Antitumor Potential and Side Effects of Natural and Synthetic Compounds
Share This Topical Collection
Editor
 Prof. Dr. Hiroshi Sakagami
Prof. Dr. Hiroshi Sakagami
 Prof. Dr. Hiroshi Sakagami
Prof. Dr. Hiroshi Sakagami
E-Mail
Website
Guest Editor
Meikai University Research Institute of Odontology (M-RIO), 1-1 Keyakidai, Sakado, Saitama 350-0283, Japan
Interests: informatics; information network; pharmacy; biological pharmacy; basic medicine; general pharmacology; boundary medicine; laboratory medicine; dentistry
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Although many antitumor substances have been isolated from the natural kingdom, most authors have shown their cytotoxicity against malignant cells, but not against non-malignant cells. Recently, synthetic compounds of chromones and α,β-unsaturated ketones have shown very high antitumor potential, sometimes exceeding that of anticancer drugs.
This Topical Collection calls for papers exploring the antitumor potential of natural and synthetic compounds against both malignant and non-malignant cells and their adverse effects. The papers that focus on the exploration of preparative or synthetic methods and the elucidation of action mechanisms are also within the scope of this issue.
Prof. Dr. Hiroshi Sakagami
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Medicines is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1400 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (4 papers)
Open AccessArticle
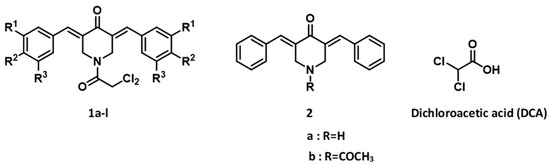
Dichloroacetyl Amides of 3,5-Bis(benzylidene)-4-piperidones Displaying Greater Toxicity to Neoplasms than to Non-Malignant Cells
by
Mohammad Hossain, Praveen K. Roayapalley, Hiroshi Sakagami, Keitaro Satoh, Kenjiro Bandow, Umashankar Das and Jonathan R. Dimmock
Cited by 1 | Viewed by 2863
Abstract
A series of 3,5-bis(benzylidene)-1-dichloroacetyl-4-piperidones
1a–
l was evaluated against Ca9-22, HSC-2, HSC-3, and HSC-4 squamous cell carcinomas. Virtually all of the compounds displayed potent cytotoxicity, with 83% of the CC
50 values being submicromolar and several CC
50 values being in the
[...] Read more.
A series of 3,5-bis(benzylidene)-1-dichloroacetyl-4-piperidones
1a–
l was evaluated against Ca9-22, HSC-2, HSC-3, and HSC-4 squamous cell carcinomas. Virtually all of the compounds displayed potent cytotoxicity, with 83% of the CC
50 values being submicromolar and several CC
50 values being in the double digit nanomolar range. The compounds were appreciably less toxic to human HGF, HPLF, and HPC non-malignant cells, which led to some noteworthy selectivity index (SI) figures. From these studies,
1d,
g,
k emerged as the lead molecules in terms of their potencies and SI values. A Quantitative Structure-Activity Relationship (QSAR) study revealed that cytotoxic potencies and potency–selectivity expression figures increased when the magnitude of the sigma values in the aryl rings was elevated. The modes of action of the representative cytotoxins in Ca9-22 cells were found to include G2/M arrest and stimulation of the cells to undergo mitosis and cause poly(ADP-ribose) polymerase (PARP) and procaspase 3 cleavage.
Full article
►▼
Show Figures
Open AccessArticle
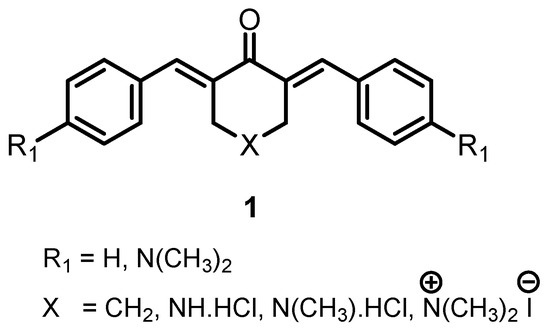
Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring
by
Praveen K. Roayapalley, Hiroshi Sakagami, Keitaro Satoh, Shigeru Amano, Kenjiro Bandow, Renato J. Aguilera, Karla G. Cano Hernandez, Austre Y. Schiaffino Bustamante, Stephen G. Dimmock, Rajendra K. Sharma, Umashankar Das and Jonathan R. Dimmock
Cited by 3 | Viewed by 3454
Abstract
A series of 3,5-bis(benzylidene)-4-piperidones
2a–
u were prepared as candidate cytotoxic agents. In general, the compounds are highly toxic to human gingival carcinoma (Ca9-22), human squamous carcinoma-2 (HSC-2) and human squamous carcinoma-4 (HSC-4) neoplasms, but less so towards non-malignant human gingival fibroblast
[...] Read more.
