Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation
Abstract
1. Introduction
2. Results
2.1. Middle-Aged Mice Exhibit Visceral Adiposity, Prediabetes, and Perivascular Adipocyte Hypertrophy
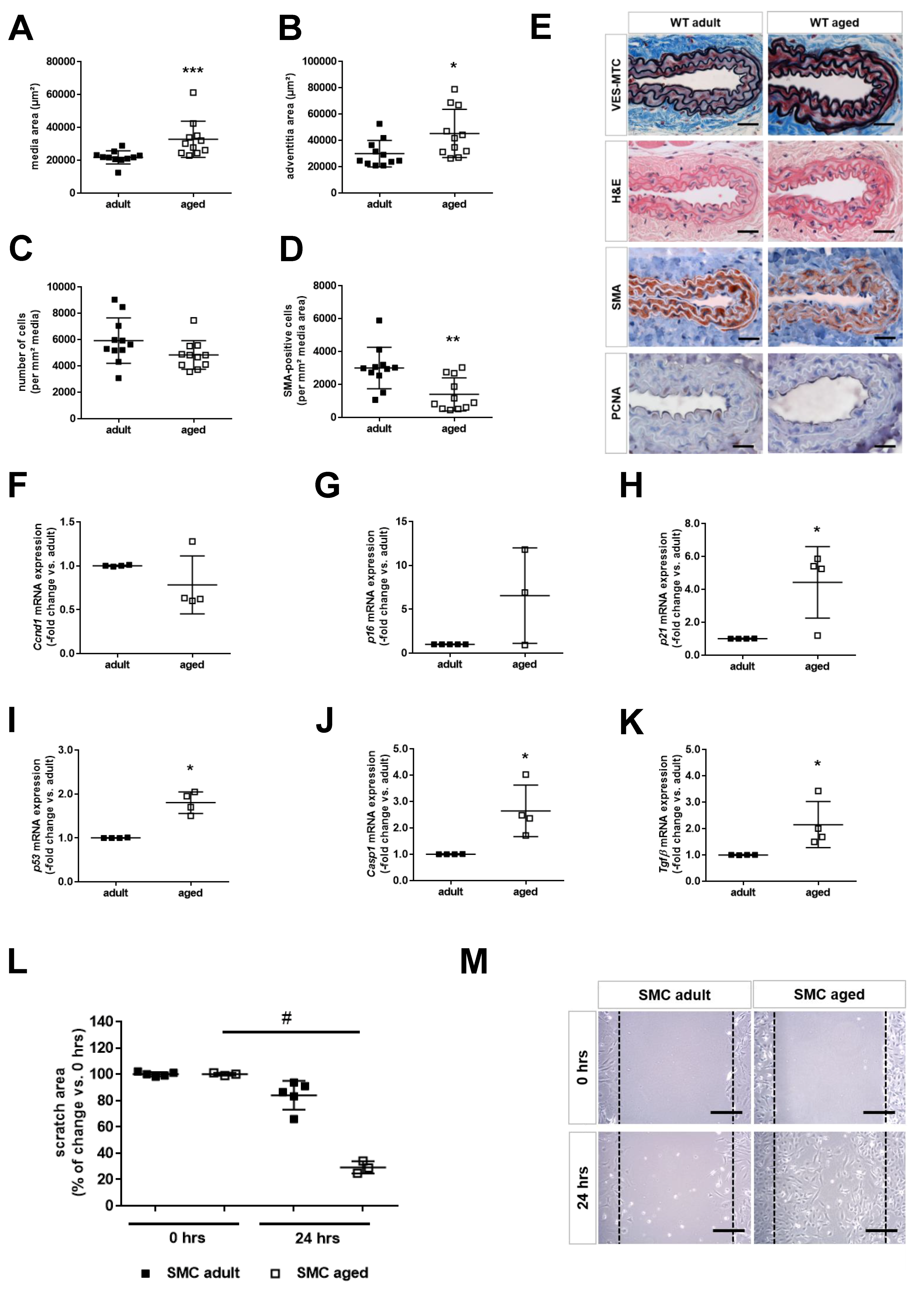
2.2. Age is Associated with Smooth Muscle Cell Senescence and De-Differentiation
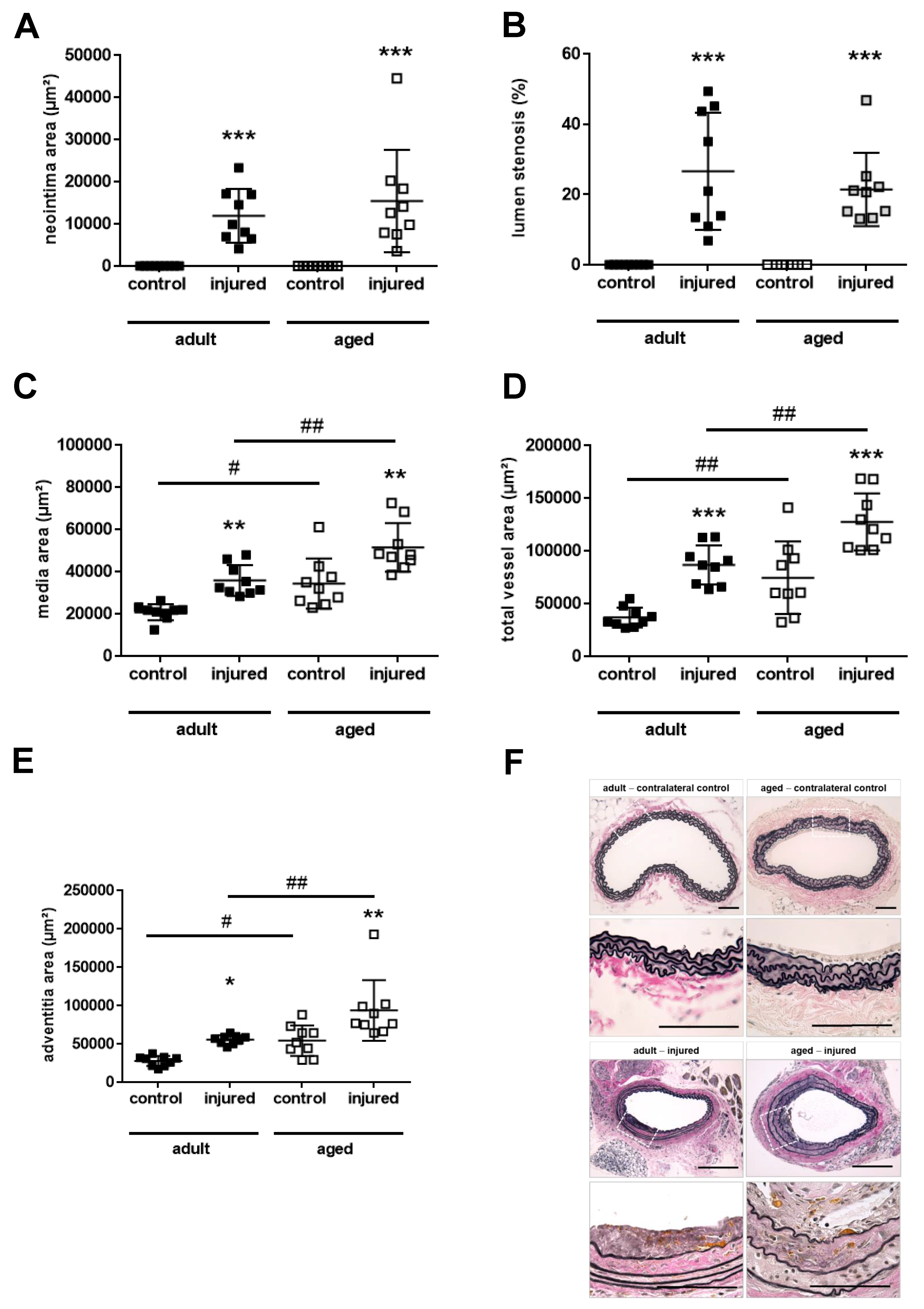
2.3. Aging Aggravates Media Thickening and Outward Remodeling after Arterial Injury
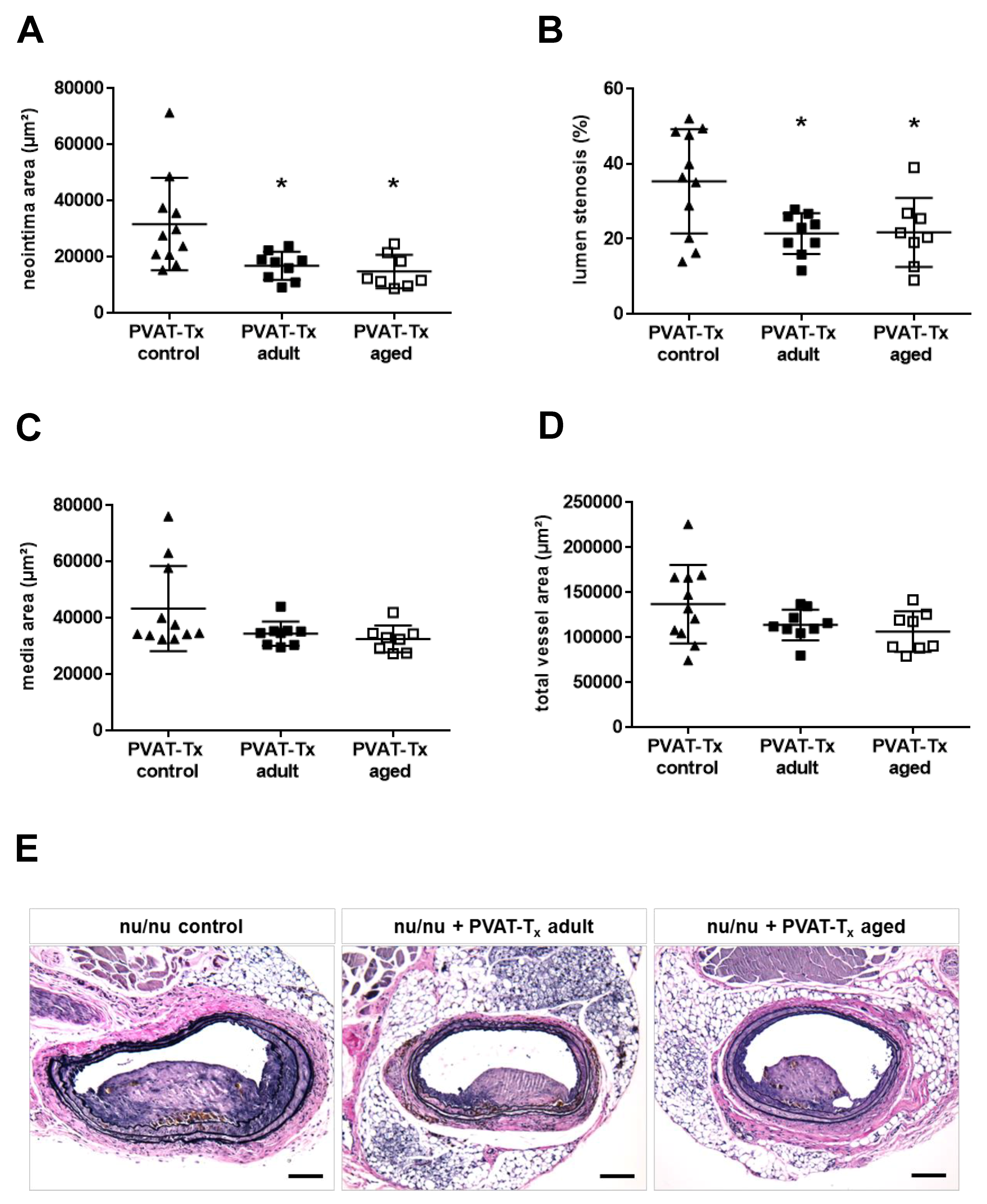
2.4. Transplantation of Perivascular Adipose Tissue Does Not Mimic the Vascular Aging Phenotype, but Age-Independently Reduces Neointima Formation and Lumen Stenosis
2.5. Differential Expression of Potential Mediators of Neointima Formation in Perivascular and Visceral Adipose Tissue
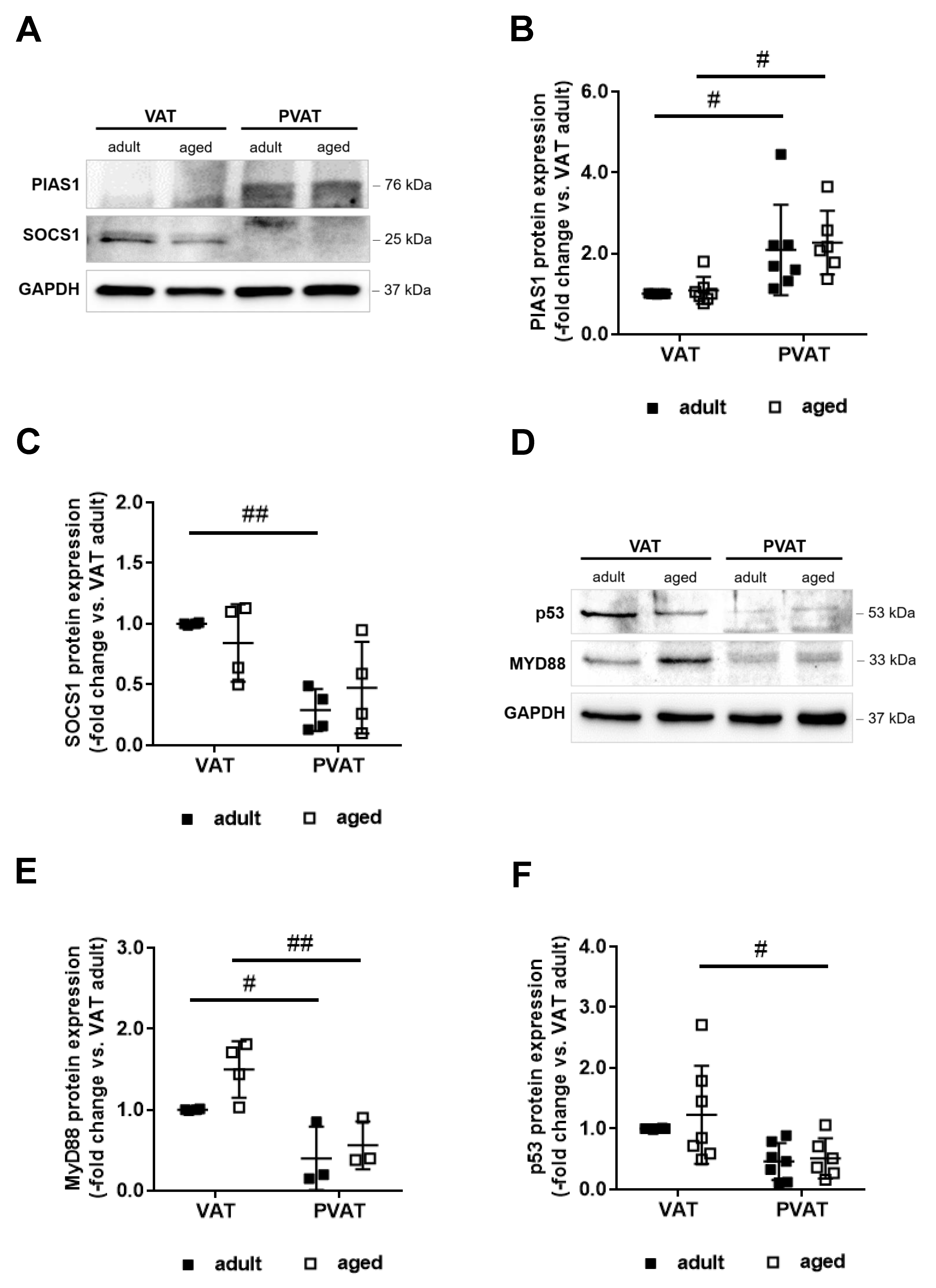
2.6. Increased Expression of PIAS1 May Mediate the Anti-Inflammatory Expression Profile of Perivascular Fat
2.7. Differences in Adipocyte Differentiation May Underlie Higher PIAS1 Expression in PVAT
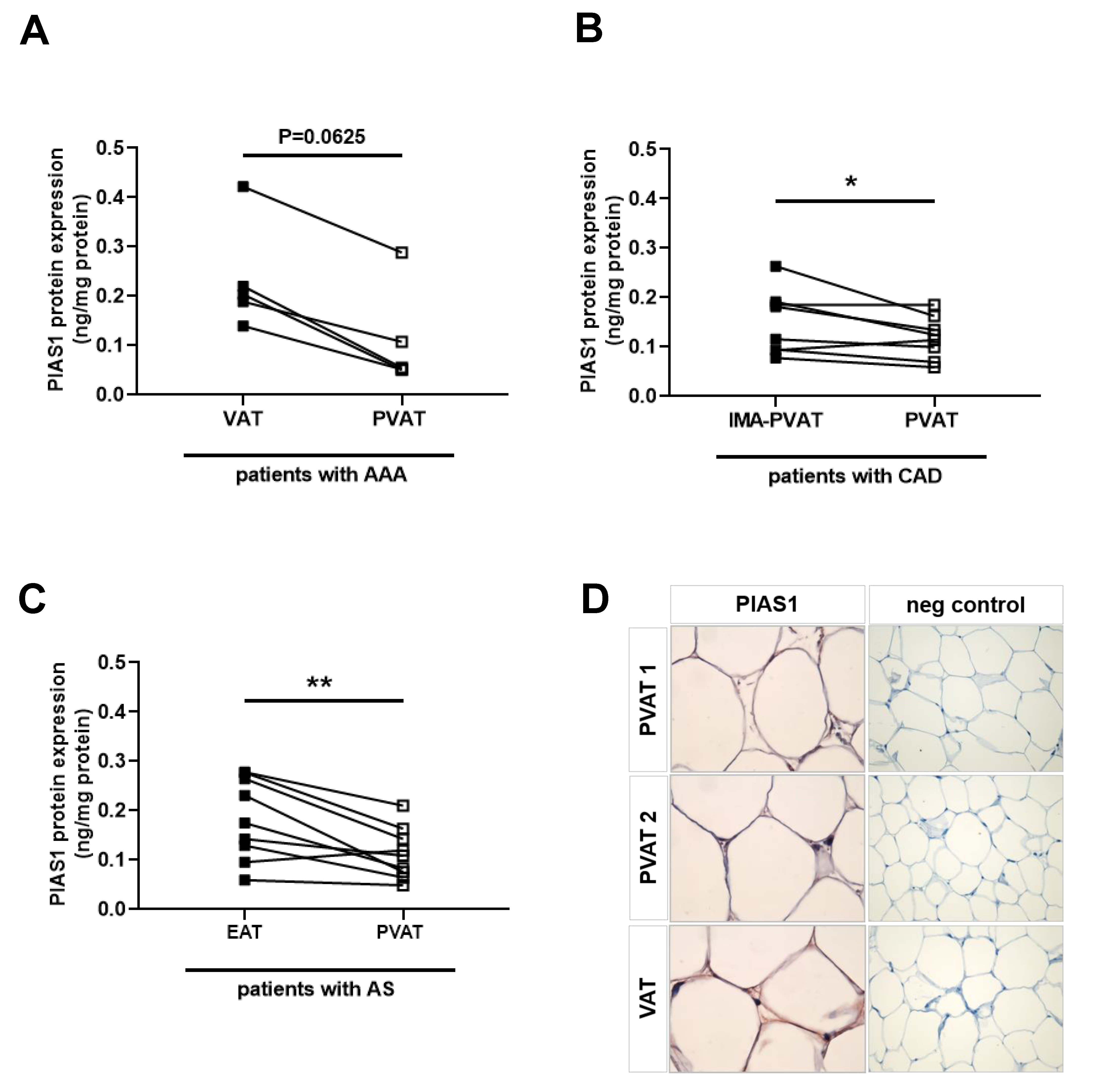
2.8. Reduced PIAS Levels in Human Perivascular Adipose Tissue Surrounding Atherosclerotic Vessels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Serum Analysis
4.3. Vascular Tissue Harvest and Characterization
4.4. Adipose Tissue Harvest and Characterization
4.5. Carotid Artery INJURY
4.6. Perivascular Adipose Tissue Harvest and Transplantation
4.7. Morphometry of Neointima Formation
4.8. Immunohistochemistry
4.9. Isolation and Analysis of Primary Smooth Muscle Cells
4.10. Adipogenic Differentiation and Analysis of Murine 3T3L1 Fibroblasts
4.11. RNA Isolation and PCR Analysis
4.12. Protein Isolation and Western Blot Analysis
4.13. Human (Perivascular) Adipose Tissue Samples
4.14. Enzyme-Linked Immunosorbent Assay
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| AS | aortic valve stenosis |
| AT | adipose tissue |
| BAT | brown adipose tissue |
| CAD | coronary artery disease |
| FBS | fetal bovine serum |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| MTC | Masson trichrome |
| NC | normal chow |
