Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion
Abstract
1. Introduction
2. Results
2.1. Physiology
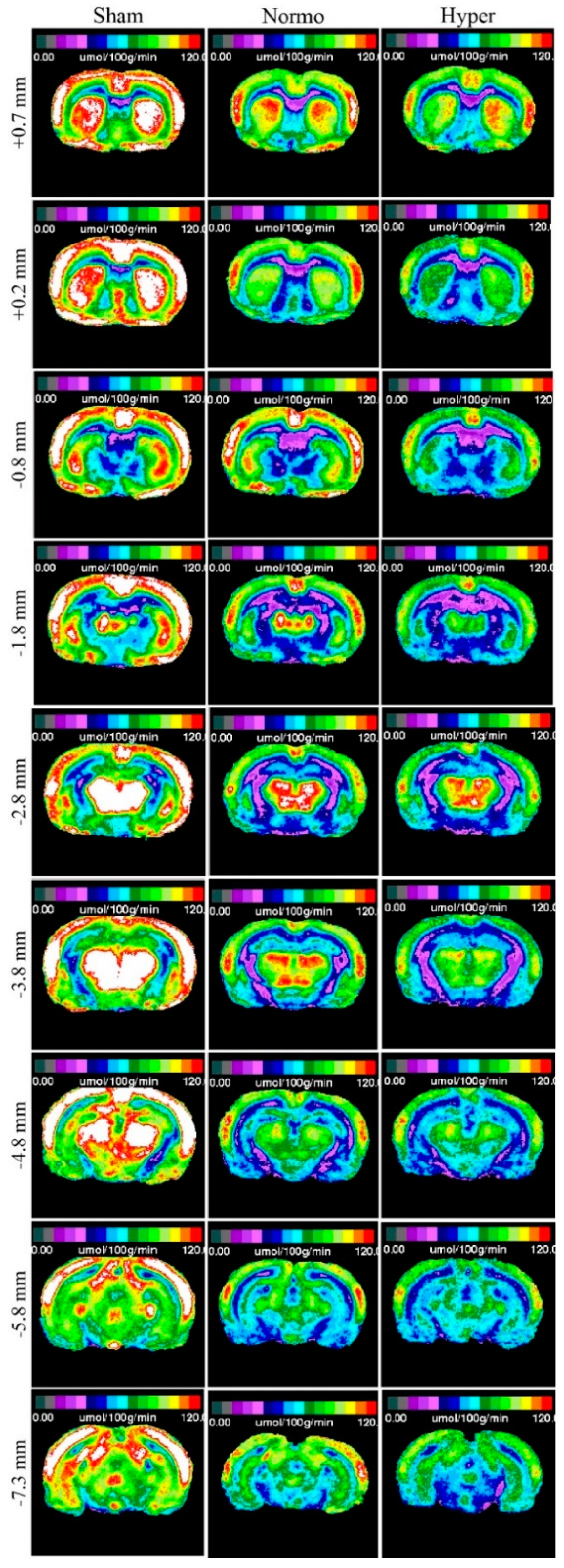
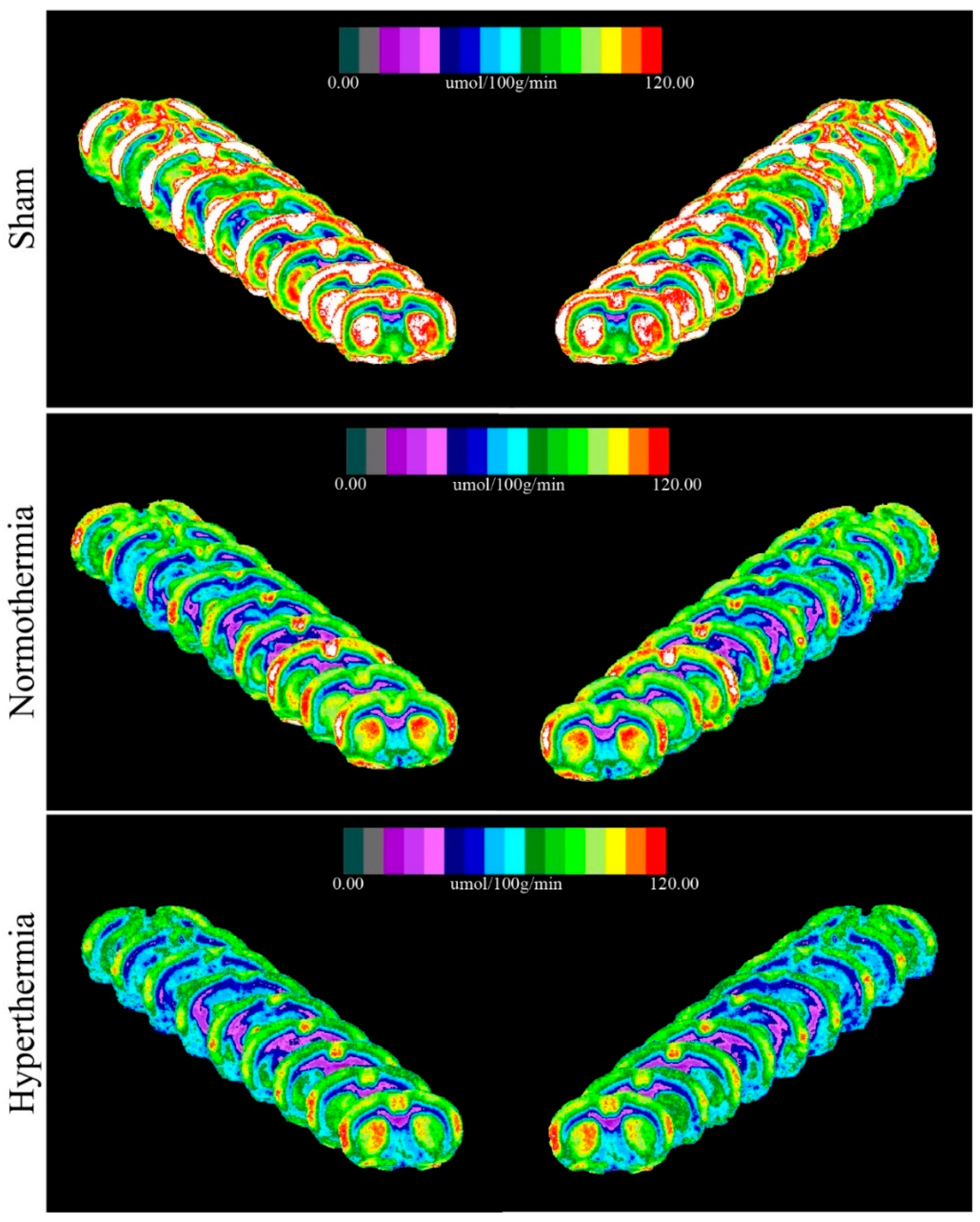
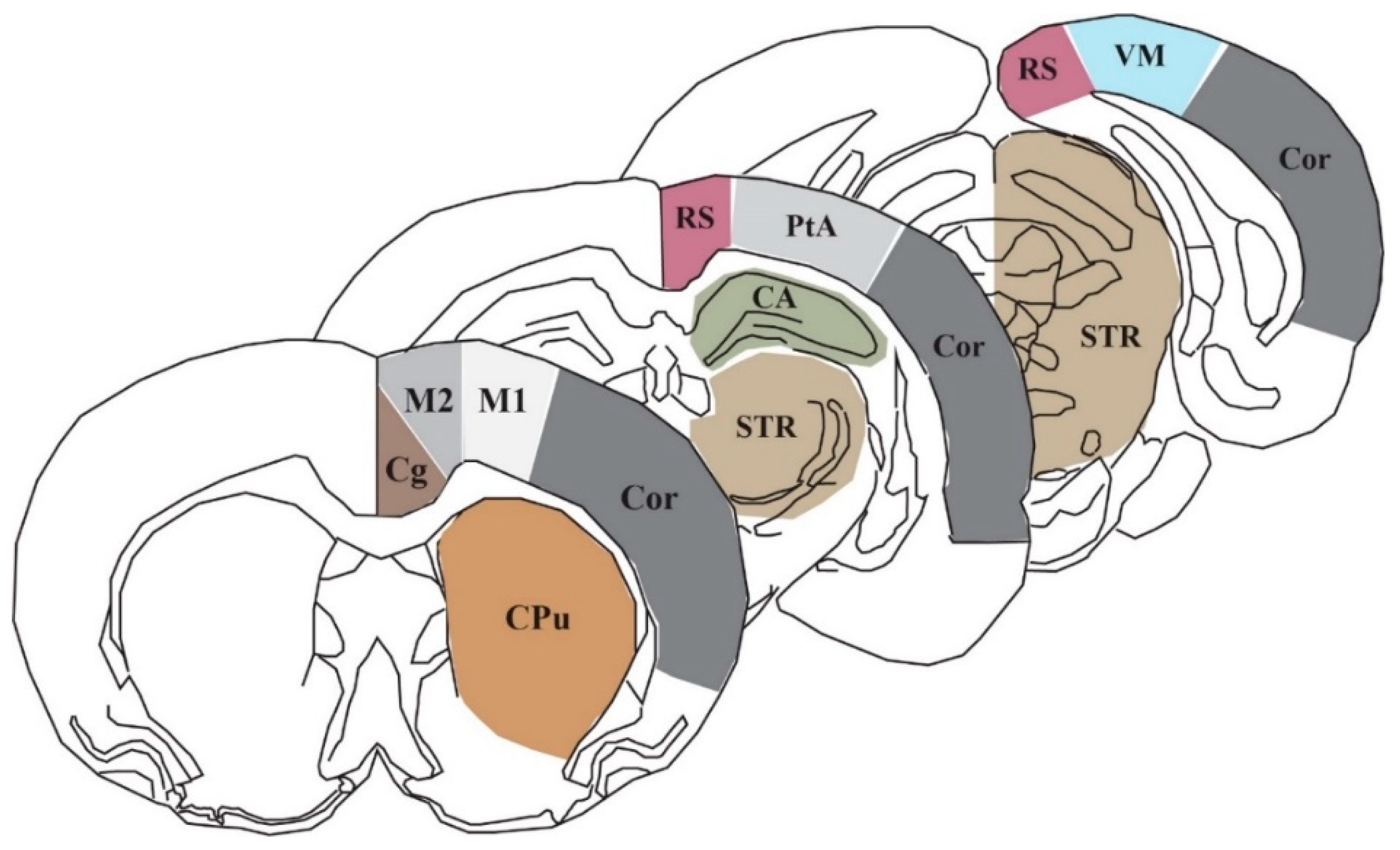
2.2. Qualitative Metabolic Consequences
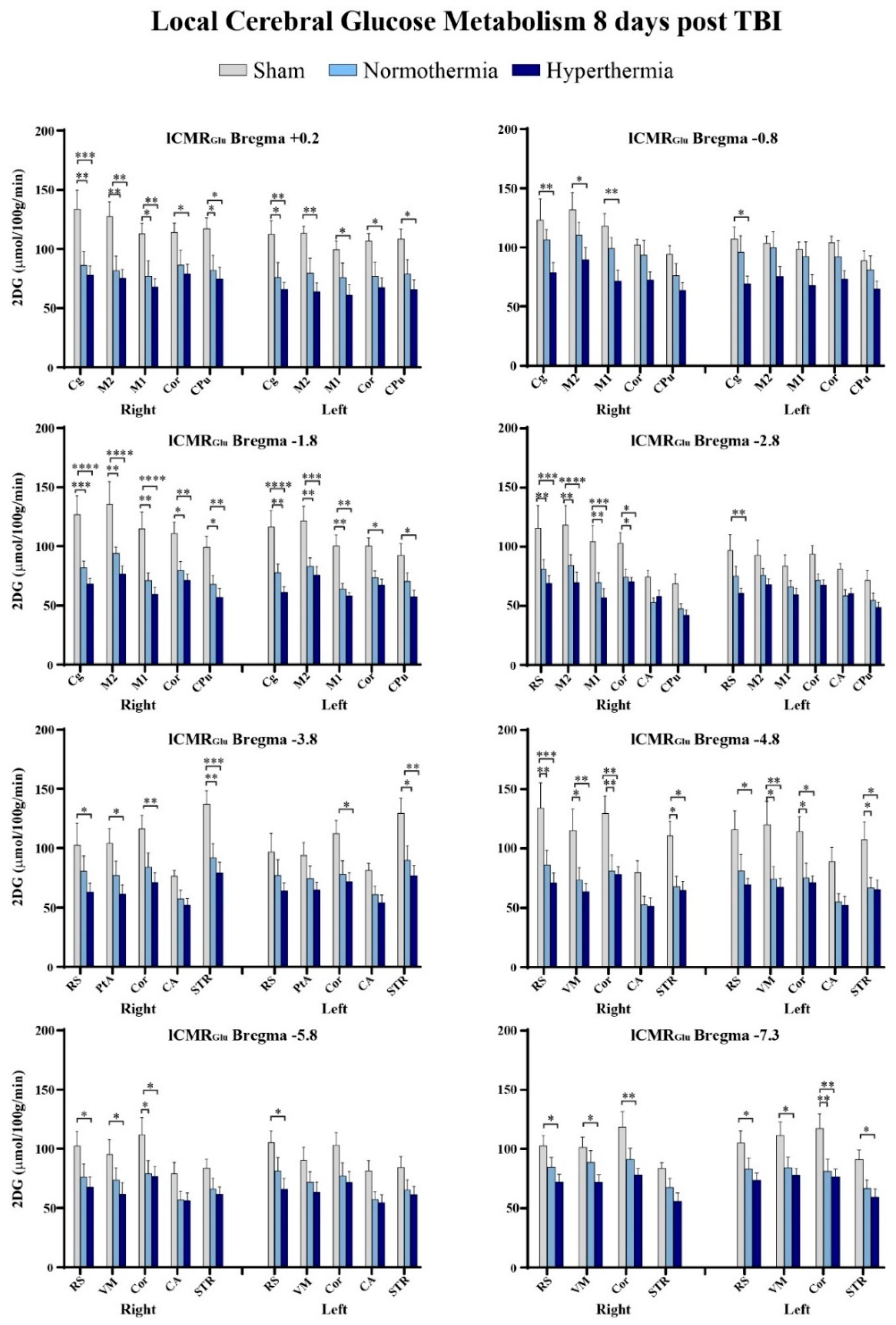
2.3. Quantitative Findings
3. Discussion
4. Methods
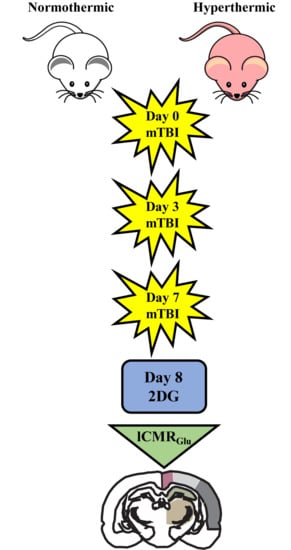
4.1. Weight Drop Injury
4.2. 14C-2-Deoxy-d-Glucose (2DG) Surgical Methods
4.3. Image Processing
4.4. Region of Interest Analysis and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| 2DG | 14C-2-deoxy-d-glucose |
| mTBI | Mild traumatic brain injury |
| lCMRGlc | Local cerebral metabolic rate of glucose |
| CBF | Cerebral blood flow |
| CNS | Central nervous system |
| ICU | Intensive care unit |
| ROI | Region of interest |
| Cg | Cingulate cortex |
| M1 | Primary motor cortex |
