Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Assay for Cytotoxic Activity
2.4. Calculation of Tumor-Specificity Index (TS)
2.5. Calculation of Potency-Selectivity Expression (PSE)
2.6. Cell Cycle Analysis
2.7. Western Blot Analysis
2.8. Assay for Anti-Human Immunodeficiency Virus (HIV) Activity
2.9. Assay for Anti-Herpes Simplex Virus (HSV) Activity
2.10. Statistical Treatment
3. Results
3.1. Tumor-Specificity of Pyokanin (PB)
3.2. Potent Neurotoxicity of PB
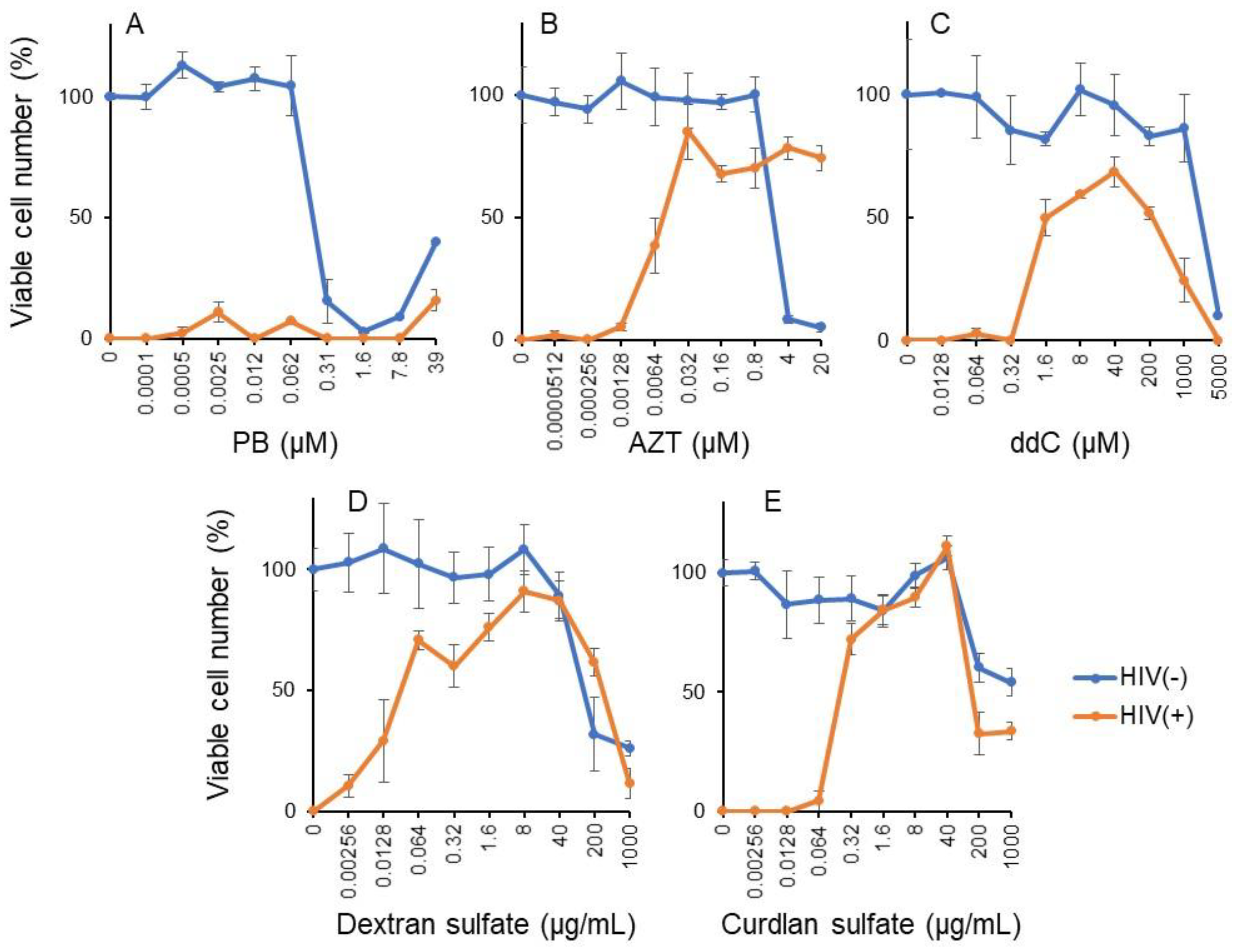
3.3. Re-Evaluation of Antiviral Activity of PB
3.3.1. Pyoktanin (PB) failed to induce anti-HIV activity
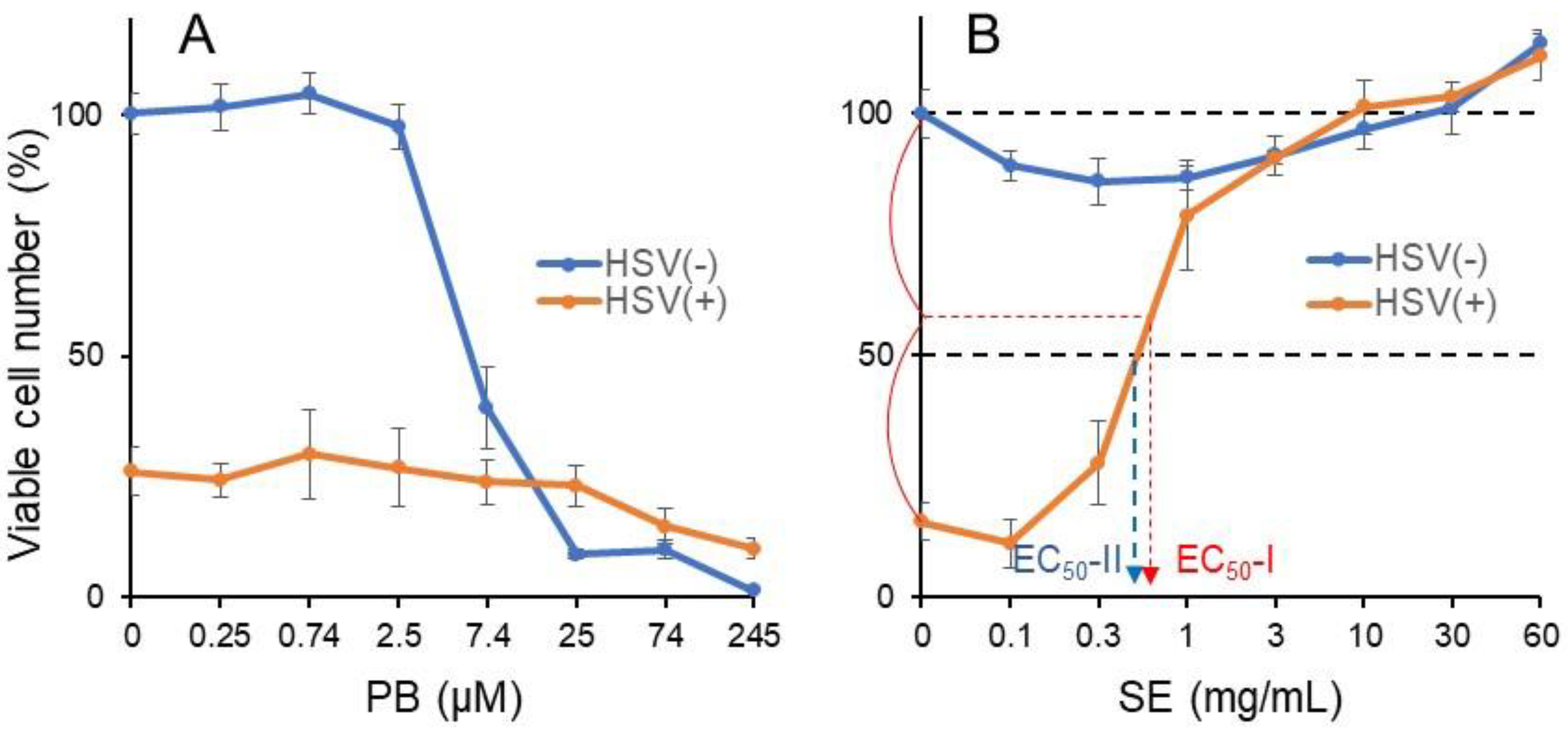
3.3.2. Pyoktanin (PB) failed to induce anti-HSV activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanadan, A.; Kamyab-Hesari, K.; Daneshpajouh, M.; Balighi, K.; Normohammadpour, P. Nodular colloid degeneration of the skin: Report of three cases with review and update. Indian Dermatol. Online J. 2014, 5, S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Sasaki, T.; Tomura, N.; Okada, H.; Kuwata, T. Removal of a malignant cystic brain tumor utilizing pyoktanin blue and fibrin glue: Technical note. Surg. Neurol. Int. 2017, 8, 24. [Google Scholar] [CrossRef]
- Otani, N.; Wada, K.; Toyooka, T.; Takeuchi, S.; Tomiyama, A.; Mori, K. Usefulness of dural surface tracing of the cortical vessels with indocyanine green videoangiography just prior to dural opening for various cerebrovascular diseases. Surg. Neurol. Int. 2017, 8, 201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takamiya, S.; Seki, T.; Yamazaki, K.; Sasamori, T.; Houkin, K. Intraoperative Visualization of a Spinal Arachnoid Cyst Using Pyoktanin Blue. World Neurosurg. 