Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration
Abstract
1. Introduction
2. Results
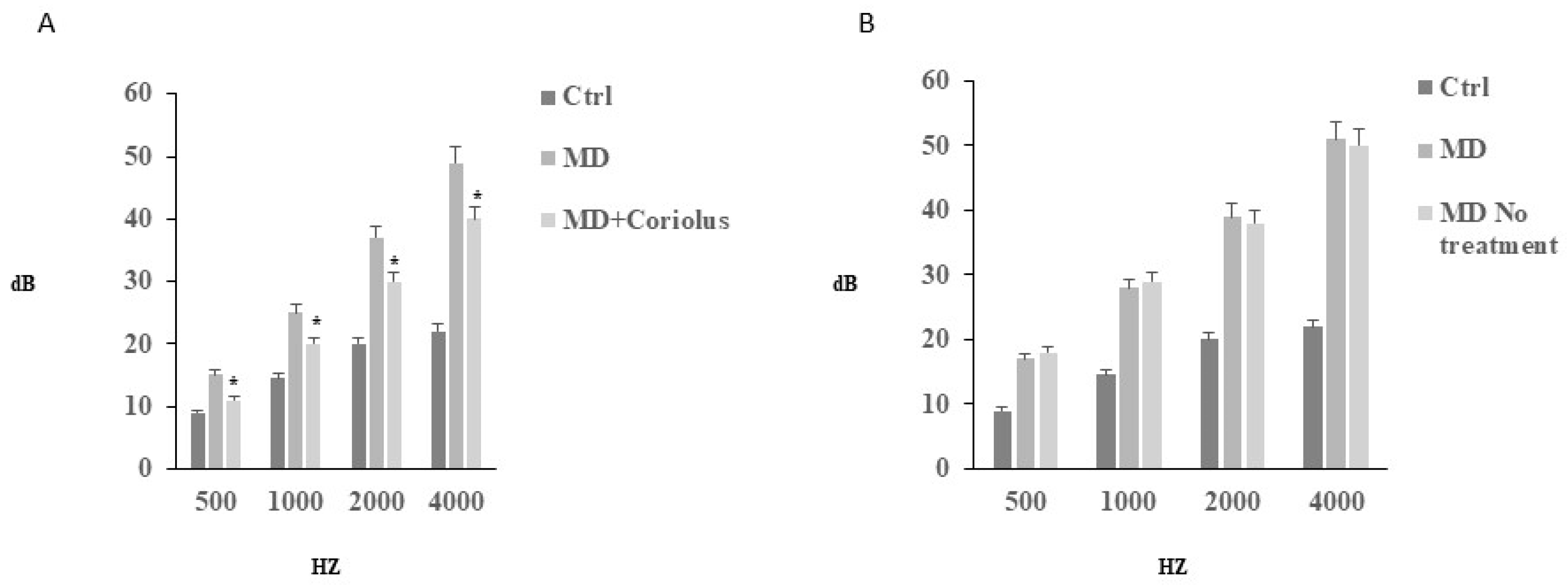
2.1. Auditory Function Analysis
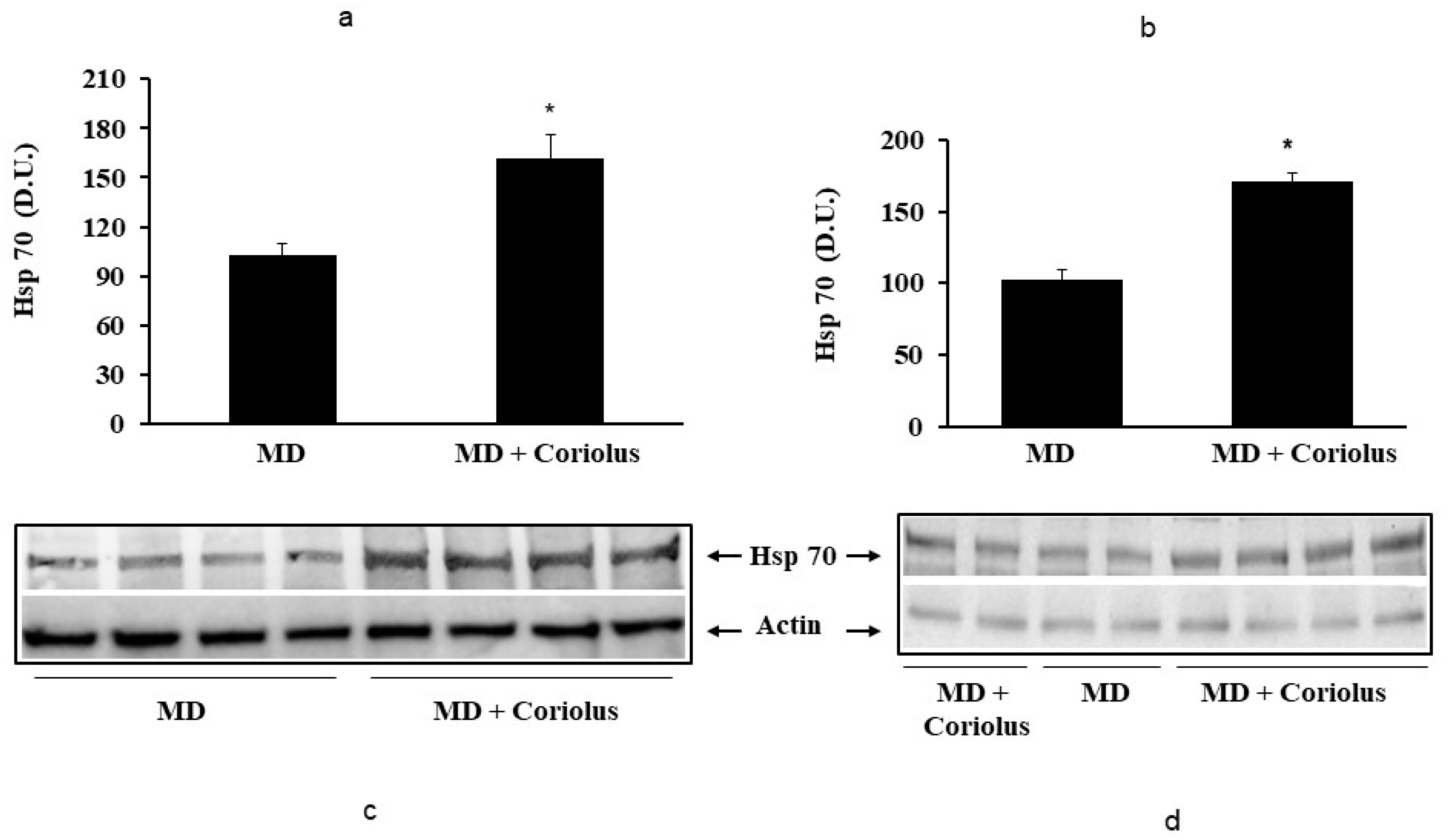
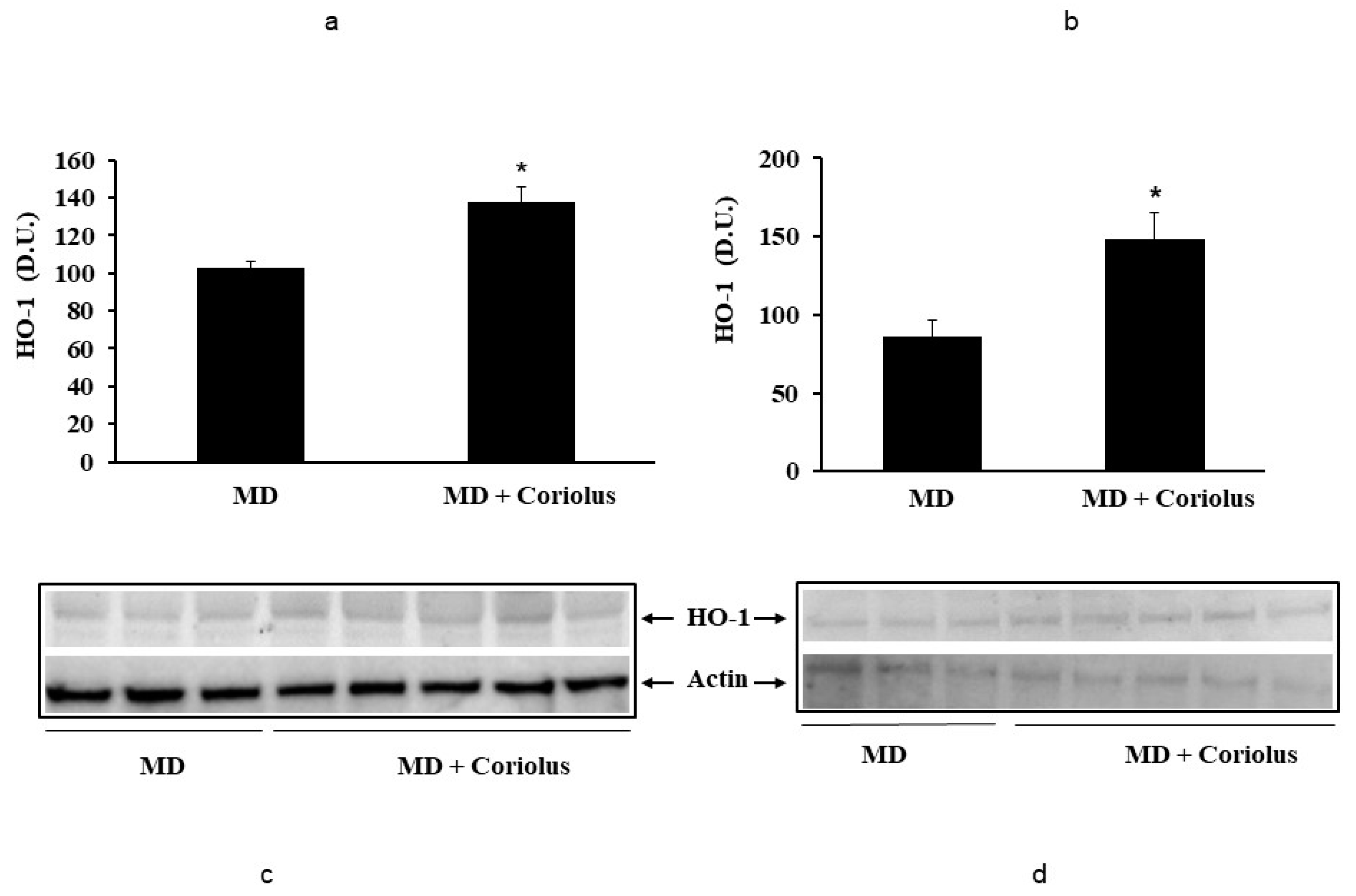
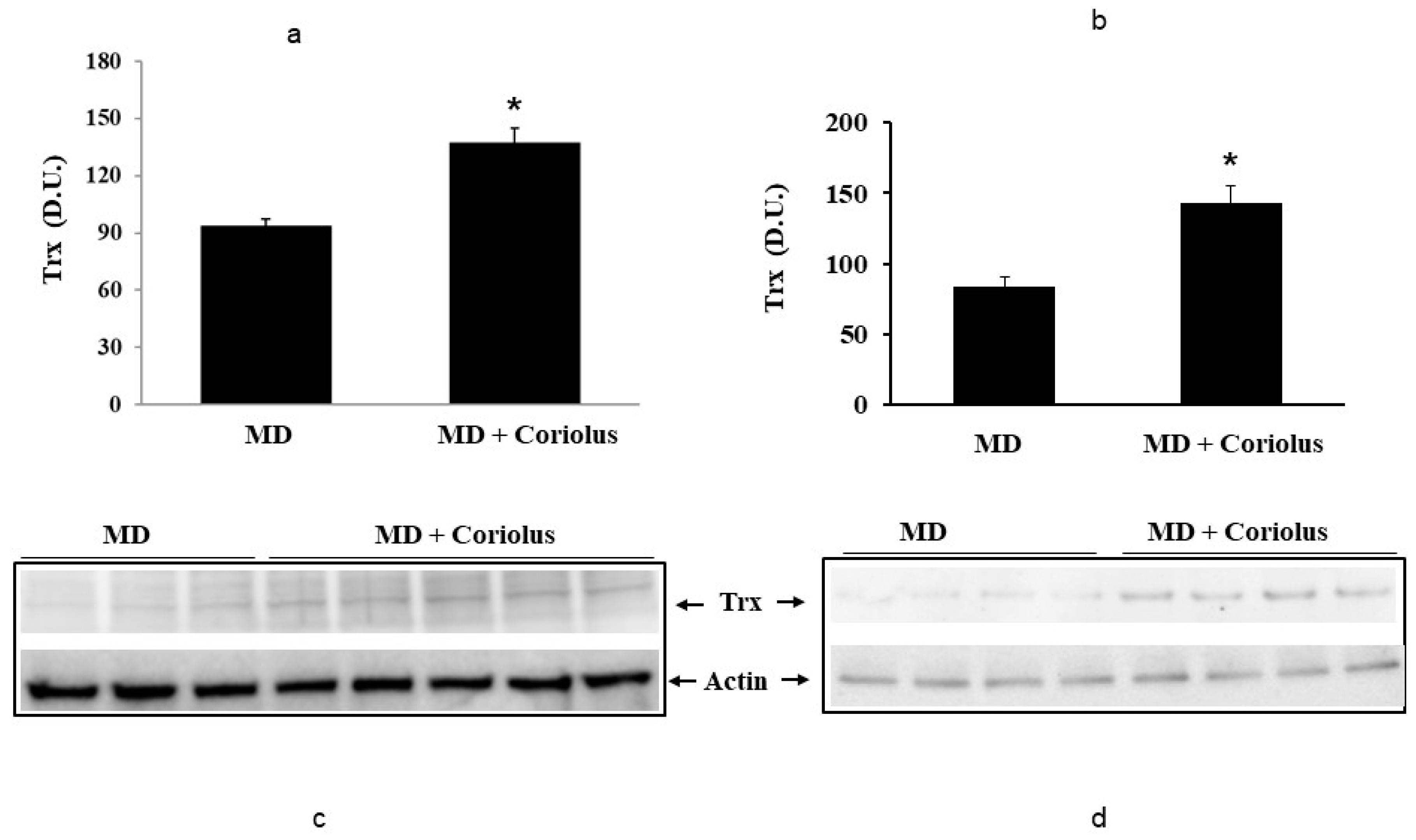
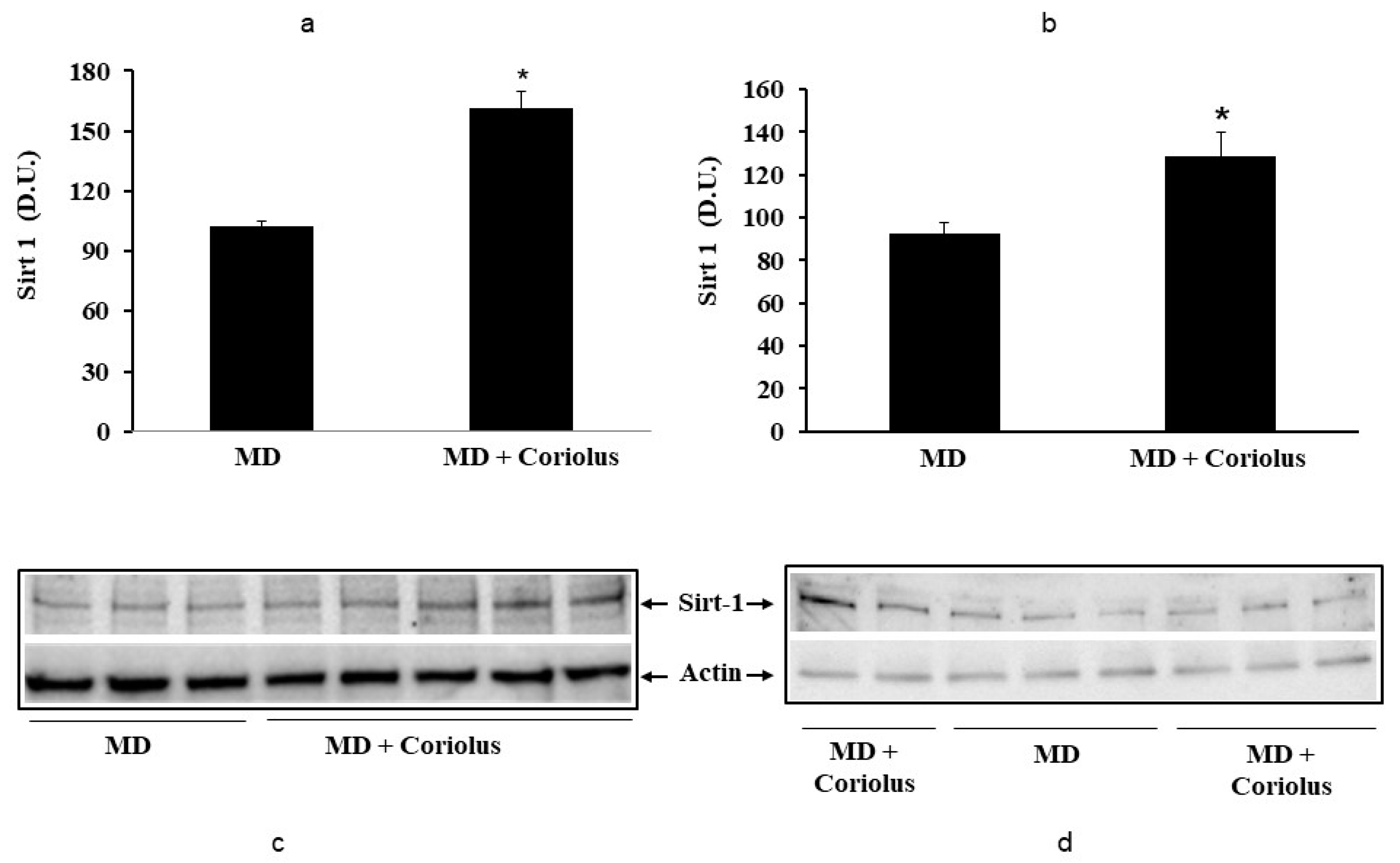
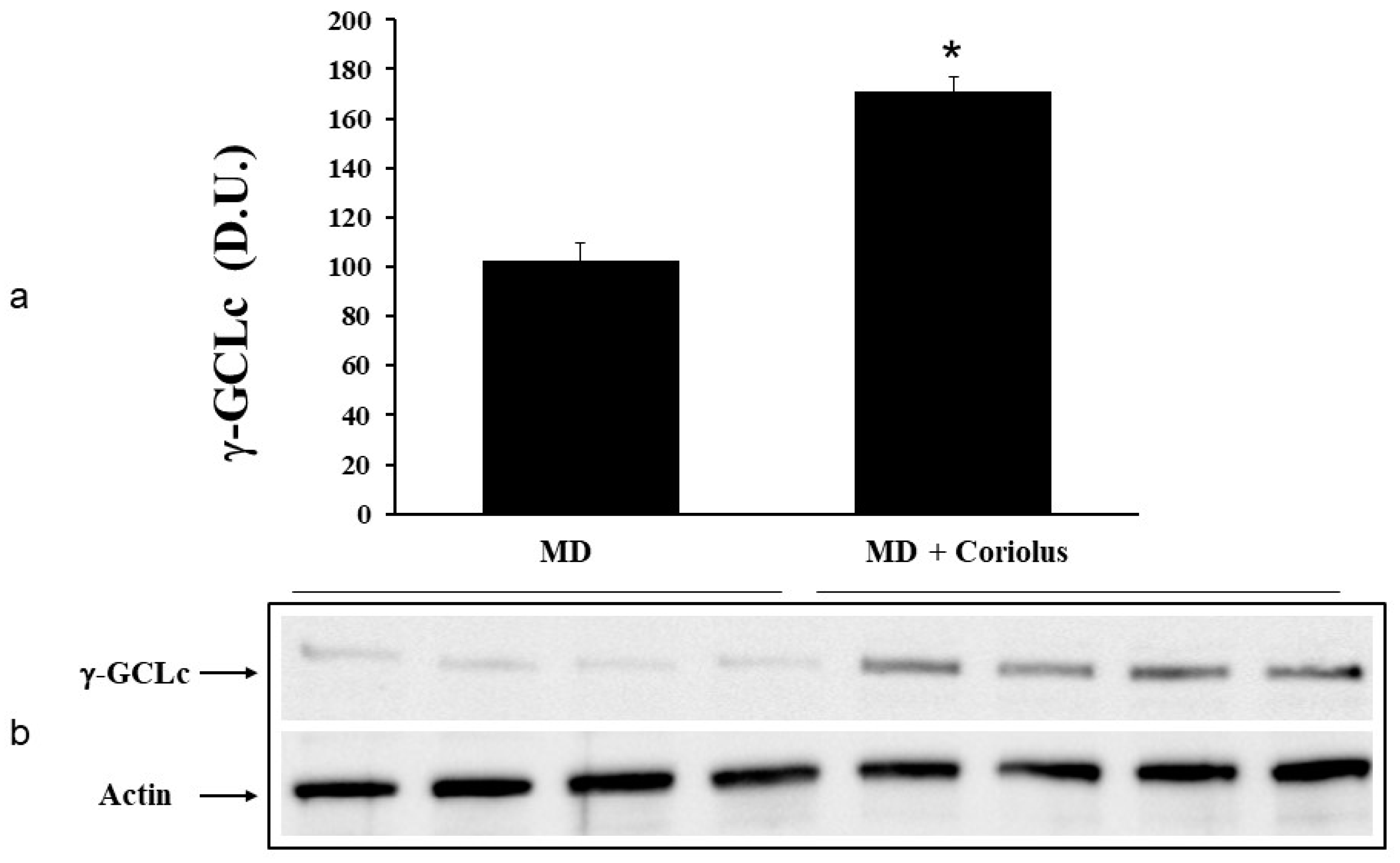
2.2. Redoxomics
Modulation of Hsp72, HO-1, Thioredoxin, Sirtuins and γ-GC Liase, in MD Patients after Coriolus Mushroom Supplementation
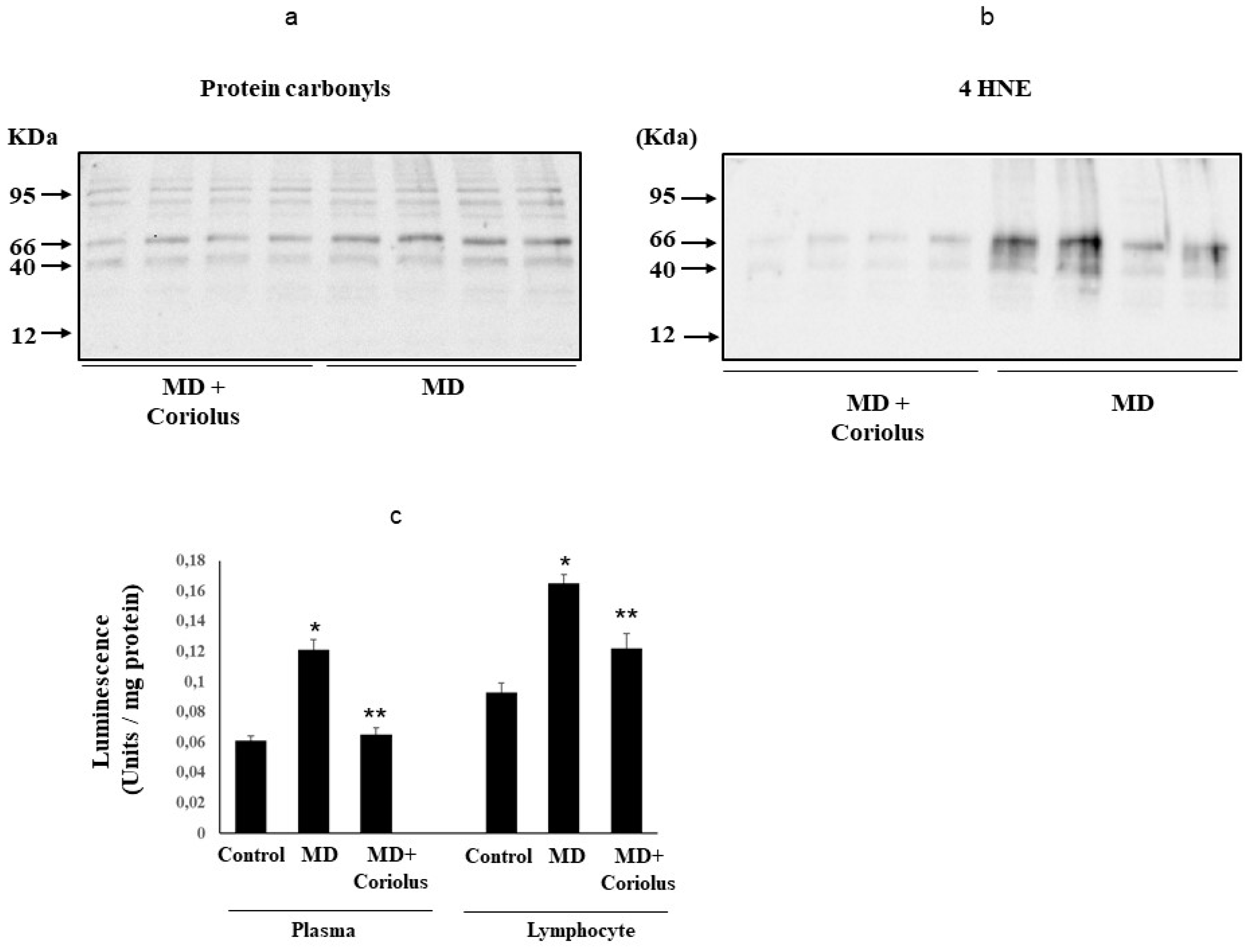
2.3. Assessment of Systemic Oxidative Status
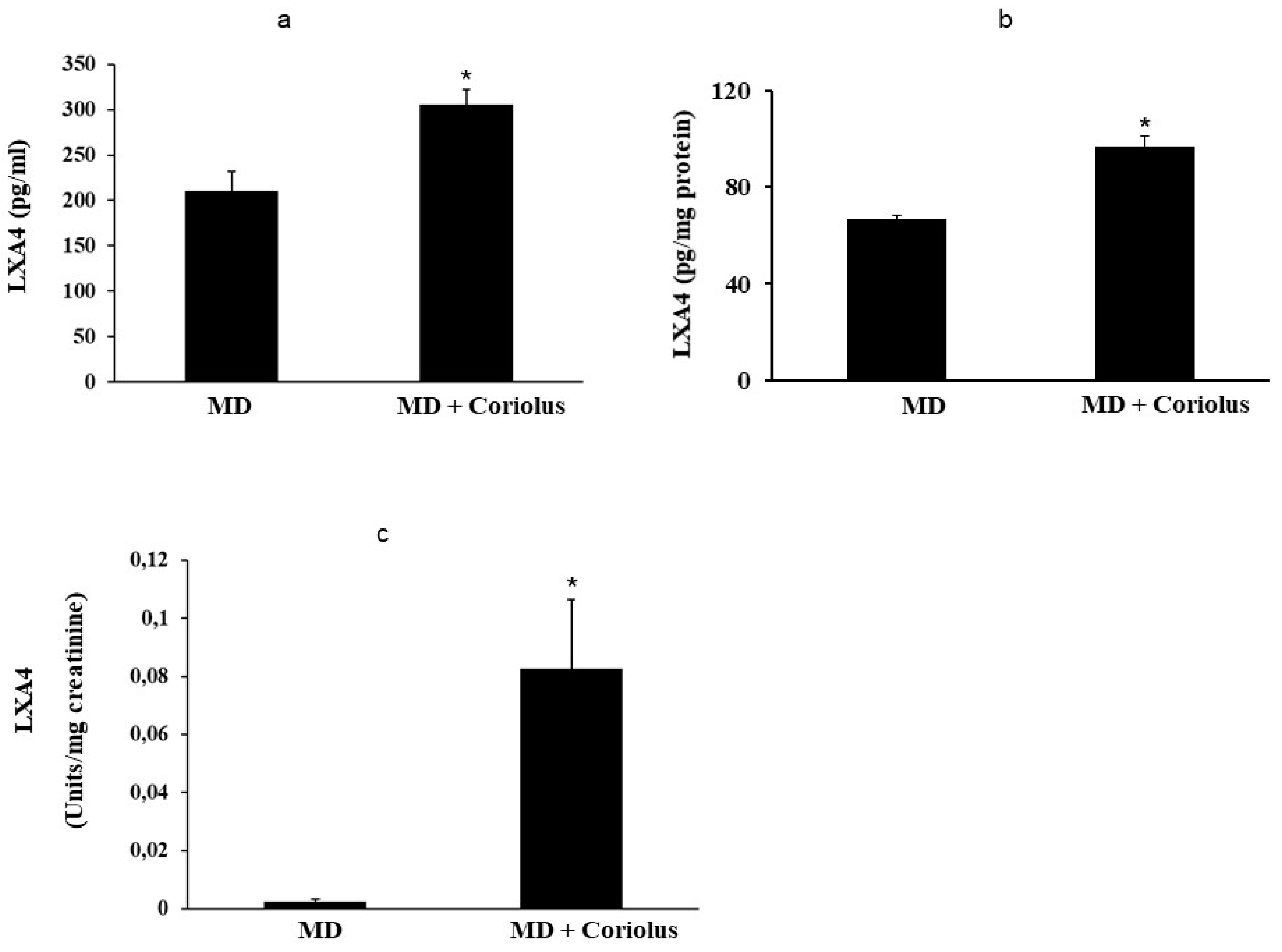
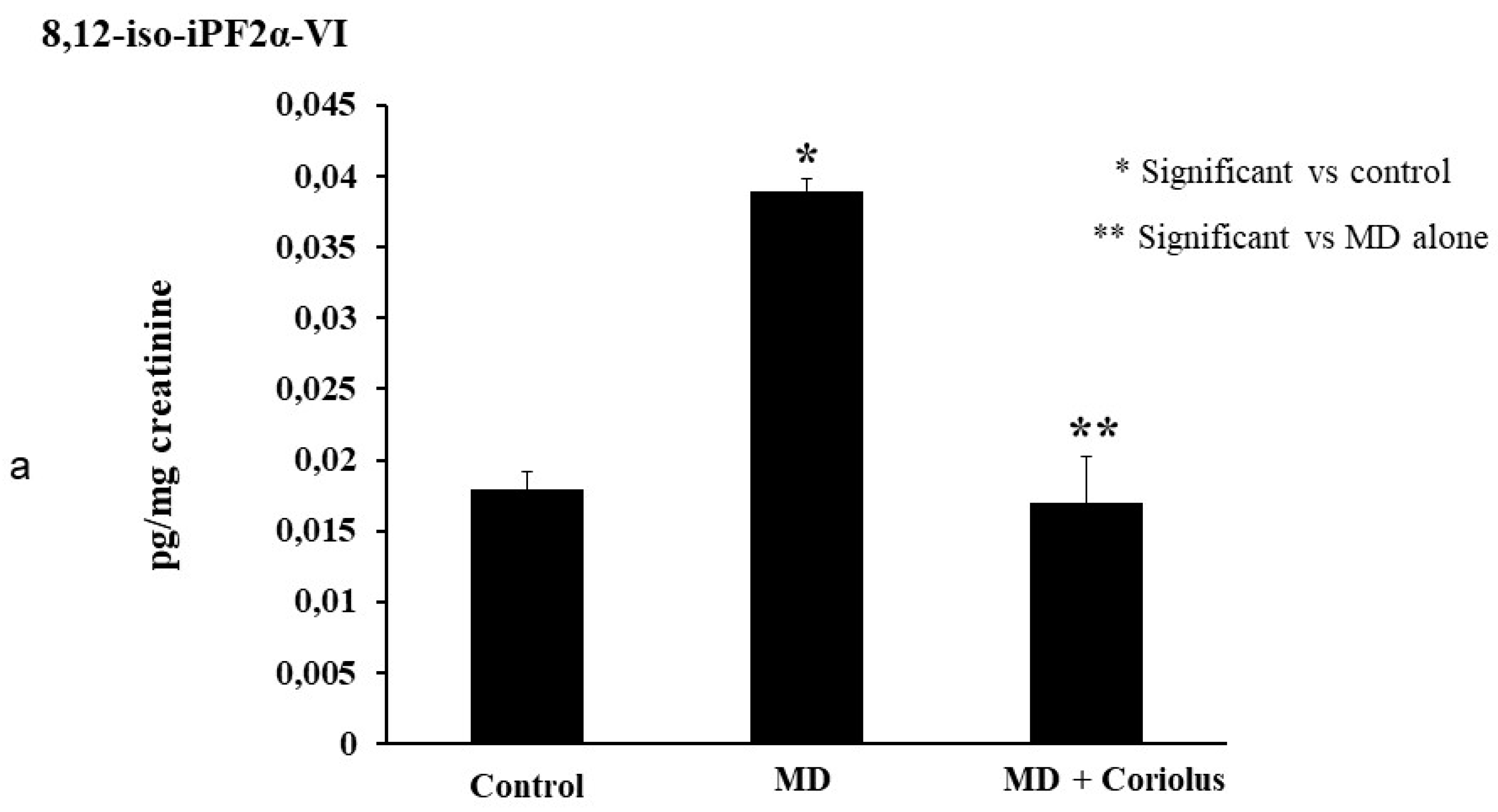
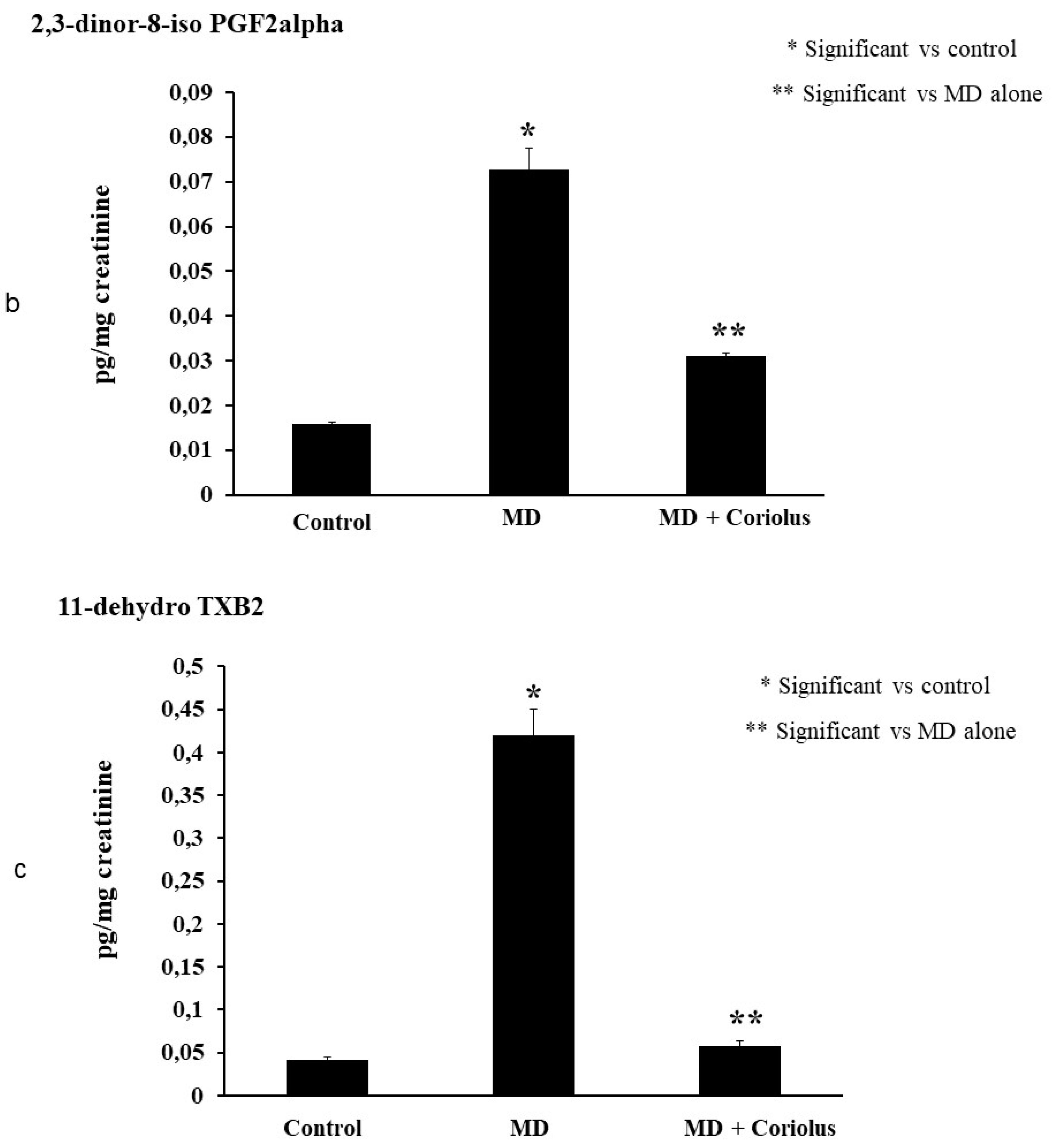
2.4. Lipidomics Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Coriolus Versicolor Biomass Preparation
4.3. Ethical Permission
4.4. Patients
4.5. Sampling
4.6. Lymphocytes Purification
4.7. Western Blot Analysis
4.8. Glutathione and Glutathione Disulfide Assay
4.9. Spontaneous Ultraweak Chemiluminescence Assay
4.10. Lipidomic Analysis
4.11. Lipoxin A4 Assay
4.12. Determination of Protein
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MD | Meniere’s disease |
| HNE | 4-hydroxynonenal |
| HSP72 | Heat Shock Proteins 70 |
| HO-1 | Heme Oxygenase-1 |
| TRX | Thioredoxin |
| γ-GC | gamma-glutamylcysteine liase |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| LXA4 | Lipoxin A4 |
| AD | Alzheimer’s disease |
| FPRL1 | Formyl-Peptide Receptor-Like 1 |
| BRM | Biological Response Modifiers |
| TA | Tension–Anxiety |
| D | Depression–Discouragement |
| AH | Anger–Hostility |
| V | Vigor–Activity |
| F | Fatigue |
| C | Confusion–Loss |
| POMS | Profile of Mood States |
| MTHFR | Methylenetetrahydrofolate reductase |
| TLR | Toll-like receptor coding genes |
| ROS | Reactive Oxygen Species |
References
- Hallpike, S.C.; Cairns, H. Observations on the pathology of Meniere’s syndrome. J. Laryngol. Otol. 1938, 53, 625–655. [Google Scholar] [CrossRef]
- Megerian, C.A.; Cliff, A. Diameter of the cochlear nerve in endolymphatic hydrops: Implications for the etiology of hearing loss in Meniere’s disease. Laryngoscope 2005, 9, 1525–1535. [Google Scholar] [CrossRef]
- Capaccio, P.; Pignataro, L.; Gaini, L.M.; Sigismund, P.E.; Novembrino, C.; De Giuseppe, R. Unbalanced oxidative status in idiopathic sudden sensorineural hearing los. Eur. Arch. Otorhinolaryngol. 2012, 269, 449–453. [Google Scholar] [CrossRef]
