Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies
Abstract
:1. Introduction
2. Results
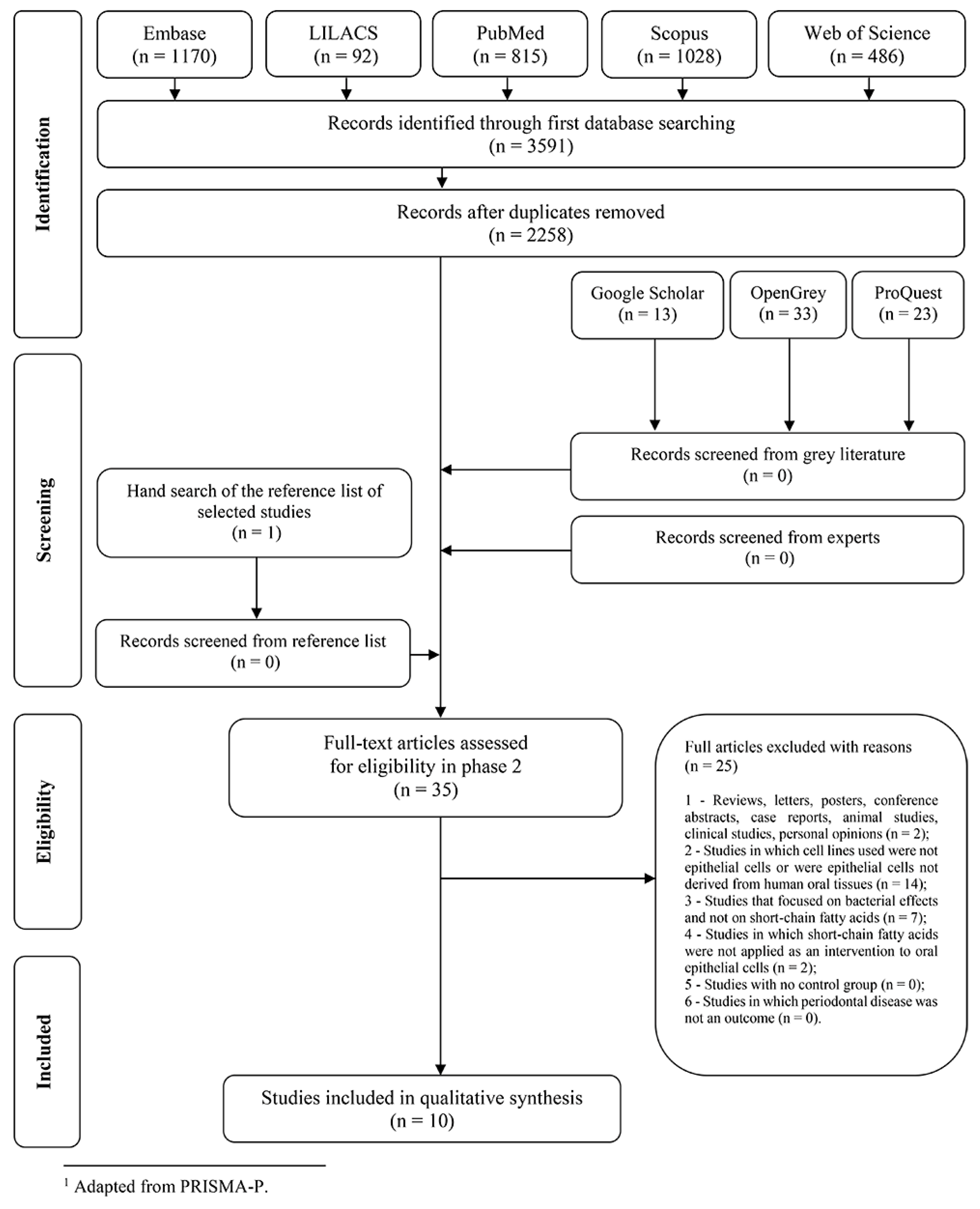
2.1. Selection of Studies and Screening Process
2.2. Characteristics of the Studies
2.3. Risk of Bias in Individual Studies
2.4. Synthesis of Results
2.5. Certainty in Cumulative Eevidence and Risk of Bias across Studies
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Focused Question
4.3. Eligibility Criteria
- Studies in which other cells apart from human oral epithelial cells were used, such as cells of animal origin, from other organs (lung, skin, liver, kidney, colon, etc.), and derived from other tissues (fibroblasts, neutrophils, macrophages, etc.);
- Studies in which SCFA were not used as a treatment to epithelial cells or studies without a control group;
- Studies in which the molecular and cellular effects of SCFA on cells potentially related to periodontal disease were not evaluated;
- Clinical and animal studies;
- Pilot studies and research projects.