| M2 | Secondary motor cortex |
| RS | Retrosplenial cortex |
| PtA | Parietal association cortex |
| VM | Visual cortex |
| CPu | Caudate putamen |
| STR | Striatum |
| CA | Cornu ammonis/hippocampus |
| COR | Cortical strip |
| GLUT | Glucose transporter-3 |
| FDG-PET | [18F]fluorodeoxyglucose-positron emission tomography |
| RCTs | Randomized controlled trials |
References
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths-United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Xu, L.; Wald, M.M.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2010.
- Coronado, V.G.; Thurman, D.J.; Greenspan, A.I.; Weissman, B.M. Epidemiology. In Neurotrauma and Critical Care of the Brain; Jallo, J., Loftus, C.M., Eds.; Thieme: New York, NY, USA, 2009. [Google Scholar]
- Giza, C.C.; Difiori, J.P. Pathophysiology of sports-related concussion: An update on basic science and translational research. Sports Health 2011, 3, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Mittenberg, W.; Canyock, E.M.; Condit, D.; Patton, C. Treatment of post-concussion syndrome following mild head injury. J. Clin. Exp. Neuropsychol. 2001, 23, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Willer, B.; Leddy, J.J. Management of concussion and post-concussion syndrome. Curr. Treat. Options Neurol. 2006, 8, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The Neurometabolic Cascade of Concussion. J. Athl. Train. 2001, 36, 228–235. [Google Scholar] [CrossRef]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: Evidence of a hyper-and subsequent hypometabolic state. Brain Res. 1991, 61, 106–119. [Google Scholar] [CrossRef]
- Prins, M.L.; Alexander, D.; Giza, C.C.; Hovda, D.A. Repeated Mild Traumatic Brain Injury: Mechanisms of Cerebral Vulnerability. J. Neurotrauma 2013, 30, 30–38. [Google Scholar] [CrossRef]
- Bergsneider, M.; Hovda, D.A.; McArthur, D.L.; Etchepare, M.; Huang, S.C.; Sehati, N.; Becker, D.P. Metabolic Recovery Following Human Traumatic Brain Injury Based on FDG-PET: Time Course and Relationship to Neurological Disability. J. Head Trauma Rehabil. 2001, 16, 135–148. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Kelly, J.P. Cumulative Effects Associated With Recurrent Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA 2003, 290, 2549–2555. [Google Scholar] [CrossRef]
- The CDC, NIH, DoD, VA Leadership Panel. Report to Congress on Traumatic Brain Injury in the United States: Understanding the Public Health Problem among Current and Former Military Personnel. 2013. Available online: https://www.cdc.gov/traumaticbraininjury/pubs/congress_military.html (accessed on 11 June 2019).