- Schreiber, B.E.; Agrup, C.; Haskard, D.O.; Luxon, L.M. Sudden sensorineural hearing loss. Lancet 2010, 375, 1203–1211. [Google Scholar] [CrossRef]
- Chau, J.K.; Lin, J.R.; Atashband, S.; Irvine, R.A.; Westerberg, B.D. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010, 120, 1011–1021. [Google Scholar] [CrossRef]
- Melki, S.J.; Heddon, C.M.; Frankel, J.K.; Levitt, A.H.; Momin, S.R.; Alagramam, K.N.; Megerian, C.A. Pharmacological protection of hearing loss in the mouse model of endolymphatic hydrops. Laryngoscope 2010, 120, 1637–1645. [Google Scholar] [CrossRef]
- Merchant, S.N.; Adams, J.C.; Nadol, J.B. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2005, 26, 151–160. [Google Scholar] [CrossRef]
- Calabrese, V.; Dattilo, S.; Petralia, A.; Parenti, R.; Pennisi, M.; Koverech, G.; Calabrese, V.; Graziano, A.; Monte, I.; Maiolino, L.; et al. Analytical approaches to the diagnosis and treatment of aging and aging-related disease: Redox status and proteomics. Free Radic. Res. 2015, 49, 511–524. [Google Scholar] [CrossRef]
- Pilipenko, V.; Narbute, K.; Amara, I.; Trovato Salinaro, A.; Scuto, M.; Pupure, J.; Jansone, B.; Poikans, J.; Bisenieks, E.; Klusa, V.; et al. GABA-containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J. Neurosci. Res. 2019, 97, 708–726. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Trovato Salinaro, A.; Modafferi, S.; Scuto, M.; Albouchi, F.; Monti, D.; Giordano, J.; Zappia, M.; Franceschi, C.; et al. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018, 96, 1641–1662. [Google Scholar] [CrossRef]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Ontario, M.L.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 13, 86–103. [Google Scholar] [CrossRef]
- Cornelius, C.; Trovato Salinaro, A.; Scuto, M.; Fronte, V.; Cambria, M.T.; Pennisi, M.; Bella, R.; Milone, P.; Graziano, A.; Crupi, R.; et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: Role of vitagenes. Immun. Ageing 2013, 10, 41. [Google Scholar] [CrossRef]
- Calabrese, V.; Scapagnini, G.; Davinelli, S.; Koverech, G.; Koverech, A.; De Pasquale, C.; Trovato Salinaro, A.; Scuto, M.; Calabrese, E.J.; Genazzani, A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell Commun. Signal. 2014, 8, 369–384. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2017, 115, 80–91. [Google Scholar] [CrossRef]