4.4. Search Strategy
4.5. Selection of Studies
4.6. Data Extraction
4.7. Assessment of Risk of Bias in Individual Studies
4.8. Summary Measures and Synthesis of Results
4.9. Grading of Cumulative Evidence and Risk of Bias Across Studies
5. Conclusions
- SCFA impair the viability of oral epithelial cells at mM concentrations via apoptosis, autophagy, and pyroptosis.
- SCFA impair the integrity and presumably the transmigration of leucocytes through the epithelial layer by changing junctional and adhesion protein expression, respectively.
- SCFA affect the expression of chemokines and cytokines in oral epithelial cells.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodontal Res. 1991, 26 Pt 2, 230–242. [Google Scholar] [CrossRef]
- Scott, D.A.; Krauss, J. Neutrophils in periodontal inflammation. Front. Oral Biol. 2012, 15, 56–83. [Google Scholar] [PubMed] [Green Version]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.; Eftimiadi, C.; Damiani, G.; Buffa, P.; Buffa, D.; Botta, G.A. Short chain fatty acids present in periodontal pockets may play a role in human periodontal diseases. J. Periodontal Res. 1987, 22, 190–191. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Lu, R.; Meng, H.; Gao, X.; Xu, L.; Feng, X. Effect of non-surgical periodontal treatment on short chain fatty acid levels in gingival crevicular fluid of patients with generalized aggressive periodontitis. J. Periodontal Res. 2014, 49, 574–583. [Google Scholar] [CrossRef]
- Cueno, M.E.; Ochiai, K. Re-discovering periodontal butyric acid: New insights on an old metabolite. Microb. Pathogen. 2015, 94, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Meng, S.; Wu, J.; Johnson, J.; Tang, R.; Hodin, R. Butyrate inhibits colon carcinoma cell growth through two distinct pathways. Surgery 1998, 124, 248–253. [Google Scholar] [CrossRef]
- Roy, M.J.; Dionne, S.; Marx, G.; Qureshi, I.; Sarma, D.; Levy, E.; Seidman, E.G. In vitro studies on the inhibition of colon cancer by butyrate and carnitine. Nutrition 2009, 25, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Pattayil, L.; Balakrishnan-Saraswathi, H.T. Evaluation of Apoptotic Induction of Butyric Acid Derivatives in Colorectal Carcinoma Cells. Anticancer Res. 2019, 39, 3795–3801. [Google Scholar] [CrossRef]
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.E.; Persson, G.R. The impact of the stone age diet on gingival conditions in the absence of oral hygiene. J. Periodontol. 2009, 80, 759–768. [Google Scholar] [CrossRef]
- Hujoel, P. Dietary carbohydrates and dental-systemic diseases. J. Dent Res. 2009, 88, 490–502. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Van Esch, B.C.A.M.; Henricks, P.A.J.; Garssen, J.; Folkerts, G. Time and concentration dependent effects of short chain fatty acids on lipopolysaccharide- or Tumor Necrosis Factor α-induced endothelial activation. Front. Pharmacol. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, H.G.; Takeo Sato, F.; Curi, R.; Vinolo, M.A.R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 2016, 785, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Mamoto, R.; Yamaguchi, S.; Nishida, M.; Ito, M.; Fujioka, Y. Butyrate attenuates lipolysis in adipocytes co-cultured with macrophages through non-prostaglandin E2-mediated and prostaglandin E2-mediated pathways. Lipids Health Dis. 2016, 15, 213. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Encarnação, J.C.; Abrantes, A.M.; Pires, A.S.; Botelho, M.F. Revisit dietary fiber on colorectal cancer: Butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015, 34, 465–478. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Petito, V.; Lopetuso, L.; Bruno, G.; Gerardi, V.; Ianiro, G.; Sgambato, A.; Gasbarrini, A.; Cammarota, G. Pre- and posttherapy assessment of intestinal soluble mediators in IBD: Where we stand and future perspectives. Mediators Inflamm. 