2018, 109, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Sapra, A. Gram Staining. In StatPearls, StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Churchman, J.W. The Selective Bactericidal Action of Gentian Violet. J. Exp. Med. 1912, 16, 221–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomes-de-Elvas, A.R.; Palmeira-de-Oliveira, A.; Gaspar, C.; Gouveia, P.; Palmeira-de-Oliveira, R.; Pina-Vaz, C.; Rodrigues, A.G.; Martinez-de-Oliveira, J. In vitro assessment of gentian violet anti- candida activity. Gynecol. Obs. Investig. 2012, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Sakagami, H.; Horiike, M.; Kadokura, H.; Yamasaki, T.; Klokkevold, P.R.; Takei, H.H.; Yokose, S. Photodynamic Therapy with Pyoktanin Blue and Diode Laser for Elimination of Enterococcus faecalis. In Vivo 2018, 32, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and antibiofilm photodynamic therapy against vancomycin resistant Staphylococcus aureus (VRSA) induced infection in vitro and in vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.B.; Costa, D.H.; Miyakawa, W.; Delgado, M.G.T.; Garcez, A.S.; Yoshimura, T.M.; Ribeiro, M.S.; Nunez, S.C. Photodynamic Activity on Biofilm in Endotracheal Tubes of Patients Admitted to an Intensive Care Unit. Photochem. Photobiol. 2020, 96, 618–624. [Google Scholar] [CrossRef]
- Kazuno, K.; Kinoshita, H.; Hori, M.; Yosizaki, T.; Tamura, A.; Sato, H.; Murata, S. Endovascular treatment for mycotic aneurysm using pyoktanin- applied devices. CVIR Endovasc. 2020, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Maley, A.M.; Arbiser, J.L. Gentian violet: A 19th century drug re-emerges in the 21st century. Exp. Derm. 2013, 22, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Aljofan, M.; Sganga, M.L.; Lo, M.K.; Rootes, C.L.; Porotto, M.; Meyer, A.G.; Saubern, S.; Moscona, A.; Mungall, B.A. Antiviral activity of gliotoxin, gentian violet and brilliant green against Nipah and Hendra virus in vitro. Virol. J. 2009, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, A. Inactivation of influenza A virus by gentian violet (GV) and GV-dyed cotton cloth, and bactericidal activities of these agents. J. Infect. Chemother. 