- Wu, J.; Wang, A.; Min, Z.; Xiong, Y.; Yan, Q. Lipoxin A4 inhibits the production of proinflammatory cytokines induced by beta-amyloid in vitro and in vivo. Biochem. Biophys. Res. Commun. 2011, 408, 382–387. [Google Scholar] [CrossRef]
- Medeiros, R.; Kitazawa, M.; Passos, G.F.; Baglietto-Vargas, D.; Cheng, D.; Cribbs, D.H.; La Ferla, F.M. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am. J. Pathol. 2013, 182, 1780–1789. [Google Scholar] [CrossRef]
- Jean-Louis, T.; Rockwell, P.; Figueiredo-Pereira, M.E. Prostaglandin J2 promotes O-GlcNAcylation raising APP processing by α- and β-secretases: Relevance to Alzheimer’s disease. Neurobiol. Aging 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Praticò, D. The 5-lipoxygenase pathway: Oxidative and inflammatory contributions to the Alzheimer’s disease phenotype. Front. Cell. Neurosci. 2015, 8, 436. [Google Scholar] [CrossRef]
- Dunn, H.C.; Ager, R.R.; Baglietto-Vargas, D.; Cheng, D.; Kitazawa, M.; Cribbs, D.H.; Medeiros, R. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. J. Alzheimers Dis. 2015, 43, 893–903. [Google Scholar] [CrossRef]
- Gangemi, S.; Pescara, L.; D’Urbano, E.; Basile, G.; Nicita-Mauro, V.; Davì, G.; Romano, M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp. Gerontol. 2005, 40, 612–614. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wu, S.H.; Zhou, Y.; Tang, Y.R. Lipoxin A4-induced heme oxygenase-1 protects cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE complex. PLoS ONE 2013, 8, e67120. [Google Scholar] [CrossRef]
- Amara, I.; Timoumi, R.; Annabi, E.; Di Rosa, G.; Scuto, M.; Najjar, M.F.; Calabrese, V.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate targets the thioredoxin system and the oxidative branch of the pentose phosphate pathway in liver of Balb/c mice. Environ. Toxicol. 2019, 5, 78–86. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Cornelius, C.; Koverech, G.; Koverech, A.; Scuto, M.; Lodato, F.; Fronte, V.; Muccilli, V.; Reibaldi, M.; Longo, A.; et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder linked to Alzheimer’s disease. Front. Pharmacol. 2014, 5, 129. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Mancuso, C.; Ientile, R.; Stella, A.M.; Butterfield, D.A. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol. Biol. 2010, 610, 285–308. [Google Scholar]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms a potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- El Elsayed, H.; Elsayed, E.A.; Aziz, R.; Wadaan, M.A. Mushrooms and truffles: Historical biofactories for complementary medicine in Africa and in the Middle East. Evid. Based Complement. Altern. Med. 2013, 2013, 620451. [Google Scholar] [CrossRef]