2013, 2013, 391473. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, M.; Wicksteed, B.; Schiltz, G.E.; Gilchrist, A.; Layden, B.T. SCFA Receptors in Pancreatic β Cells: Novel Diabetes Targets? Trends Endocrinol. Metab. 2016, 27, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fiber intake and risk of first stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, R.E.; Buckner, B.A. Butyrate and propionate: Important components of toxic dental plaque extracts. Infect. Immun. 1981, 32, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Shirasugi, M.; Nishioka, K.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Normal human gingival fibroblasts undergo cytostasis and apoptosis after long-term exposure to butyric acid. Biochem. Biophys. Res. Commun. 2017, 482, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, A.W.; Wentz, F.M.; Orban, B. Dimensions and Relations of the Dentogingival Junction in Humans. J. Periodontol. 1961, 32, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Magrin, G.L.; Di Summa, F.; Strauss, F.J.; Panahipour, L.; Mildner, M.; Magalhães Benfatti, C.A.; Gruber, R. Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 1679. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Meng, H.; Yu, J.; Lu, H.; Li, W.; Lu, R.; Zhao, Y.; Li, Q.; Su, L. Butyrate rather than LPS subverts gingival epithelial homeostasis by downregulation of intercellular junctions and triggering pyroptosis. J. Clin. Periodontol. 2019, 46, 894–907. [Google Scholar] [CrossRef]
- Pöllänen, M.T.; Salonen, J.I. Effect of short chain fatty acids on human gingival epithelial cell keratins in vitro. Eur. J. Oral Sci. 2000, 108, 523–529. [Google Scholar] [CrossRef]
- Takigawa, S.; Sugano, N.; Nishihara, R.; Koshi, R.; Murai, M.; Yoshinuma, N.; Ochiai, K.; Ito, K. The effect of butyric acid on adhesion molecule expression by human gingival epithelial cells. J. Periodontal Res. 2008, 43, 386–390. [Google Scholar] [CrossRef]
- Takigawa, S.; Sugano, N.; Ochiai, K.; Arai, N.; Ota, N.; Ito, K. Effects of sodium bicarbonate on butyric acid-induced epithelial cell damage in vitro. J. Oral Sci. 2008, 50, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, Y.; Kikuchi, K.; González-Alva, P.; Inoue, H.; Noguchi, Y.; Tsuchiya, H.; Hayashi, J.; Shin, K.; Ochiai, K.; Kusama, K. Association of butyric acid produced by periodontopathic bacteria with progression of oral cancer. J. Cancer Sci. Ther. 2010, 2, 26–32. [Google Scholar]
- Evans, M.; Murofushi, T.; Tsuda, H.; Mikami, Y.; Zhao, N.; Ochiai, K.; Kurita-Ochiai, T.; Yamamoto, M.; Otsuka, K.; Suzuki, N. Combined effects of starvation and butyrate on autophagy-dependent gingival epithelial cell death. J. Periodontal Res. 2017, 52, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kashket, S. Cytotoxic effects of short-chain carboxylic acids on human gingival epithelial cells. Oral Microbiol. Immunol. 1997, 12, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, B.C.; Niederman, R. Short chain carboxylic acids decrease human gingival keratinocyte proliferation and increase apoptosis and necrosis. J. Clin. Periodontol. 1998, 25, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Gardner, M.; Higgins, J.; Holly, J.M.P.; Gaunt, T.R.; Perks, C.M.; Turner, S.D.; Rinaldi, S.; Thomas, S.; Harrison, S.; et al. Developing the WCRF International/University of Bristol Methodology for Identifying and Carrying Out Systematic Reviews of Mechanisms of Exposure-Cancer Associations. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1667–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touw, J.J.A.; Van Steenbergen, T.J.M.; De Graaff, J. Butyrate: A cytotoxin for Vero cells produced by Bacteroides gingivalis and Bacteroides asaccharolyticus. Antonie van Leeuwenhoek 1982, 48, 315–325. [Google Scholar] [CrossRef]