| PBS | phosphate-buffered saline |
| PVAT | perivascular adipose tissue |
| SASP | senescence-associated secretory phenotype |
| SMC | smooth muscle cell |
| Tx | transplantation |
| VAT | visceral adipose tissue |
| VES | Verhoeff’s elastic stain |
| WT | wild-type |
References
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rittger, H.; Waliszewski, M.; Brachmann, J.; Hohenforst-Schmidt, W.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Schlundt, C.; et al. Long-term outcomes after treatment with a paclitaxel-coated balloon versus balloon angioplasty: Insights from the pepcad-des study (treatment of drug-eluting stent [des] in-stent restenosis with sequent please paclitaxel-coated percutaneous transluminal coronary angioplasty [ptca] catheter). JACC Cardiovasc. Interv. 2015, 8, 1695–1700. [Google Scholar] [PubMed]
- Batchelor, W.B.; Anstrom, K.J.; Muhlbaier, L.H.; Grosswald, R.; Weintraub, W.S.; O’Neill, W.W.; Peterson, E.D. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: Results in 7,472 octogenarians. National cardiovascular network collaboration. J. Am. Coll. Cardiol. 2000, 36, 723–730. [Google Scholar] [CrossRef]
- Cohen, H.A.; Williams, D.O.; Holmes, D.R., Jr.; Selzer, F.; Kip, K.E.; Johnston, J.M.; Holubkov, R.; Kelsey, S.F.; Detre, K.M. Impact of age on procedural and 1-year outcome in percutaneous transluminal coronary angioplasty: A report from the nhlbi dynamic registry. Am. Heart J. 2003, 146, 513–519. [Google Scholar] [CrossRef]
- De, G.J.; Kobayashi, Y.; Albiero, R.; Reimers, B.; Di, M.C.; Finci, L.; Colombo, A. Coronary artery stenting in the elderly: Short-term outcome and long-term angiographic and clinical follow-up. J. Am. Coll. Cardiol. 1998, 32, 577–583. [Google Scholar]
- Hariri, R.J.; Alonso, D.R.; Hajjar, D.P.; Coletti, D.; Weksler, M.E. Aging and arteriosclerosis. I. Development of myointimal hyperplasia after endothelial injury. J. Exp. Med. 1986, 164, 1171–1178. [Google Scholar] [CrossRef]