- Paterson, R.R.; Lima, N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomed, J. 2014, 37, 357–368. [Google Scholar] [CrossRef]
- Komura, D.L.; Ruthes, A.C.; Carbonero, E.R. Water-soluble polysaccharides from Pleurotus ostreatus var florida mycelial biomass. Int. J. Biol. Macromol. 2014, 70, 354–359. [Google Scholar] [CrossRef]
- Cui, J.; Goh, K.K.; Archer, R. Characterisation and bioactivity of protein-bound polysaccharides from submerged-culture fermentation of Coriolus versicolor Wr-74 and ATCC-20545 strains. J. Ind. Microbiol. Biotechnol. 2007, 34, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.F.; Leung, P.C. General review of polysaccharopeptides PSP from C versicolor Pharmacological and clinical studies. Cancer Ther. 2008, 6, 117–130. [Google Scholar]
- Trovato Salinaro, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. NeuroToxicology 2016, 53, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Trovato Salinaro, A.; Pennisi, M.; Crupi, R.; Di Paola, R.; Alario, A.; Modafferi, S.; Di Rosa, G.; Fernandes, T.; Signorile, A.; Maiolino, L.; et al. Neuroinflammation and Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease: Modulation by Coriolus Versicolor (Yun-Zhi) Nutritional Mushroom. J. Neurol. Neuromed. 2017, 2, 19–28. [Google Scholar]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida, A. Nitric oxide in the CNS: Neuroprotection vs. Neurotoxicity. Nat. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Maiolino, L.; Luca, M.; Chiaramonte, R.; Toscano, M.A.; Serra, A. Oxidative stress, redox homeostasis and cellular stress response in Ménière’s disease: Role of vitagenes. Neurochem. Res. 2010, 35, 2208–2217. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Hagiwara, A.; Suzuki, M. Polymorphic analysis of the heat-shock protein 70 gene (HSPA1A) in Me’nie`re’s disease. Acta Otolaryngol. 2008, 128, 1173–1177. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Trovato Salinaro, A.; Cambria, M.T.; Lo Cascio, M.S.; Di Rienzo, L.; Condorelli, D.; De Lorenzo, A.; Calabrese, E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010, 16, 8778–8783. [Google Scholar] [CrossRef]