- Coronado, V.G.; Haileyesus, T.; Cheng, T.A.; Bell, J.M.; Haarbauer-Krupa, J.; Lionbarger, M.R.; Flores-Herrera, J.; McGuire, L.C.; Gilchrist, J. Trends in Sports- and Recreation-Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J. Head Trauma Rehabil. 2015, 30, 185. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.; Ferguson, L.; Giza, C.; Prins, M.L. Mechanisms underlying vulnerabilities after repeat mild traumatic brain injuries. Exp. Neurol. 2019, 317, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Fehily, B.; Fitzgerald, M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- Longhi, L.; Perego, C.; Ortolano, F.; Aresi, S.; Fumagalli, S.; Zanier, E.R.; Stocchetti, N.; De Simoni, M.G. Tumor necrosis factor in traumatic brain injury: Effects of genetic deletion of p55 or p75 receptor. J. Cereb. Blood Flow Metab. 2013, 33, 1182–1189. [Google Scholar] [CrossRef]
- Weil, Z.M.; Gaier, K.R.; Karelina, K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 2014, 70, 108–116. [Google Scholar] [CrossRef]
- Hovda, D.A. The neurophysiology of concussion. Prog. Neruol. Surg. 2014, 28, 28–37. [Google Scholar]
- Thompson, H.J.; Kirkness, C.J.; Mitchell, P.H. Intensive care unit management of fever following traumatic brain injury. Intensive Crit. Care Nurs. 2007, 23, 91–96. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Gao, G.Y.; Li, W.P.; Yu, M.K.; Zhu, C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma 2002, 19, 869–874. [Google Scholar] [CrossRef]
- Stocchetti, N.; Rossi, S.; Zanier, E.R.; Colombo, A.; Beretta, L.; Citerio, G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002, 28, 1555–1562. [Google Scholar] [CrossRef]
- Truettner, J.S.; Bramlett, H.M.; Dietrich, W.D. Hyperthermia and Mild Traumatic Brain Injury: Effects on Inflammation and the Cerebral Vasculature. J. Neurotrauma 2017, 35, 940–952. [Google Scholar] [CrossRef]
- Titus, D.J.; Furones, C.; Atkins, C.M.; Dietrich, W.D. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp. Neurol. 2015, 263, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Atkins, C.M.; Alonso, O.F.; Bramlett, H.M.; Dietrich, W.D. Mild Hyperthermia Worsens the Neuropathological Damage Associated with Mild Traumatic Brain Injury in Rats. J. Neurotrauma 2012, 29, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Bramlett, H.M.; Ruenes, G.; Dietrich, W.D. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma 2004, 21, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Alonso, O.; Halley, M.; Busto, R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: A light and electron microscopic study in rats. Neurosurgery 1996, 38, 533–541. [Google Scholar] [PubMed]
- Dietrich, W.D.; Bramlett, H.M. Hyperthermia and central nervous system injury. Prog. Brain Res. 2007, 162, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Kogure, K.; Busto, R. Effects of Hypothermia and Hyperthermia on Brain Energy Metabolism. Acta Anaesthesiol. Scand. 1975, 19, 199–205. [Google Scholar] [CrossRef]
- McCulloch, J.; Savaki, H.E.; Jehle, J.; Sokoloff, L. Local Cerebral Glucose Utilization in Hypothermic and Hyperthermic Rats. J. Neurochem. 1982, 39, 255–258. [Google Scholar] [CrossRef]
- Chen, T.; Qian, Y.Z.; Di, X.; Rice, A.; Zhu, J.P.; Bullock, R. Lactate/glucose dynamics after rat fluid percussion brain injury. J. Neurotrauma 2000, 17, 135–142. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Lifshitz, F. Fasting-induced hypoglycemia in experimentally malnourished rats. J. Nutr. 1977, 107, 383–390. [Google Scholar] [CrossRef]