- Eghbalieh, S.D.; Chowdhary, P.; Muto, A.; Ziegler, K.R.; Kudo, F.A.; Pimiento, J.M.; Mirmehdi, I.; Model, L.S.; Kondo, Y.; Nishibe, T.; et al. Age-related neointimal hyperplasia is associated with monocyte infiltration after balloon angioplasty. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 109–117. [Google Scholar] [CrossRef]
- Vazquez-Padron, R.I.; Lasko, D.; Li, S.; Louis, L.; Pestana, I.A.; Pang, M.; Liotta, C.; Fornoni, A.; Aitouche, A.; Pham, S.M. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J. Vasc. Surg. 2004, 40, 1199–1207. [Google Scholar] [CrossRef]
- Chajara, A.; Delpech, B.; Courel, M.N.; Leroy, M.; Basuyau, J.P.; Levesque, H. Effect of aging on neointima formation and hyaluronan, hyaluronidase and hyaluronectin production in injured rat aorta. Atherosclerosis 1998, 138, 53–64. [Google Scholar] [CrossRef]
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part iii: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497. [Google Scholar]
- Shi, Y.; O’Brien, J.E.; Fard, A.; Mannion, J.D.; Wang, D.; Zalewski, A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 1996, 94, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Moos, M.P.; John, N.; Grabner, R.; Nossmann, S.; Gunther, B.; Vollandt, R.; Funk, C.D.; Kaiser, B.; Habenicht, A.J. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikias, G.; Konstantinides, S.; Schäfer, K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Leifheit-Nestler, M.; Hubert, A.; Schumann, B.; Glückermann, R.; Eschholz, N.; Krüger, N.; Lutz, S.; Hasenfuss, G.; Konstantinides, S.; et al. Leptin promotes neointima formation and smooth muscle cell proliferation via nadph oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc. Res. 2013, 99, 555–565. [Google Scholar] [CrossRef][Green Version]
- Takaoka, M.; Nagata, D.; Kihara, S.; Shimomura, I.; Kimura, Y.; Tabata, Y.; Saito, Y.; Nagai, R.; Sata, M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ. Res. 2009, 105, 906–911. [Google Scholar] [CrossRef]
- Maffia, P.; Grassia, G.; Di, M.P.; Carnuccio, R.; Berrino, L.; Garside, P.; Ianaro, A.; Ialenti, A. Neutralization of interleukin-18 inhibits neointimal formation in a rat model of vascular injury. Circulation 2006, 114, 430–437. [Google Scholar] [CrossRef][Green Version]
