympathetic function by light | [64] | |||
| Decreased stimulus sensitivity | [12] | |||
| Gastrointestinal system | Increased tachygastria, arrhythmia | [62] | ||
| Delayed gastric emptying, diabetic gastroparesis | [62,65] | |||
| Correlation between salivary α-amylase level and psychiatric symptoms | [66] | |||
| Urinary system | Enhanced urinary frequency, urgency, incontinence and detrusor hyperreflexia | [67] | Wisket rat model: decreased bladder volume | [68] |
| Receptor | Activity | References |
|---|---|---|
| α7nAChRs | antagonist | [154,155,156,157] |
| AHR | agonist | [158] |
| AMPAR | antagonist | [148,149,150] |
| GPR35 | agonist | [30,32,159,160] |
| KAR | antagonist | [149,150] |
| NMDAR | antagonist | [121,149,150,157,161,162,163,164,165,166,167,168,169,170,171,172] |
| ANS Assessment | Preclinical Findings | References |
|---|---|---|
| Hypothalamus | HPA axis stimulates the formation of KYNA and other KP metabolites | [22,209,210,211,212,213,217] |
| KYNA in the PVN decreased mean arterial blood pressure and heart rate | [217] | |
| Brainstem | L-KYN decreases the elevated blood pressure | [219,220,221,222,223] |
| Chemoreflex: KYNA blocked the parasympathetic component (bradycardia), pressor response was reduced | [224] | |
| Gastrointestinal system | Activated LC by colon distension antagonized by administered KYNA | [207] |
| KYNA decreased the motility index Colitis model: KYNA and SZR-72 decreased the motility index, normalized the smooth muscle tone, had an inhibitory effect on the colitis-induced high reactive oxygen species activities | [33,225,226,227] | |
| Urinary system | KYNA prevented LC elicited by bladder distention KYNA inhibited the urinary bladder contractions evoked by stimulating the pontine micturition center | [228,229] |
| Molecule/Therapy | Action Mechanism | Effect | Preclinical Model | Ref. | Clinical Study | Ref. |
|---|---|---|---|---|---|---|
| 1-methyl-d-tryptophan | IDO inhibitor | Decreased neuroinflammation and oxidative stress | Ketamine-induced schizophrenia rat model | [130] | ||
| Allopurinol | TDO inhibitor | Antioxidant effect | Ketamine-induced schizophrenia rat model | [130] | ||
| Improved depressive-like behavior | Chronic stress-induced depression mice model | [261] | ||||
| d-cycloserine | KAT inhibitor | Improved cognitive functions | Schizophrenia | [262,263] | ||
| PF-04859989 | KAT inhibitor | Restored glutamate release events | Rat model exhibiting elevated KYNA levels | [264] | ||
| Angiotensin receptor blockers | KAT inhibitor | Reduced KYNA production | Rat cortical slice | [215,265,266,267] | ||
| Ro 61-8048 | KMO inhibitor | Neuroprotection | Gerbil and rat model of brain ischemia | [268] | ||
| Leucine | Large neutral amino acid transporter 1 blocker | Prevented systemic KYN from entering into the brain | Depression-like mice model | [198] | ||
| VNS | Anti-inflammatory, Improved heart rate variability, low moods and emotional symptoms | Depression | [269,270] | |||
| Anti-inflammatory via α7nAChRs | Schizophrenia | [247] | ||||
| Dipyridamol | Adenosine reuptake inhibitor | Greater improvement in positive symptoms and general psychopathology symptoms | Schizophrenia | [271] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büki, A.; Kekesi, G.; Horvath, G.; Vécsei, L. A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 10016. https://doi.org/10.3390/ijms221810016
Büki A, Kekesi G, Horvath G, Vécsei L. A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia. International Journal of Molecular Sciences. 2021; 22(18):10016. https://doi.org/10.3390/ijms221810016
Chicago/Turabian StyleBüki, Alexandra, Gabriella Kekesi, Gyongyi Horvath, and László Vécsei. 2021. "A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia" International Journal of Molecular Sciences 22, no. 18: 10016. https://doi.org/10.3390/ijms221810016