- Suslu, N.; Yilmaz, T.; Gursel, B. Utility of anti-HSP 70, TNF alpha, ESR, antinuclear antibody, and antiphospholipid antibodies in the diagnosis and treatment of sudden sensorineural hearing loss. Laryngoscope 2009, 119, 341–346. [Google Scholar] [CrossRef]
- Feng, W.; Rosca, M.; Fan, Y.; Hu, Y.; Feng, P.; Lee, H.G.; Monnier, V.M.; Fan, X. Gclc deficiency in mouse CNS causes mitochondrial damage and neurodegeneration. Hum. Mol. Genet. 2017, 26, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Ottaviani, F.; Cuccarini, V.; Ambrosetti, U.; Fagnani, E.; Bottero, A. Methylenetetrahydrofolate reductase gene mutations as risk factors for sudden hearing loss. Am. J. Otolaryngol. 2005, 26, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Ottaviani Cuccarini, V.; Bottero, A.; Schindler, A.; Cesana, B.M. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope 2007, 117, 547–551. [Google Scholar] [CrossRef]
- Capaccio, P.; Cuccarini, V.; Ottaviani, F.; Fracchiolla, N.S.; Bossi, A.; Pignataro, L. Prothrombotic gene mutations in patients with sudden sensorineural hearing loss and cardiovascular thrombotic disease. Ann. Otol. Rhinol. Laryngol. 2009, 118, 205–210. [Google Scholar] [CrossRef]
- Furuta, T.; Teranishi, M.; Uchida, Y.; Nishio, N.; Kato, K.; Otake, H. Association of interleukin-1 gene polymorphisms with sudden sensorineural hearing loss and Meniere’s disease. Int. J. Immunogenet. 2011, 38, 249–254. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Teranishi, M.; Uchida, Y.; Nishio, N.; Suzuki, H.; Kato, K. Polymorphisms in genes involved in inflammatory pathways in patients with sudden sensorineural hearing loss. J. Neurogenet. 2012, 26, 387–396. [Google Scholar] [CrossRef]
- Uchida, Y.; Sugiura, S.; Ando, F.; Shimokata, H.; Nakashima, T. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with sudden sensorineural hearing loss. Laryngoscope 2010, 120, 791–795. [Google Scholar] [CrossRef]
- Uchida, Y.; Sugiura, S.; Nakashima, T.; Ando, F.; Shimokata, H. Contribution of 1425G/A polymorphism in protein kinase C-Eta (PRKCH) gene and brain white matter lesions to the risk of sudden sensorineural hearing loss in a Japanese nested case-control study. J. Neurogenet. 2011, 25, 82–87. [Google Scholar] [CrossRef]