- Levine, M. The role for butyrate and propionate in mediating HeLa-cells growth inhibition by human dental plaque fluid from adult periodontal disease. Arch. Oral Biol. 1985, 30, 155–159. [Google Scholar] [CrossRef]
- Pöllänen, M.T.; Overman, D.O.; Salonen, J.I. Bacterial metabolites sodium butyrate and propionate inhibit epithelial cell growth in vitro. J. Periodontal Res. 1997, 32, 326–334. [Google Scholar] [CrossRef]
- Shah, H.N.; Gharbia, S.E.; O’Toole, C.M. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J. Periodontol. 1992, 63, 44–51. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Ochiai, K.; Suzuki, N.; Otsuka, K.; Fukushima, K. Human gingival fibroblasts rescue butyric acid-induced T-cell apoptosis. Infect. Immun. 2002, 70, 2361–2367. [Google Scholar] [CrossRef] [Green Version]
- Kurita-Ochiai, T.; Seto, S.; Suzuki, N.; Yamamoto, M.; Otsuka, K.; Abe, K.; Ochiai, K. Butyric acid induces apoptosis in inflamed fibroblasts. J. Dent Res. 2008, 87, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.P.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.O.; Vieira, A.; Sernaglia, E.M.; Lancellotti, M.; Vieira, A.T.; Avila-Campos, M.J.; Rodrigues, H.G.; Vinolo, M.A.R. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell. Microbiol. 2017, 19, e12720. [Google Scholar] [CrossRef] [Green Version]
- Eftimiadi, C.; Stashenko, P.; Tonetti, M.; Mangiante, P.E.; Massara, R.; Zupo, S.; Ferrarini, M. Divergent effect of the anaerobic bacteria by-product butyric acid on the immune response: Suppression of T-lymphocyte proliferation and stimulation of interleukin-1 beta production. Oral Microbiol. Immunol. 1991, 6, 17–23. [Google Scholar] [CrossRef]
- Seto, S.; Kurita-Ochiai, T.; Ochiai, K. Increased susceptibility to tumor necrosis factor-alpha in butyric acid-induced apoptosis is caused by downregulation of cFLIP expression in Jurkat T cells. Microbiol. Immunol. 2008, 52, 188–196. [Google Scholar] [CrossRef]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal. Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Mikami, D.; Uwada, J.; Yazawa, T.; Kamiyama, K.; Kimura, H.; Taniguchi, T.; Iwano, M. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 2018, 9, 31342–31354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhou, Z.; Hu, Y.; Dong, S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J. Genet. Genomics 2012, 39, 375–384. [Google Scholar] [CrossRef]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef]
- Niederman, R.; Buyle-Bodin, Y.; Lu, B.-Y.; Robinson, P.; Naleway, C. Short-chain carboxylic acid concentration in human gingival crevicular fluid. J. Dent. Res. 1997, 76, 575–579. [Google Scholar] [CrossRef]
- Merchant, A.T.; Pitiphat, W.; Franz, M.; Joshipura, K.J. Whole-grain and fiber intakes and periodontitis risk in men. Am. J. Clin. Nutr. 2006, 83, 1395–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jiang, C.; Liu, G.; Wang, P.; Shi, M.; Yang, M.; Zhong, Z.; Ding, S.; Li, Y.; Liu, B.; et al. Sodium butyrate protects against oxidative stress in human nucleus pulposus cells via elevating PPARgamma-regulated Klotho expression. Int. Immunopharmacol. 2020, 85, 106657. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, N.; Matei, N.; McBride, D.W.; Ding, Y.; Liang, H.; Tang, J.; Zhang, J.H. Sodium butyrate attenuated neuronal apoptosis via GPR41/Gbetagamma/PI3K/Akt pathway after MCAO in rats. J. Cereb. Blood Flow Metab. 2020, 271678X20910533. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral butyrate for mildly to moderately active Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Butzner, J.D.; Parmar, R.; Bell, C.J.; Dalal, V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut 1996, 38, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, S.; Omata, Y.; Hofmann, J.; Bottcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Kronke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 52–69. [Google Scholar] [CrossRef] [Green Version]