- Sahara, M.; Ikutomi, M.; Morita, T.; Minami, Y.; Nakajima, T.; Hirata, Y.; Nagai, R.; Sata, M. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc. Res. 2014, 101, 236–246. [Google Scholar] [CrossRef]
- Fujishima, Y.; Maeda, N.; Matsuda, K.; Masuda, S.; Mori, T.; Fukuda, S.; Sekimoto, R.; Yamaoka, M.; Obata, Y.; Kita, S.; et al. Adiponectin association with t-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017, 31, 1571–1583. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.N.; Chen, H.Z.; Gao, P.; Zhu, L.H.; Li, H.L.; Lv, X.; Zhang, Q.J.; Zhang, R.; Wang, Z.; et al. Sirt1 acts as a modulator of neointima formation following vascular injury in mice. Circ. Res. 2011, 108, 1180–1189. [Google Scholar] [CrossRef]
- Chen, S.; Kapturczak, M.H.; Wasserfall, C.; Glushakova, O.Y.; Campbell-Thompson, M.; Deshane, J.S.; Joseph, R.; Cruz, P.E.; Hauswirth, W.W.; Madsen, K.M.; et al. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Schütz, E.; Bochenek, M.L.; Riehl, D.R.; Bosmann, M.; Münzel, T.; Konstantinides, S.; Schäfer, K. Absence of Transforming Growth Factor beta 1 in murine platelets reduces neointima formation without affecting arterial thrombosis. Thromb. Haemost. 2017, 17, 1782–1797. [Google Scholar]
- Manka, D.; Chatterjee, T.K.; Stoll, L.L.; Basford, J.E.; Konaniah, E.S.; Srinivasan, R.; Bogdanov, V.Y.; Tang, Y.; Blomkalns, A.L.; Hui, D.Y.; et al. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: Role of monocyte chemoattractant protein-1. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Cox, J.H.; Bellac, C.L.; Doucet, A.; Starr, A.E.; Overall, C.M. Macrophage-specific metalloelastase (mmp-12) truncates and inactivates elr+ cxc chemokines and generates ccl2, -7, -8, and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 2008, 112, 3455–3464. [Google Scholar] [CrossRef]
- Liu, B.; Liao, J.; Rao, X.; Kushner, S.A.; Chung, C.D.; Chang, D.D.; Shuai, K. Inhibition of stat1-mediated gene activation by pias1. Proc. Natl. Acad. Sci. USA 1998, 95, 10626–10631. [Google Scholar] [CrossRef]