- Loepke, A.W.; McCann, J.C.; Kurth, C.D.; McAuliffe, J.J. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth. Analg. 2006, 102, 75–80. [Google Scholar] [CrossRef]
- Lee, D.H.; Chung, M.Y.; Lee, J.U.; Kang, D.G.; Paek, Y.W. Changes of glucose transporters in the cerebral adaptation to hypoglycemia. Diabetes Res. Clin. Pract. 2000, 47, 15–23. [Google Scholar] [CrossRef]
- Mannino, C.; Glenn, T.C.; Hovda, D.A.; Vespa, P.M.; McArthur, D.L.; Van Horn, J.D.; Wright, M.J. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J. Neurosci. Res. 2018, 96, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Panach, J.; Lull, N.; Lull, J.J.; Ferri, J.; Martinez, C.; Sopena, P.; Robles, M.; Chirivella, J.; Noe, E. A voxel-based analysis of FDG-PET in traumatic brain injury: Regional metabolism and relationship between the thalamus and cortical areas. J. Neurotrauma 2011, 28, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Mickley, G.A.; Cobb, B.L.; Farrell, S.T. Brain hyperthermia alters local cerebral glucose utilization: A comparison of hyperthermic agents. Int. J. Hyperth. 1997, 13, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.L.; McCue, P.M.; Petrie, M.A.; Shields, R.K. Whole body heat exposure modulates acute glucose metabolism. Int. J. Hyperth. 2018, 35, 644–651. [Google Scholar] [CrossRef]
- Nunneley, S.A.; Martin, C.C.; Slauson, J.W.; Hearon, C.M.; Nickerson, L.D.; Mason, P.A. Changes in regional cerebral metabolism during systemic hyperthermia in human. J. Appl. Physiol. 1985, 92, 846–851. [Google Scholar] [CrossRef]
- Polderman, K.H. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008, 37, 1955–1969. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: Experimental and clinical experience. Brain Circ. 2017, 3, 186–198. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. Therapeutic hypothermia and targeted temperature management in traumatic brain injury: Clinical challenges for successful translation. Brain Res. 2016, 1640, 94–103. [Google Scholar] [CrossRef]
- Walter, A.; Finelli, K.; Bai, X.; Johnson, B.; Neuberger, T.; Seidenberg, P.; Bream, T.; Hallett, M.; Slobounov, S. Neurobiological effect of selective brain cooling after concussive injury. Brain Imaging Behav. 2018, 12, 891–900. [Google Scholar] [CrossRef]
- Foda, M.A.; Marmarou, A. A new model of diffuse brain injury in rats. J. Neurosurg. 1994, 80, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.; Guskiewicz, K.; Randolph, C.; Barr, W.B.; Hammeke, T.A.; Marshall, S.W.; Kelly, J.P. Effects of symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery 2009, 65, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D.; Zhao, W.; Alonso, O.F.; Loor-Estades, J.Y.; Dietrich, W.D.; Busto, R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am. J. Physiol. 1997, 272, H2859–H2868. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Zhao, W.; Young, T.Y.; Ginsberg, M.D. Registration and three-dimensional reconstruction of autoradiographic images by the disparity analysis method. IEEE Trans. Med. Imaging 1993, 12, 782–791. [Google Scholar] [CrossRef]
- Zhao, W.; Ginsberg, M.D.; Smith, D.W. Three-dimensional quantitative autoradiography by disparity analysis: Theory and application to image-averaging of local cerebral glucose utilization. J. Cereb. Blood Flow Metab. 1995, 15, 552–565. [Google Scholar] [CrossRef]
- Zhao, W.; Belayev, L.; Ginsberg, M.D. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J. Cereb. Blood Flow Metab. 1997, 17, 1281–1290. [Google Scholar] [CrossRef]