- Sukseree, S.; Schwarze, U.Y.; Gruber, R.; Gruber, F.; Quiles Del Rey, M.; Mancias, J.D.; Bartlett, J.D.; Tschachler, E.; Eckhart, L. ATG7 is essential for secretion of iron from ameloblasts and normal growth of murine incisors during aging. Autophagy 2020, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Fan, M.; Yu, R.; Zhang, Y.; Liu, J.; Zhou, X.; Cai, Y.; Huang, S.; Hu, Z.; et al. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 2020, 34, 4266–4282. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zeng, C.; Liu, J.; Yuan, L.; Liu, W.; Wang, L.; Zhu, H.; Xu, Y.; Luo, Y.; Xie, D.; et al. Sodium butyrate induces autophagic apoptosis of nasopharyngeal carcinoma cells by inhibiting AKT/mTOR signaling. Biochem. Biophys Res. Commun. 2019, 514, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ke, X.; Yan, F.; Lei, L.; Li, H. Necroptosis in the periodontal homeostasis: Signals emanating from dying cells. Oral Dis. 2018, 24, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Gao, H.; Liu, H.; Wu, B.; Zhang, B.; Gu, M.; Yang, W. Chidamide induces necroptosis via regulation of cFLIPL expression in Jurkat and HUT78 cells. Mol. Med. Rep. 2020, 21, 936–944. [Google Scholar] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; John Wiley & Sons, Ltd.: England, UK, 2011. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needleman, I.G. A guide to systematic reviews. J. Clin. Periodontol. 2011, 29 (Suppl. 3), 6–9, discussion 37–38. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Pavan, L.M.; Rêgo, D.F.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N. In vitro Anti-Tumor Effects of Statins on Head and Neck Squamous Cell Carcinoma: A Systematic Review. PLoS ONE 2015, 10, e0130476. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author (Year); Country | Cell Type (Origin) | Treatment/ Concentration | Treatment Regime | Methods: Assays | Results | Main Conclusions | Clinical Application |
|---|---|---|---|---|---|---|---|
| Evans et al. (2017); Japan [42] | Ca9-22 (HOSCC) | Sodium butyrate/ 5 mM; RPMI1640 (control) | 48 h incubation a; 24 h incubation b; 0, 2, 4 and 6 h incubation c; 0, 2, 4 and 8 h incubation d | SYTOX Green cell death assay a; Caspase-3 assay b; Caspase-3/7 assay b; qRT-PCR analysis c; Western blot b,d | Butyrate induced cell death in starved and sufficient nutrient conditions.Although butyrate treatment induced about 10 times higher caspase-3 activity (as a measure for apoptosis) compared to the non-treated cells, butyrate-induced apoptosis accounted for only 29.4 or 12.3% of total cell death induced by butyrate in the starved and fed conditions, respectively.The combined effects of histone H3 acetilatilation, AMPK activation, and LC3 upregulation during starvation and butyrate exposure resulted in induction of cell autophagy. | Starvation enhances butyrate induced cell death and autophagy of gingival epithelial cells. | The combined stimuli of butyrate exposure and cell starvation is may be involved in tissue destruction at the dentogingival junction. |