- Liu, B.; Yang, R.; Wong, K.A.; Getman, C.; Stein, N.; Teitell, M.A.; Cheng, G.; Wu, H.; Shuai, K. Negative regulation of nf-kappab signaling by pias1. Mol. Cell. Biol. 2005, 25, 1113–1123. [Google Scholar] [CrossRef]
- Schmidt, D.; Muller, S. Members of the pias family act as sumo ligases for c-jun and p53 and repress p53 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 2872–2877. [Google Scholar] [CrossRef]
- Richard, A.J.; Stephens, J.M. The role of jak-stat signaling in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 431–439. [Google Scholar] [CrossRef]
- Sims, F.H. A comparison of coronary and internal mammary arteries and implications of the results in the etiology of arteriosclerosis. Am. Heart J. 1983, 105, 560–566. [Google Scholar] [CrossRef]
- Calfa, M.; Aitouche, A.; Vazquez-Padron, R.I.; Gay-Rabinstein, C.; Lasko, D.; Badell, J.; Farji, A.; El-Haddad, A.; Liotta, C.; Louis, L.B.; et al. Aging and transplant arteriosclerosis in absence of alloreactivity and immunosuppressive drugs in a rat aortic model: Recipient age’s contribution. Transplantation 2005, 79, 1683–1690. [Google Scholar] [CrossRef]
- Stemerman, M.B.; Weinstein, R.; Rowe, J.W.; Maciag, T.; Fuhro, R.; Gardner, R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc. Natl. Acad. Sci. USA 1982, 79, 3863–3866. [Google Scholar] [CrossRef]
- Wang, M.; Spinetti, G.; Monticone, R.E.; Zhang, J.; Wu, J.; Jiang, L.; Khazan, B.; Telljohann, R.; Lakatta, E.G. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS ONE 2011, 6, e16653. [Google Scholar] [CrossRef]
- Urano, Y.; Shirai, K.; Watanabe, H.; Miyashita, Y.; Hashiguchi, S. Vascular smooth muscle cell outgrowth, proliferation, and apoptosis in young and old rats. Atherosclerosis 1999, 146, 101–105. [Google Scholar] [CrossRef]
- Torella, D.; Leosco, D.; Indolfi, C.; Curcio, A.; Coppola, C.; Ellison, G.M.; Russo, V.G.; Torella, M.; Li Volti, G.; Rengo, F.; et al. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2850–H2860. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Evan, G.I.; Schwartz, S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Investig. 1995, 95, 2266–2274. [Google Scholar] [CrossRef]