A series of 3,5-bis(benzylidene)-4-piperidones
2a–
u were prepared as candidate cytotoxic agents. In general, the compounds are highly toxic to human gingival carcinoma (Ca9-22), human squamous carcinoma-2 (HSC-2) and human squamous carcinoma-4 (HSC-4) neoplasms, but less so towards non-malignant human gingival fibroblast (HGF), human periodontal ligament fibroblast (HPLF) and human pulp cells (HPC), thereby demonstrating tumour-selective toxicity. A further study revealed that most of the compounds in series
2 were more toxic to the human Colo-205 adenocarcinoma cell line (Colo-205), human HT29 colorectal adenocarcinoma cells (HT-29) and human CEM lymphoid cells (CEM) neoplasms than towards non-malignant human foreskin Hs27 fibroblast line (Hs27) cells. The potency of the cytotoxins towards the six malignant cell lines increased as the sigma and sigma star values of the aryl substituents rose. Attempts to condense various aryl aldehydes with 2,2,6,6-tetramethyl-4-piperidone led to the isolation of some 1,5-diaryl-1,4-pentadien-3-ones. The highest specificity for oral cancer cells was displayed by
2e and
2r. In the case of
2r, its selective toxicity exceeded that of doxorubicin and melphalan. The enones
2k,
m, o have the highest SI values towards colon cancer and leukemic cells. Both
2e,
r inhibited mitosis and increased the subG1 population (with a transient increase in G2/M phase cells). Slight activation of caspase-3, based on the cleavage of poly(ADP-ribose)polymerase (PARP) and procaspase 3, was detected.
Full article
►▼
Show Figures
Open AccessArticle
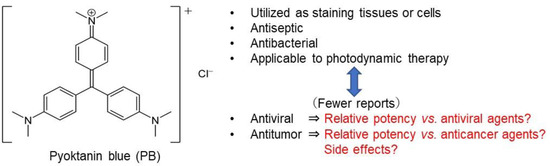
Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue
by
Hiroshi Sakagami, Toshiko Furukawa, Keitaro Satoh, Shigeru Amano, Yosuke Iijima, Takuro Koshikawa, Daisuke Asai, Kunihiko Fukuchi, Hiromu Takemura, Taisei Kanamoto and Satoshi Yokose
Cited by 3 | Viewed by 4508
Abstract
Background: Pyoktanin blue (PB) is used for staining tissues and cells, and it is applied in photodynamic therapy due to its potent bactericidal activity. However, clinical application of PB as an antiviral and antitumor agent has been limited due to its potent toxicity.
[...] Read more.
Background: Pyoktanin blue (PB) is used for staining tissues and cells, and it is applied in photodynamic therapy due to its potent bactericidal activity. However, clinical application of PB as an antiviral and antitumor agent has been limited due to its potent toxicity. For clinical application, the antitumor and antiviral activity as well as the neurotoxicity of PB were re-evaluated with a chemotherapeutic index.
Methods: Tumor-specificity (TS) was determined by the ratio of CC
50 against normal oral cells/oral squamous cell carcinoma (OSCC); neurotoxicity by that of normal oral/neuronal cells; antiviral activity by that of mock-infected/virus-infected cells; and potency-selectivity expression (PSE) by dividing TS by CC
50 (OSCC).
Results: Antitumor activity of PB (assessed by TS and PSE) was comparable with that of DXR and much higher than that of 5-FU and melphalan. PB induced caspase-3 activation and subG1 cell accumulation in an OSCC cell line (Ca9-22). PB and anticancer drugs showed comparable cytotoxicity against both neuronal cells and OSCC cell lines. PB showed no detectable anti-HIV/HSV activity, in contrast to reverse transferase inhibitors, sulfated glucans, and alkaline extract of leaves of S.P.
Conclusions: PB showed first-class anticancer activity and neurotoxicity, suggesting the importance of establishing the safe treatment schedule.
Full article
►▼
Show Figures
Open AccessArticle
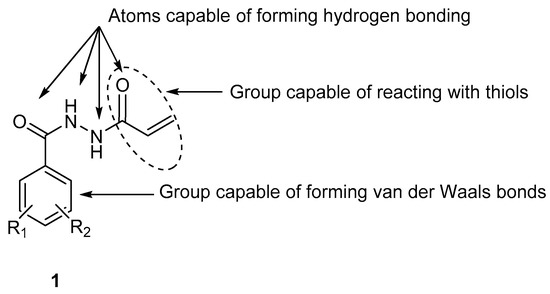
Design, Syntheses, and Bioevaluations of Some Novel N2-Acryloylbenzohydrazides as Chemostimulants and Cytotoxic Agents
by
Kinjal Lakhani, Edgar A. Borrego, Karla G. Cano, Jonathan R. Dimmock, Renato J. Aguilera, Swagatika Das, Praveen K. Roayapalley, Rajendra K. Sharma and Umashankar Das
Viewed by 3985
Abstract
A series of novel
N2-acryloylhydrazides
1a–m and a related series of compounds
6a–c were prepared as potential chemostimulants. In general, these compounds are cytotoxic to human HCT 116 colon cancer cells, as well as human MCF-7 and MDA-MB-231 breast cancer cell
[...] Read more.
A series of novel
N2-acryloylhydrazides
1a–m and a related series of compounds
6a–c were prepared as potential chemostimulants. In general, these compounds are cytotoxic to human HCT 116 colon cancer cells, as well as human MCF-7 and MDA-MB-231 breast cancer cell lines. A representative compound
N1-(3,4-dimethoxyphenylcarbonyl)-
N2-acryloylhydrazine
1m sensitized HCT 116 cells to the potent antineoplastic agent 3,5-bis(benzylidene)-4-piperidone
2a, and also to 5-fluorouracil. A series of compounds was prepared that incorporated some of the molecular features of
2a and related compounds with various
N2-acryloylhydrazides in series 1. These compounds are potent cytotoxins. Two modes of action of representative compounds are the lowering of mitochondrial membrane potential and increasing the concentration of reactive oxygen species.
Full article
►▼
Show Figures