| Liu et al. (2019); China [37] | Primary gingival epithelial cells (freshly isolated) | Sodium butyrate/ 10 mM; LPS/ 1 μg/mL; KGM (control) | 48 h incubation | Transepithelial electrical resistance; FITC-dextran transport assay; Flow cytometry; qRT-PCR analysis; Western blot; Immunohisto-chemestry; Immuno-cytochemistry; Im-munofluorescence; FE-SEM; TEM | Butyrate had a stronger effect on cell membrane damage than LPS, altered cell morphology, increased cell death and down regulated the intercellular junction markers. Sodium butyrate, rather than LPS, increased inflammatory chemokines of periodontitis, such as IL8 and MCP1, suggesting a role as virulence factor in gingival epithelial cells. During butyrate treatment, mitochondria lost their morphologies, which may indicate a triggering for the cascade of cell pyroptosis. | Butyrate rather than LPS subverts the gingival epithelial barrier function by triggering gingival epithelial cell pyroptosis and downregulating the expression of intercellular junction proteins. | Butyrate acts in the destruction of the gingival epithelial barrier, and may play a role in initiating periodontitis. |
| Magrin et al. (2020); Austria [36] | HSC-2 (HOSCC); TR146 (HOSCC); Primary gingival epithelial cells (freshly isolated) | Acetate/ 10 mM; Propionate/ 10 mM; Butyrate/ 10 mM; DMEM (control) | 24 h incubation, 3 h exposure to TNFα + IL1β a; 24 h incubation (butyrate only), 3 h exposure to TNFα + IL1β b; 24 h incubation (butyrate only), 30 min exposure to TNFα + IL1β c; 1 h exposure to TNFα + IL1β, 3 h incubation (butyrate only) d | MTT assay; qRT-PCR analysis a,d; Western blot b; Immuno-fluorescence c | Butyrate suppressed in a dose-dependent manner the cytokine-induced ICAM1 expression in HSC-2 and primary gingival epithelial cells but not in TR146 cells. Acetate and propionate failed to cause a significant suppression of cytokine-induced ICAM1 expression. Butyrate inhibited the nuclear translocation of p65 on HSC-2 cells. Butyrate failed to reverse cytokine-induced ICAM1 increase in HSC-2 cells for an acute inflammation protocol. | Butyrate but not acetate or propionate attenuates the cytokine-induced ICAM1 expression in oral epithelial cells. | Butyrate can modulate epithelial cell responses in the inflamed periodontium and thereby possibly influencing the ICAM1-dependent transmigration of leucocytes and immune cells. |
| Miyazaki et al. (2010); Japan [41] | Ca9-22 (HOSCC); HSC-2 (HOSCC); HSC-3 (HOSCC); HSC-2 (HOSCC) | Sodium butyrate/ 0.3, 2.5 and 20 mM; RPMI1640 (control) | 24 h incubation; 8 h incubation a; 4, 8, 12 and 24 h incubation b | MTT assay; “Scratch” assay a; qRT-PCR analysis b; Western blot | The proliferative activities of HSC-2,-3 and -4 cells decreased with butyrate in a dose-dependent manner, whereas in Ca9-22 cells slightly increased in 0.3 mM concentration. Expression of podoplanin (oral cancer biomarker) was enhanced by butyrate in HSC-2 and HSC-3 cells. Cell migration was inhibited in the presence of butyrate for HSC-2 and HSC-4 cells. | Sodium butyrate increases podoplanin expression and cell migration in certain HOSCC cell lines, suggesting that the progression of periodontal disease may promote the progression of oral squamous cell carcinomas via a podoplanin-dependent pathway. | It is suggested an association of butyrate produced by periodontopathic bacteria with the progression of oral cancers. |
| Pöllänen & Salonen (2000); Finland [38] | Immortalized human oral epithelial cells (gingival keratinocytes); Primary gingival epithelial cells (freshly isolated) | Sodium butyrate/ 8 mM; Sodium propionate/ 8 mM; KBM (control) | 24 h incubation | Western blot; Immunohisto-chemestry | Propionate did not affected keratinocyte cell numbers, whereas butyrate reduced cell numbers by about 30%. The expression of the cytoplasmic keratin K17 was markedly increased with propionate and especially butyrate, further confirmed in primary gingival epithelial cells. | Butyrate and propionate increase the relative amount of keratin proteins in the cells, most strikingly keratin K17, a protein related to periodontal pocket formation. | The increased expression of K17 after SCFA exposure may contribute to detachment of the junctional epithelium from tooth surface and to the formation of periodontal pockets. |