- O’Brien, E.R.; Alpers, C.E.; Stewart, D.K.; Ferguson, M.; Tran, N.; Gordon, D.; Benditt, E.P.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ. Res. 1993, 73, 223–231. [Google Scholar] [CrossRef]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- McCaffrey, T.A.; Nicholson, A.C.; Szabo, P.E.; Weksler, M.E.; Weksler, B.B. Aging and arteriosclerosis. The increased proliferation of arterial smooth muscle cells isolated from old rats is associated with increased platelet-derived growth factor-like activity. J. Exp. Med. 1988, 167, 163–174. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Wang, M.; Lakatta, E.G.; Sonntag, W.E.; Ungvari, Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate macaca mulatta: Reversal by resveratrol treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 811–820. [Google Scholar] [CrossRef]
- Chignier, E.; Eloy, R. Adventitial resection of small artery provokes endothelial loss and intimal hyperplasia. Surg. Gynecol. Obstet. 1986, 163, 327–334. [Google Scholar]
- Takaoka, M.; Suzuki, H.; Shioda, S.; Sekikawa, K.; Saito, Y.; Nagai, R.; Sata, M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1576–1582. [Google Scholar] [CrossRef]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Lipinski, M.J.; Doran, A.C.; Skaflen, M.D.; Fuster, V.; McNamara, C.A. Lymphocytes and the adventitial immune response in atherosclerosis. Circ. Res. 2012, 110, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Downs, L.C.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: A paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 780–792. [Google Scholar] [CrossRef]
- Barandier, C.; Montani, J.P.; Yang, Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: Effects of aging and obesity. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1807–H1813. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Dou, X.; Guo, L.; Liu, Y.; Zhou, S.R.; Wei, X.B.; Qian, S.W.; Huang, H.Y.; Xu, C.J.; et al. Protein inhibitor of activated stat 1 (pias1) protects against obesity-induced insulin resistance by inhibiting inflammation cascade in adipose tissue. Diabetes 2015, 64, 4061–4074. [Google Scholar] [CrossRef]