| TBI #1 (Day 0) | TBI #2 (Day 3) | TBI #3 (Day 7) | 2DG (Day 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | |
| Sham | 335 ± 8 | 38.09 ± 0.39 | 38.2 ± 0.35 | 327 ± 5 | 38.14 ± 0.42 | 38.03 ± 0.38 | 348 ± 8 | 38.4 ± 0.36 | 38.3 ± 0.37 | 344 ± 3 |
| Normothermia | 307 ± 3 | 36.85 ± 0.05 | 36.68 ± 0.06 | 304 ± 3 | 36.88 ± 0.04 | 36.73 ± 0.07 | 314 ± 3 | 36.86 ± 0.04 | 36.78 ± 0.05 | 319 ± 4 |
| Hyperthermia | 309 ± 2 | 38.85 ± 0.03 | 38.79 ± 0.06 | 310 ± 4 | 38.84 ± 0.03 | 38.7 ± 0.05` | 313 ± 6 | 38.85 ± 0.02 | 38.56 ± 0.28 | 315 ± 5 |
| Region | Sham | TBI Normo | TBI Hyper | |
|---|---|---|---|---|
| Bregma +0.7 mm | ||||
| Caudate putamen | R | 115.7 ± 6.6 | 92.6 ± 14.1 | 92 ± 12.9 |
| L | 108.1 ± 5.8 | 90.8 ± 12.6 | 80.8 ± 7.3 | |
| Cortical strip | R | 106.3 ± 7.1 | 92.8 ± 12.6 | 84.4 ± 7.4 |
| L | 100.6 ± 4.7 | 83 ± 12.2 | 74.6 ± 5.1 | |
| 1° motor cortex | R | 100.6 ± 9.4 | 77.2 ± 11.6 | 70.6 ± 5.9 |
| L | 95.4 ± 7.2 | 76.7 ± 11.9 | 70.2 ± 6.4 | |
| 2° motor cortex | R | 112 ± 9.6 | 85.1 ± 11.5 | 88.4 ± 9.5 |
| L | 99.6 ± 7.6 | 76.8 ± 12 | 69.4 ± 5.7 | |
| Cingulate cortex | R | 108.5 ± 7 | 87.7 ± 10.8 | 80.8 ± 7.7 |
| L | 98.5 ± 7.4 | 81.7 ± 11.9 | 69 ± 6.9 | |
| Bregma +0.2 mm | ||||
| Caudate putamen | R | 117.1 ± 9.2 | 81.9 ± 12.8 * | 74.9 ± 10 * |
| L | 108.3 ± 8.6 | 78.7 ± 12.3 | 65.9 ± 8.4 * | |
| Cortical strip | R | 114.1 ± 8.1 | 86.5 ±12.4 | 79 ± 8.1 * |
| L | 106.7 ± 6.5 | 77.1 ± 11.7 | 67.5 ± 8.3 * | |
| 1° motor cortex | R | 112.9 ± 9 | 77.1 ± 12.8 * | 68 ± 7.2 ** |
| L | 99.2 ± 7.3 | 75.9 ± 12.3 | 60.9 ± 9 * | |
| 2° motor cortex | R | 127.2 ± 12.8 | 81.6 ± 12.7 ** | 75.4 ± 7.5 ** |
| L | 113.4 ± 5.9 | 79.4 ± 12.9 | 63.9 ± 7.4 ** | |
| Cingulate cortex | R | 133.5 ± 16.3 | 86.3 ± 11.5 ** | 77.8 ± 7.9 *** |
| L | 112.7 ± 11.3 | 76.1 ± 12.5 * | 66.1 ± 5.7 ** | |
| Bregma −0.8 mm | ||||
| Caudate putamen | R | 94.2 ± 7.6 | 76.2 ± 10.2 | 63.7 ± 6.5 |
| L | 88.7 ± 8.3 | 81.1 ± 12 | 64.8 ± 6.4 | |
| Cortical strip | R | 102.2 ± 4.6 | 93.7 ± 12.1 | 72.6 ± 6.6 |
| L | 104.1 ± 5.6 | 92.2 ±13.4 | 73.5 ± 6.8 | |
| 1° motor cortex | R | 118.2 ± 10.6 | 99.1 ± 9.3 | 71.4 ± 9.6 ** |
| L | 98.2 ± 6.4 | 92.5 ± 12.3 | 67.8 ± 9.1 | |
| 2° motor cortex | R | 132 ± 14.6 | 110.6 ± 10.8 | 89.6 ± 10.5 * |
| L | 103.5 ± 6.2 | 99.7 ± 13.8 | 75.7 ± 8.5 | |
| Cingulate cortex | R | 123 ± 18.1 | 106.3 ± 8.8 | 78.5 ± 8.7 ** |
| L | 107 ± 10.3 | 95.9 ± 14.1 | 69 ± 6.9 * | |
| Bregma −1.8 mm | ||||
| Caudate putamen | R | 99 ± 9.2 | 68 ± 7.4 * | 56.9 ±7.5 ** |
| L | 92.5 ± 9.8 | 70.3 ± 7.5 | 57.6 ± 5.1 * | |
| Cortical strip | R | 110.6 ± 9.9 | 79.6 ± 7.7 * | 71.1 ± 5.5 ** |
| L | 100 ± 7.3 | 73.4 ± 6 | 67.2 ± 5 * | |
| 1° motor cortex | R | 114.9 ± 14 | 70.9 ± 6.8 ** | 59.5 ± 5.9 **** |
| L | 100.2 ± 9.3 | 63.8 ± 5 ** | 58.3 ± 2.4 ** | |
| 2° motor cortex | R | 135.3 ± 19.3 | 94.1 ± 5.2 ** | 76.7 ± 6.7 **** |
| L | 121.7 ± 12.2 | 83 ± 7.2 ** | 75.8 ± 7 *** | |
| Cingulate cortex | R | 127.7 ± 16.1 | 81.7 ± 5.8 *** | 68.1 ± 4.8 **** |
| L | 116.3 ± 13.9 | 77.9 ± 7.4 ** | 61.1 ± 5 **** | |
| Bregma −2.8 mm | ||||
| Caudate putamen | R | 68.8 ± 8.2 | 47.6 ± 4.2 | 42 ± 4.3 |
| L | 71.4 ± 8.3 | 54.4 ± 6.3 | 48.8 ± 4.3 | |
| Hippocampus | R | 74.5 ± 5.4 | 53 ± 3.8 | 58.3 ± 4.7 |
| L | 80.9 ± 5.3 | 58.8 ± 4.8 | 60.4 ± 4.6 | |
| Cortical strip | R | 102.9 ± 9.1 | 74.3 ± 6.8 * | 70.4 ± 3.5 * |
| L | 93.8 ± 7.2 | 71.3 ± 5.8 | 67.6 ± 4.3 | |
| 1° motor cortex | R | 104.5 ± 13.5 | 69.8 ± 8.6 ** | 56.9 ± 7.6 *** |