| Sorkin & Niederman (1998); USA [44] | Immortalized human oral epithelial cells (HPV-transformed cells) | “Mixed SCFA” (butyric, propionic, acetic and lactic acids)/ up to 20 mM in a mixture of equimolar concentrations of each acid; KGM or KGM + CaMg medium (control) | 30 h incubation a; 9 days incubation (medium changed every other day) b; 2, 8 and 48 h incubation c | Cell proliferation assay a; Cell survival assay b; ELISA assay c | SCFA decreased cell proliferation and cell survival in a dose-dependent manner. At all time points, ELISA assay showed more DNA in the cytoplasmic extracts and in the supernatants, suggesting that many of the cells may be initiating apoptotic cell death. | SCFA decrease gingival epithelial cell proliferation and increase apoptosis and necrosis. These effects were dose- and acid-dependent. | By decreasing the proliferative capacity of the gingival epithelium, SCFA could increase epithelial permeability over time, increasing crevicular fluid flow, and bacterial penetration. |
| Takigawa et al. (2008a); Japan [39] | Ca9-22 (HOSCC) | Butyric acid/ 3 mM; MEM (control) | 24 h incubation; 0 to 7 days incubation a; 6 h incubation b | SEM; Flow cytometry; Cell proliferation assay a; qRT-PCR analysis b | Cell growth was inhibited in a dose-dependent manner after butyric acid treatment. Butyric acid exposure for 6 h increased ICAM1 expression, decreased integrin α6 and β4 levels, and reduced cell viability. Moreover, morphological changes were observed after 24 h. | Butyric acid inhibits cell growth, reduces cell viability, suppress integrin levels and alters the expression of ICAM1 in gingival epithelial cells. | Butyric acid in periodontal pockets may augment inflammatory cell migration and disable the tight attachment among epithelial cells, leading to bacterial invasion and periodontal damage. |
| Takigawa et al. (2008b); Japan [40] | Ca9-22 (HOSCC) | Butyric acid/ 3 mM; Butyric acid + NaHCO3/ 3mM; MEM (control) | 24 h incubation; 6 and 24 h incubation a | SEM; Flow cytometry; Trypan blue exclusion assay (cell viability) a; qRT-PCR analysis a | Cell viability after butyric acid exposure was lower than that of butyric acid plus NaHCO3. ICAM1 expression was increased with butyric acid alone and suppressed in the presence of NaHCO3 after 6 h incubation. However, no differences were detected after 24 h. | NaHCO3 improves cell viability and inhibit ICAM1 expression increasing in butyric acid treated cells. | NaHCO3 may have a useful therapeutic application to reduce butyric acid damage on periodontal tissue. |
| Tsuda et al. (2010); Japan [33] | Ca9-22 (HOSCC) | Sodium butyrate/ 10 mM; RPMI1640 (control) | 48 h incubation; 24 h incubation a; 0, 4, 8, 12 and 24 h incubation b; 2, 4 and 8 h incubation c; 8 h incubation d | Microscopic observation; SYTOX Green cell death assay; Annexin V–FITC assay; Caspase-3 assay a; qRT-PCR analysis b; Western blot c; Fluorescence microscopy d | Butyrate stimulation induced apoptotic cell death in a dose- and time-dependent manner. Treatment with 3-methyl-adenine and CA-074 Me (proteins of cell autophagy), further confirmed with LC3 tests, suppressed butyrate-induced cell death, sugges-ting an autophagic pathway. | Butyrate induces the death of gingival epithelial cells primarily via caspase-independent autophagy and partly via apoptosis. | Butyrate may play an important role in killing gingival epithelial cells and breakdown the integrity of the front-line epithelial barrier of gingival tissues. |