2006, 12, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Xiao, L.Y.; Wu, P.C.; Chen, Y.K.; Lo, S.; Hu, S.C.S.; Chen, Y.H.; Chiu, C.C.C.; Yuan, S.S.F. Orabase-formulated gentian violet effectively improved oral potentially malignant disorder in vitro and in vivo. Biochem. Pharm. 2020, 171, 113713. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Vikulina, T.; Weitzmann, M.N. Gentian violet inhibits MDA-MB-231 human breast cancer cell proliferation, and reverses the stimulation of osteoclastogenesis and suppression of osteoblast activity induced by cancer cells. Oncol. Rep. 2015, 34, 2156–2162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugita, Y.; Takao, K.; Uesawa, Y.; Nagai, J.; Iijima, Y.; Sano, M.; Sakagami, H. Development of Newly Synthesized Chromone Derivatives with High Tumor Specificity against Human Oral Squamous Cell Carcinoma. Medicines 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Kantoh, K.; Ono, M.; Nakamura, Y.; Nakamura, Y.; Hashimoto, K.; Sakagami, H.; Wakabayashi, H. Hormetic and anti-radiation effects of tropolone-related compounds. In Vivo 2010, 24, 843–851. [Google Scholar] [PubMed]
- Sakagami, H.; Shi, H.; Bandow, K.; Tomomura, M.; Tomomura, A.; Horiuchi, M.; Fujisawa, T.; Oizumi, T. Search of Neuroprotective Polyphenols Using the “Overlay” Isolation Method. Molecules 2018, 23, 1840. [Google Scholar] [CrossRef]
- Iijima, Y.; Bandow, K.; Amano, S.; Sano, M.; Hino, S.; Kaneko, T.; Horie, N.; Sakagami, H. Protection of Bortezomib-induced Neurotoxicity by Antioxidants. Anticancer Res 2020, 40, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, M.; Kimura, Y.; Nagura, H.; Ono, T.; Ito, H. A new human cell line derived from human carcinoma of