| L | 83.6 ± 9.7 | 66.2 ± 5 | 59.4 ± 5.4 | |
| 2° motor cortex | R | 118.2 ± 16.8 | 84.2 ± 9.2 ** | 69.8 ± 9 **** |
| L | 93 ± 12.6 | 75.9 ± 5.7 | 68 ± 4.7 | |
| Retrosplenial cortex | R | 115.8 ± 19.2 | 80.9 ± 8.3 ** | 69 ± 6.8 *** |
| L | 97 ± 13.2 | 75.1 ± 8.1 | 60.5 ± 4.2 ** | |
| Bregma −3.8 mm | ||||
| Striatum | R | 137 ± 11.4 | 91.9 ± 11.8 ** | 79.2 ± 9.2 *** |
| L | 129.2 ± 13.1 | 89.8 ± 12.1 * | 77.1 ± 8.6 ** | |
| Hippocampus | R | 76.7 ± 4.6 | 57.5 ± 7.1 | 51.9 ± 6 |
| L | 81.2 ± 6.2 | 60.9 ± 7.4 | 53.9 ± 6.8 | |
| Cortical strip | R | 116.4 ± 11.3 | 84 ± 12 | 71.1 ± 8.3 ** |
| L | 112.1 ± 11.1 | 78.2 ± 11.2 | 71.6 ± 7.8 * | |
| Parietal association cortex | R | 103.9 ± 12.8 | 77.3 ± 11.7 | 61.2 ± 8.1 * |
| L | 93.9 ± 10.7 | 74.5 ± 10.9 | 64.9 ± 6.5 | |
| Retrosplenial cortex | R | 102.3 ± 18.7 | 80.6 ± 12.7 | 63.1 ± 7.5 * |
| L | 96.9 ± 15.4 | 77.2 ± 13 | 64.1 ± 6.7 | |
| Bregma −4.8 mm | ||||
| Striatum | R | 110.6 ± 12.1 | 68.2 ± 8.7 * | 64.7 ± 7.5 * |
| L | 107.5 ± 14.8 | 67.1 ± 8.6 * | 65.6 ± 7.8 * | |
| Hippocampus | R | 79.8 ± 9.9 | 52.5 ± 7.5 | 51.4 ± 7.1 |
| L | 89.1 ± 11.8 | 55 ± 6.9 | 52.1 ± 7.7 | |
| Cortical strip | R | 129.5 ± 14.8 | 81 ± 13.4 ** | 78.2 ± 6.7 ** |
| L | 114.1 ± 12.8 | 75.5 ± 12.3 * | 71.2 ± 6 * | |
| Visual cortex | R | 115.1 ± 18.1 | 73.3 ± 10.9 * | 63.6 ± 7 ** |
| L | 120.1 ± 19.6 | 74.4 ± 10.9 * | 67.6 ± 7.4 ** | |
| Retrosplenial cortex | R | 134.1 ± 21.6 | 86.4 ± 12.1 ** | 70.9 ± 8.7 *** |
| L | 116 ± 15.6 | 81.1 ± 13.7 | 69.6 ± 5.4 * | |
| Bregma −5.8 mm | ||||
| Striatum | R | 83.5 ± 7.6 | 66.2 ± 8.9 | 61.5 ± 6.4 |
| L | 84.3 ± 9.1 | 65.3 ± 8.2 | 61.3 ± 7 | |
| Hippocampus | R | 78.9 ± 9.7 | 56.9 ± 6.9 | 56.3 ± 6.3 |
| L | 81.3 ± 8.7 | 57.3 ± 6.3 | 54.4 ± 6.7 | |
| Cortical strip | R | 111.9 ± 14.6 | 78.9 ± 11 * | 76.8 ± 8.4 * |
| L | 103.1 ± 10.7 | 77.1 ± 11.1 | 71.4 ± 9.4 | |
| Visual cortex | R | 95.4 ± 12.3 | 73.4 ± 10.5 | 61.4 ± 9.8 * |
| L | 90.1 ± 11 | 71.6 ± 9 | 63 ± 8.8 | |
| Retrosplenial cortex | R | 102.2 ± 12.7 | 76.3 ± 10.9 | 67.6 ± 8.9 * |
| L | 105.4 ± 9.6 | 81.2 ± 11.4 | 66.1 ± 9.1 * | |
| Bregma −7.3 mm | ||||
| Striatum | R | 83.7 ± 5 | 67.6 ± 7.8 | 55.8 ± 7 |
| L | 91 ± 8.2 | 67 ± 6.7 | 59.4 ± 7.2 * | |
| Cortical strip | R | 118.3 ± 13.6 | 91 ± 9.5 | 78.1 ± 5.4 ** |
| L | 117.4 ± 12.2 | 81.1 ± 10.2 ** | 76.5 ± 6.6 ** | |
| Visual cortex | R | 101.2 ± 8.8 | 88.7 ± 9.8 | 71.7 ± 6.7 * |
| L | 111.5 ± 11.6 | 84.3 ± 9 | 77.9 ± 5.5 * | |
| Retrosplenial cortex | R | 102.6 ± 8.7 | 84.7 ± 8.2 | 72 ± 6.8 * |
| L | 105.2 ± 10.3 | 83 ± 9.3 | 73.6 ± 6.1 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaya, M.; Truettner, J.; Zhao, W.; Bramlett, H.; Dietrich, W.D. Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. Int. J. Mol. Sci. 2020, 21, 609. https://doi.org/10.3390/ijms21020609
Blaya M, Truettner J, Zhao W, Bramlett H, Dietrich WD. Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. International Journal of Molecular Sciences. 2020; 21(2):609. https://doi.org/10.3390/ijms21020609
Chicago/Turabian StyleBlaya, Meghan, Jessie Truettner, Weizhao Zhao, Helen Bramlett, and William Dalton Dietrich. 2020. "Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion" International Journal of Molecular Sciences 21, no. 2: 609. https://doi.org/10.3390/ijms21020609
APA StyleBlaya, M., Truettner, J., Zhao, W., Bramlett, H., & Dietrich, W. D. (2020). Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. International Journal of Molecular Sciences, 21(2), 609. https://doi.org/10.3390/ijms21020609