| Zhang & Kashket (1997); USA [43] | Immortalized human oral epithelial cells (HPV-transformed cells) | “Mixed SCFA” (acetic, formic, lactic and propionic acids)/ up to 100 mM in a mixture of equimolar concentrations of each acid; NaCl (salt control); KGM (control) | 2, 16, 24, 40, 48 and up to 64 h incubation; 16 h incubation, change medium to KGM for another 16, 24, 40 or 48 h incubation (reversibility protocol) | MTT assay; Trypan blue exclusion assay | Cell growth was completely inhibited in the presence of 50 mM or above of mixed SCFA. Few cells were dead in the control or in cultures with up to 20 mM mixed SCFA. Cells exposed to 12.5 mM SCFA or less began to grow shortly after acids removal. Cells exposed to 25 mM mixed SCFA began to grow only after a recovery period of about 16 h. | Cell growth is progressively inhibited with increasing concentrations of SCFA. | SCFA can damage the integrity of gingival epithelium in situ. |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Total (% Score Yes) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Evans et al. (2017) [42] | Yes | Unclear | No | Yes | Yes | Not applicable | 50,0 | Moderate |
| Liu et al. (2019) [37] | Yes | Yes | Yes | Yes | Yes | Not applicable | 83,3 | Low |
| Magrin et al. (2020) [36] | Unclear | Yes | Yes | Yes | Unclear | No | 50,0 | Moderate |
| Miyazaki et al. (2010) [41] | Yes | Unclear | No | Yes | Yes | Yes | 66,6 | Moderate |
| Pöllänen & Salonen (2000) [38] | Yes | Unclear | Yes | Yes | Unclear | No | 50,0 | Moderate |
| Sorkin & Niederman (1998) [44] | Unclear | Yes | No | No | Yes | Not applicable | 33,3 | High |
| Takigawa et al. (2008a) [39] | Yes | Yes | No | Yes | Yes | Not applicable | 66,6 | Moderate |
| Takigawa et al. (2008b) [40] | Yes | No | No | Yes | Yes | Not applicable | 50,0 | Moderate |
| Tsuda et al. (2010) [33] | Yes | Yes | No | Yes | Unclear | Not applicable | 50,0 | Moderate |
| Zhang & Kashket (1997) [43] | Unclear | Yes | No | No | Yes | Not applicable | 33,3 | High |
| Outcomes | Impact | Number of Studies | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| SCFA induce cell death of human oral epithelial cells | Increase in periodontal epithelial barrier destruction | 8 studies | ⊕⊕◯◯ LOW a,b,c |
| SCFA modulate intercellular junction proteins expression | Contribute to epithelial detachment and periodontal pocket formation | 3 studies | ⊕⊕◯◯ LOW a,b,e |
| SCFA influence the transmigration of leucocytes and immune cells | Modulation on immune cell migration | 3 studies | ⊕◯◯◯ VERY LOW a,b,c,d,e |
| Butyrate increases podoplanin expression in human oral epithelial cells | Butyrate produced by periodontopathic bacteria is associated with the progression of oral cancers | 1 study | ⊕◯◯◯ VERY LOW b,e,f,g |
| Sodium bicarbonate reduces butyric acid detrimental effects | Sodium bicarbonate have a useful therapeutic application to reduce butyric acid damage on periodontal tissue | 1 study | ⊕◯◯◯ VERY LOW b,c,e,f,g |
| PICOS | |
|---|---|
| Participants | Human oral epithelial cells |
| Intervention | Short-chain fatty acids |
| Comparison | No treatment with short-chain fatty acids (control) |
| Outcomes | Molecular and cellular parameters related to periodontal disease * |
| Types of Studies included | In vitro studies |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magrin, G.L.; Strauss, F.J.; Benfatti, C.A.M.; Maia, L.C.; Gruber, R. Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 4895. https://doi.org/10.3390/ijms21144895
Magrin GL, Strauss FJ, Benfatti CAM, Maia LC, Gruber R. Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies. International Journal of Molecular Sciences. 2020; 21(14):4895. https://doi.org/10.3390/ijms21144895
Chicago/Turabian StyleMagrin, Gabriel Leonardo, Franz Josef Strauss, Cesar Augusto Magalhães Benfatti, Lucianne Cople Maia, and Reinhard Gruber. 2020. "Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies" International Journal of Molecular Sciences 21, no. 14: 4895. https://doi.org/10.3390/ijms21144895