- Nishio, N.; Teranishi, M.; Uchida, Y.; Sugiura, S.; Ando, F.; Shimokata, H. Contribution of complement factor H Y402H polymorphism to sudden sensorineural hearing loss risk and possible interaction with diabetes. Gene 2012, 499, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.; Minor, L.B. Physiologic effects on the vestibular system in Meniere’s disease. Otolaryngol. Clin. N. Am. 2010, 43, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Gürkov, R.; Jerin, C.; Flatz, W.; Maxwell, R. Clinical manifestations of hydropic ear disease (Menière’s). Eur. Arch. Otorhinolaryngol. 2019, 276, 27–40, Erratum in Eur. Arch. Otorhinolaryngol. 2019, 276, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Derebery, M.J. Allergic and immunologic features of Meniere’s disease. Otolaryngol. Clin. N. Am. 2011, 44, 655–666. [Google Scholar] [CrossRef]
- Di Renzo, L.; Bianchi, A.; Saraceno, R.; Calabrese, V.; Cornelius, C.; Iacopino, L.; Chimenti, S.; De Lorenzo, A. 174G/C IL-6 gene promoter polymorphism predicts therapeutic response to TNF-α blockers. Pharmacogenet. Genom. 2012, 22, 134–142. [Google Scholar] [CrossRef]
- Requena, T.; Gazquez, I.; Moreno, A.; Batuecas, A.; Aran, I.; Soto-Varela, A.; Santos-Perez, S.; Perez, N.; Perez-Garrigues, H.; Lopez-Nevot, A.; et al. Allelic variants in TLR10 gene may influence bilateral affectation and clinical course of Meniere’s disease. Immunogenetics 2013, 65, 345–355. [Google Scholar] [CrossRef]
- Monro, J.A. Treatment of cancer with mushroom products. Arch. Environ. Health 2003, 58, 533–537. [Google Scholar] [CrossRef]
- Hoffer, M. Annual Meeting of the American Academy of Otolaryngology-Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 202–203. [Google Scholar] [CrossRef]
- Flecha, B.; Llesuy, S.; Boveris, A. Hydroperoxideinitiated chemiluminescence: An assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic. Biol. 1991, 10, 93–100. [Google Scholar] [CrossRef]
- Wolfer, A.M.; Gaudin, M.; Taylor-Robinson, S.D.; Holmes, E.; Nicholson, J.K. Development and Validation of a High-hroughput Ultrahigh-Performance Liquid Chromatography-Mass Spectrometry Approach for Screening of Oxylipins and Their Precursors. Anal. Chem. 2015, 87, 11721–11731. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Crupi, R.; Di Paola, R.; Ruggieri, M.; Bianchini, R.; Ontario, M.L.; Cuzzocrea, S.; Calabrese, E.J. Hormesis, cellular stress response and neuroinflammation in schizophrenia: Early onset versus late onset state. J. Neurosci. Res. 2017, 95, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Wu, S.H.; Liao, P.Y.; Dong, L.; Chen, Z.Q. Signal pathway involved in inhibition by lipoxin A4 of production of interleukins in endothelial cells by lipopolysaccharide. Inflamm. Res. 2008, 57, 430–437. [Google Scholar] [CrossRef]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017, 95, 1360–1372. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Xu, T.; Beelman, R.B.; Lambert, J.D. The cancer preventive effects of edible mushrooms. Anticancer Agents Med. Chem. 2012, 12, 1255–1263. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science current perspectives advances evidences and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Lindequist, U.; Kim, H.W.; Tiralongo, E.; van Griensven, L. Medicinal Mushrooms. Evid Based Complement. Altern. Med. 2014, 2014, 806180. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Sartori, D.; Macedo, F.C., Jr.; Ribeiro, L.R.; Fungaro, M.H.; Mantovani, M.S. Effects of glucan extracted from Agaricus blazei on the expression of ERCC5 CASP9 and CYP1A1 genes and metabolic profile in HepG2 cells. Hum. Exp. Toxicol. 2013, 32, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor physiological activity uses and production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef]
- Ferreiro, E.; Pita, I.; Mota, S.; Valero, J.; Ferreira, N.; Fernandes, T.; Calabrase, V.; Fontes-Ribeiro, C.; Pereira, F.; Rego, A. Coriolus versicolor biomass increases dendritic arboraization of newly-generated neurons in mouse hippocampal dentate gyrus. Oncotarget 2018, 8, 32929–32942. [Google Scholar]
- Nam, S.I.; Yu, G.I.; Kim, H.J.; Park, K.O.; Chung, J.H.; Ha, E.; Shin, D.H. A polymorphism at-1607 2G in the matrix metalloproteinase-1 (MMP-1) increased risk of sudden deafness in Korean population but not at—519A/G in MMP-1. Laryngoscope 2011, 121, 171–175. [Google Scholar] [CrossRef] [PubMed]
| Pre-Therapy (T0) | Post-Therapy (T1) | |||
|---|---|---|---|---|
| Score | Score | |||
| Group | A | B | A | B |
| Anger (0–48) | 28 | 29 | 22 | 29 |
| Confusion (0–28) | 17 | 17 | 10 | 16 |
| Depression (0–60) | 41 | 39 | 25 | 37 |
| Fatigue (0–28) | 16 | 19 | 10 | 19 |
| Tension (0–36) | 31 | 29 | 13 | 28 |
| Vigor (0–32) | 19 | 17 | 19 | 16 |