the gingiva. I. Its establishment and morphological studies. Nihon Koku Geka Gakkai Zasshi 1974, 20, 100–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamali, C.; Sakagami, H.; Uesawa, Y.; Kurosaki, K.; Satoh, K.; Masuda, Y.; Yokose, S.; Ece, A.; Bua, S.; Angeli, A.; et al. Comprehensive study on potent and selective carbonic anhydrase inhibitors: Synthesis, bioactivities and molecular modelling studies of 4-(3-(2-arylidenehydrazine-1-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-1-yl) benzenesulfonamides. Eur. J. Med. Chem. 2021, 217, 113351. [Google Scholar] [CrossRef]
- Miyoshi, I.; TAGUCHI, H.; Kubonishi, I.; Yoshimoto, S.; Ohtsuki, Y.; Shiraishi, Y.; Akagi, T. Type C virus-producing cell lines derived from adult T cell leukemia. Gann Monogr. Cancer Res. 1982, 28, 219–229. [Google Scholar]
- Fukuchi, K.; Sakagami, H.; Sugita, Y.; Takao, K.; Asai, D.; Terakubo, S.; Takemura, H.; Ohno, H.; Horiuchi, M.; Suguro, M.; et al. Quantification of the Ability of Natural Products to Prevent Herpes Virus Infection. Medicines 2020, 7, 64. [Google Scholar] [CrossRef]
- Sakagami, H.; Fukuchi, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Natori, T.; Suguro-Kitajima, M.; Oizumi, H.; Yasui, T.; Oizumi, T. Synergism of Alkaline Extract of the Leaves of Sasa senanensis Rehder and Antiviral Agents. In Vivo 2016, 30, 421–426. [Google Scholar]
- Sakagami, H.; Okudaira, N.; Masuda, Y.; Amano, O.; Yokose, S.; Kanda, Y.; Suguro, M.; Natori, T.; Oizumi, H.; Oizumi, T. Induction of Apoptosis in Human Oral Keratinocyte by Doxorubicin. Anticancer Res. 2017, 37, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Bandow, K.; Sano, M.; Hino, S.; Kaneko, T.; Horie, N.; Sakagami, H. In Vitro Assessment of Antitumor Potential and Combination Effect of Classical and Molecular-targeted Anticancer Drugs. Anticancer Res. 2019, 39, 6673–6684. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, N.A.; Blackwell, B.N.; Hewitt, C.C.; Gaylor, D.W. Chronic toxicity and carcinogenicity studies of gentian violet in mice. Fundam. Appl. Toxicol. 1985, 5, 902–912. [Google Scholar] [CrossRef]