- Cai, Z.; Ding, Y.; Zhang, M.; Lu, Q.; Wu, S.; Zhu, H.; Song, P.; Zou, M.H. Ablation of adenosine monophosphate-activated protein kinase alpha1 in vascular smooth muscle cells promotes diet-induced atherosclerotic calcification in vivo. Circ. Res. 2016, 119, 422–433. [Google Scholar] [CrossRef]
- Woo, C.H.; Shishido, T.; McClain, C.; Lim, J.H.; Li, J.D.; Yang, J.; Yan, C.; Abe, J. Extracellular signal-regulated kinase 5 sumoylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ. Res. 2008, 102, 538–545. [Google Scholar] [CrossRef]
- Heo, K.S.; Chang, E.; Takei, Y.; Le, N.T.; Woo, C.H.; Sullivan, M.A.; Morrell, C.; Fujiwara, K.; Abe, J. Phosphorylation of protein inhibitor of activated stat1 (pias1) by mapk-activated protein kinase-2 inhibits endothelial inflammation via increasing both pias1 transrepression and sumo e3 ligase activity. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 321–329. [Google Scholar] [CrossRef]
- Liu, B.; Mink, S.; Wong, K.A.; Stein, N.; Getman, C.; Dempsey, P.W.; Wu, H.; Shuai, K. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat. Immunol. 2004, 9, 891–898. [Google Scholar] [CrossRef]
- Constanzo, J.D.; Deng, M.; Rindhe, S.; Tang, K.; Zhang, C.C.; Scaglioni, P.P. Pias1 is essential for erythroid and vascular development in the mouse embryo. Dev. Biol. 2016, 414, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Bochenek, M.L.; Schütz, E.; Gogiraju, R.; Münzel, T.; Schäfer, K. Selective deletion of leptin signaling in endothelial cells enhances neointima formation and phenocopies the vascular effects of diet-induced obesity in mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1683–1697. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Adult | Middle-Aged |
|---|---|---|
| number | 20 | 20 |
| sex | male | male |
| age (weeks) | 16 | 52 |
| diet | 10% NC | 10% NC |