| Total Mood Disturbance (−32 to 200) | 114 ± 9.8 | 116 ± 8.6 | 61 ± 6.11 | 113 ± 8.1 |
| T0 | Group A | Group B |
|---|---|---|
| Vertigo Attack Frequency | ||
| <2 crisis/year | 4 (18.1%) | 3 (16.6%) |
| From 3 to 5 crisis/year | 10 (45.4%) | 8 (44.4%) |
| From 6 to 8 crisis/year | 8 (36.3%) | 7 (38.8%) |
| Crisis Duration | ||
| <1 h | 4 (18.1%) | 4 (22.2%) |
| From 1 to 7 h | 12 (54.5%) | 9 (50%) |
| >24 h | 6 (27.2%) | 5 (27.7%) |
| Duration of Symptoms | ||
| A few days | 15 (68.1%) | 11 (61.1%) |
| Some weeks | 6 (27.2%) | 6 (33.3%) |
| A month | 1 (4.5%) | 1 (5.5%) |
| Tinnitus Handicap | Inventory | ||
|---|---|---|---|
| Pre-Therapy Score | (T0) | Post-Therapy Score | (T1) |
| Group A | Group B | Group A | Group B |
| 74 ± 2.46 | 78 ± 2.73 | 52 ± 1.73 * | 74 ± 2.65 |
| Plasma (nmol/mL) | Lymphocyte (nmol/mg Protein) | |||||
|---|---|---|---|---|---|---|
| Control | MD | MD + Coriolus | Control | MD | MD + Coriolus | |
| Total GSH | 16.7 ± 2.1 | 8.33 ± 3.0 * | 14.23 ± 2.4 ** | 9.81 ± 0.8 | 5.3 ± 0.7 * | 7.3 ± 0.5 ** |
| GSH | 15.62 ± 2.0 | 8.44 ± 1.7 * | 13.44 ± 1.7 ** | 9.58 ± 0.6 | 4.27 ± 0.4 * | 7.20 ± 0.5 ** |
| GSSG | 0.138 ± 0.01 | 0.169 ± 0.01 * | 0.146 ± 0.01 ** | 0.093 ± 0.01 | 0.118 ± 0.01 ** | 0.096 ± 0.006 ** |
| Ratio GSH/GSSG | 113.2 ± 11 | 56.9 ± 15* | 92.05 ± 13 ** | 96.5 ± 10 | 42.6 ± 7.9 * | 75.0 ± 9.6 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2020, 21, 284. https://doi.org/10.3390/ijms21010284
Scuto M, Di Mauro P, Ontario ML, Amato C, Modafferi S, Ciavardelli D, Trovato Salinaro A, Maiolino L, Calabrese V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. International Journal of Molecular Sciences. 2020; 21(1):284. https://doi.org/10.3390/ijms21010284
Chicago/Turabian StyleScuto, Maria, Paola Di Mauro, Maria Laura Ontario, Chiara Amato, Sergio Modafferi, Domenico Ciavardelli, Angela Trovato Salinaro, Luigi Maiolino, and Vittorio Calabrese. 2020. "Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration" International Journal of Molecular Sciences 21, no. 1: 284. https://doi.org/10.3390/ijms21010284
APA StyleScuto, M., Di Mauro, P., Ontario, M. L., Amato, C., Modafferi, S., Ciavardelli, D., Trovato Salinaro, A., Maiolino, L., & Calabrese, V. (2020). Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. International Journal of Molecular Sciences, 21(1), 284. https://doi.org/10.3390/ijms21010284