- Meng Sun, K.R.; Gwendolyn Osborne, M.; Marder, E.; Schmitz, R. Proposition 65 Evidence on the Carcinogenicity of Gentian Violet; Branch, R.a.C.H.A., Ed.; Office of Environmental Health Hazard, Assessment California Environmental Protection Agency: Sacramento, CA, USA, 2018. [Google Scholar]
- Nakaya, G.; Sakagami, H.; Koga-Ogawa, Y.; Shiroto, A.; Nobesawa, T.; Ueda, D.; Nakatani, S.; Kobata, K.; Iijima, Y.; Tone, S.; et al. Augmentation of Neurotoxicity of Anticancer Drugs by X-ray Irradiation. In Vivo 2020, 34, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Sakagami, H.; Shi, H.; Abe, T.; Tamura, N.; Takeshima, H.; Horie, N.; Kaneko, T.; Shiratsuchi, H.; Kaneko, T. Partial Protection of Paclitaxel-induced Neurotoxicity by Antioxidants. In Vivo 2018, 32, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Tsuji, M.; Tomomura, M.; Masuda, Y.; Iwama, S.; Nakagawa, M.; Suzuki, H.; Tanaka, K.; Abe, T.; Tamura, N.; et al. Protection of Differentiating Neuronal Cells from Amyloid β Peptide-induced Injury by Alkaline Extract of Leaves of Sasa senanensis Rehder. In Vivo 2018, 32, 231–239. [Google Scholar] [CrossRef]
- Sakagami, H.; Hara, Y.; Shi, H.; Iwama, S.; Nakagawa, M.; Suzuki, H.; Tanaka, K.; Abe, T.; Tamura, N.; Takeshima, H.; et al. Change in Anticancer Drug Sensitivity During Neuronal Differentiation of PC12 Cells. In Vivo 2018, 32, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Mollman, J.E.; Hogan, W.M.; Glover, D.J.; McCluskey, L.F. Unusual presentation of cis-platinum neuropathy. Neurology 1988, 38, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Bellm, L.A.; Epstein, J.B.; Rose-Ped, A.; Martin, P.; Fuchs, H.J. Patient reports of complications of bone marrow transplantation. Support. Care Cancer 2000, 8, 33–39. [Google Scholar] [CrossRef]