| body weight (g) | 32.0 ± 0.98 | 36.7 ± 0.83 *** |
| BAT weight (mg) | 0.34 ± 0.04 | 0.30 ± 0.03 |
| PVAT weight (mg) | n.d. | n.d. |
| SCATax weight (mg) | 0.29 ± 0.04 | 0.35 ± 0.05 |
| SCATing weight (mg) | 0.52 ± 0.06 | 0.65 ± 0.08 |
| VAT weight (mg) | 0.74 ± 0.10 | 1.12 ± 0.14 * |
| number | 15 | 15 |
| leptin (ng/mL) | 12.1 ± 3.9 | 13.1 ± 4.0 |
| glucose (mg/dL) | 160 ± 19 | 232 ± 21 * |
| insulin (µIU/mL) | 6.2 ± 0.7 | 5.1 ± 0.2 |
| HOMA-IR | 2.6 ± 0.5 | 2.9 ± 0.3 |
| total cholesterol (mg/dL) | 103 ± 4.7 | 106 ± 6.7 |
| HDL cholesterol (mg/dL) | 82 ± 4.7 | 80 ± 4.9 |
| LDL cholesterol (mg/dL) | 17 ± 2.0 | 15 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schütz, E.; Gogiraju, R.; Pavlaki, M.; Drosos, I.; Georgiadis, G.S.; Argyriou, C.; Rim Ben Hallou, A.; Konstantinou, F.; Mikroulis, D.; Schüler, R.; et al. Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation. Int. J. Mol. Sci. 2020, 21, 282. https://doi.org/10.3390/ijms21010282
Schütz E, Gogiraju R, Pavlaki M, Drosos I, Georgiadis GS, Argyriou C, Rim Ben Hallou A, Konstantinou F, Mikroulis D, Schüler R, et al. Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation. International Journal of Molecular Sciences. 2020; 21(1):282. https://doi.org/10.3390/ijms21010282
Chicago/Turabian StyleSchütz, Eva, Rajinikanth Gogiraju, Maria Pavlaki, Ioannis Drosos, George S. Georgiadis, Christos Argyriou, Amina Rim Ben Hallou, Fotios Konstantinou, Dimitrios Mikroulis, Rebecca Schüler, and et al. 2020. "Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation" International Journal of Molecular Sciences 21, no. 1: 282. https://doi.org/10.3390/ijms21010282
APA StyleSchütz, E., Gogiraju, R., Pavlaki, M., Drosos, I., Georgiadis, G. S., Argyriou, C., Rim Ben Hallou, A., Konstantinou, F., Mikroulis, D., Schüler, R., Bochenek, M. L., Gachkar, S., Buschmann, K., Lankeit, M., Karbach, S. H., Münzel, T., Tziakas, D., Konstantinides, S., & Schäfer, K. (2020). Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation. International Journal of Molecular Sciences, 21(1), 282. https://doi.org/10.3390/ijms21010282