- Vera-Llonch, M.; Oster, G.; Ford, C.M.; Lu, J.; Sonis, S. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support. Care Cancer 2007, 15, 491–496. [Google Scholar] [CrossRef]
- Harrold, K.; Gould, D.; Drey, N. The management of cytotoxic chemotherapy extravasation: A systematic review of the literature to evaluate the evidence underpinning contemporary practice. Eur. J. Cancer Care 2015, 24, 771–800. [Google Scholar] [CrossRef]
- Kreidieh, F.Y.; Moukadem, H.A.; El Saghir, N.S. Overview, prevention and management of chemotherapy extravasation. World J. Clin. Oncol. 2016, 7, 87–97. [Google Scholar] [CrossRef]
- Sand, L.; Jalouli, J. Viruses and oral cancer. Is there a link? Microbes Infect. 2014, 16, 371–378. [Google Scholar] [CrossRef]
- Kim, R.H.; Yochim, J.M.; Kang, M.K.; Shin, K.H.; Christensen, R.; Park, N.H. HIV-1 Tat enhances replicative potential of human oral keratinocytes harboring HPV-16 genome. Int. J. Oncol. 2008, 33, 777–782. [Google Scholar] [PubMed]

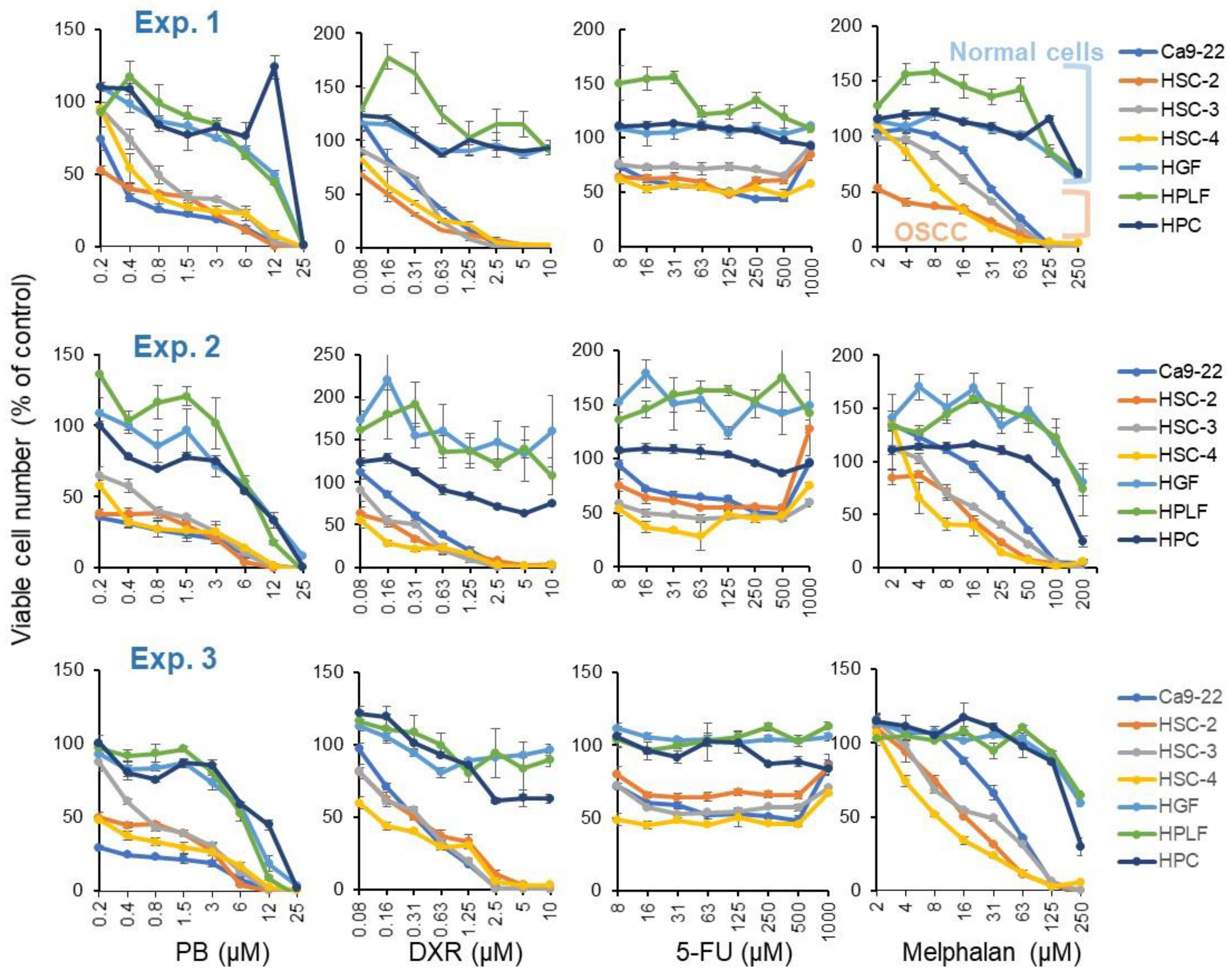


| CC50 (μM) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human Oral Squamous Carcinoma Cell | Human Normal Oral Cells | TS | PSE | ||||||||||
| Ca9-22 | HSC-2 | HSC-3 | HSC-4 | Mean | HGF | HPLF | HPC | Mean | |||||
| A | B | C | D | D/B | C/A | 100D/B2 | 100C/A2 | ||||||
| PB | 0.30 | 0.26 | 0.81 | 0.46 | 0.46 | 12.1 | 10.5 | 19.6 | 14.1 | 30.8 | 40.0 | 6722 | 13,197 |
| DXR | 0.41 | 0.16 | 0.43 | 0.20 | 0.30 | 10.0 | 7.1 | 10.0 | 9.0 | 30.0 | 24.4 | 9986 | 5930 |
| 5-FU | 124 | 109 | 1000 | 73 | 326 | 1000 | 100 | 1000 | 1000 | 3.1 | 8.1 | 1 | 7 |
| Mel | 27 | 12 | 19 | 9 | 17 | 200 | 200 | 200 | 200 | 11.8 | 7.4 | 70 | 27 |
| PB | 0.19 | 0.19 | 0.54 | 0.23 | 0.29 | 8.3 | 7.6 | 7.2 | 7.7 | 26.6 | 43.5 | 9203 | 22,734 |
| DXR | 0.45 | 0.18 | 0.27 | 0.09 | 0.25 | 10.0 | 10.0 | 10.0 | 10.0 | 40.7 | 22.1 | 16,525 | 4902 |
| 5-FU | 314 | 1000 | 20 | 9 | 336 | 1000 | 1000 | 1000 | 1000 | 3.0 | 3.2 | 1 | 1 |
| Mel | 39 | 11 | 18 | 5 | 18 | 200 | 200 | 155 | 185 | 10.3 | 5.2 | 57 | 13 |
| PB | 0.19 | 0.24 | 0.61 | 0.23 | 0.32 | 7.3 | 6.4 | 10.4 | 8.0 | 25.4 | 37.9 | 8067 | 19,850 |
| DXR | 0.33 | 0.34 | 0.38 | 0.14 | 0.30 | 10.0 | 10.0 | 10.0 | 10.0 | 33.5 | 29.9 | 11,192 | 8964 |
| 5-FU | 136 | 1000 | 1000 | 31 | 542 | 1000 | 1000 | 1000 | 1000 | 1.8 | 7.4 | 0.3 | 5 |
| Mel | 38 | 13 | 23 | 6 | 20 | 200 | 200 | 165 | 188 | 9.4 | 5.2 | 46 | 14 |
| CC50 (μM). | |||||||
|---|---|---|---|---|---|---|---|
| Neuronal Cells | |||||||
| PC12 | SH-SY5Y | LY-PPB6 | Diff.PC-12 | Mean | OSCC | Neurotoxicity | |
| E | B | B/E | |||||
| PB | 0.179 | 0.162 | 0.132 | 0.252 | 0.181 1 | 0.36 2 | 2.0 |
| DXR | 0.069 | 0.02 | 0.323 | 0.215 | 0.157 | 0.28 | 1.8 |
| 5-FU | 14 | 5.85 | 125 | 297 | 110 | 401 | 3.6 |
| Melphalan | 7.72 | 5.89 | 12.3 | 26.4 | 13.1 | 18.3 | 1.4 |
| CC50 (µg/mL) | EC50 (µg/mL) | SI | |
|---|---|---|---|
| PB | 0.168 μM | >39 μM | <1 |
| Positive controls: | |||
| AZT | 48.3 μM | 0.0095 μM | 5082 |
| ddC | 2163 μM | 1.61 μM | 1340 |
| Dextran sulfate | 120 μg/mL | 0.0286 μg/mL | 4200 |
| Curdlan sulfate | >1000 μg/mL | 0.189 μg/mL | >5294 |
| Test Sample | Viability of HSV-Infected Cells | CC50 | EC50-I | EC50-II | Anti-HSV Activity | Max. Cell Recovery | |
|---|---|---|---|---|---|---|---|
| SI-I | SI-II | ||||||
| PB | 26.0% | 5.88 μM | >245 μM | >245 μM | <0.02 | <0.02 | 29.7% |
| Positive control: | |||||||
| SE | 14.2% | >60 mg/mL | >0.78 mg/mL | 0.67 mg/mL | >76.9 | >89.6 | 101.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakagami, H.; Furukawa, T.; Satoh, K.; Amano, S.; Iijima, Y.; Koshikawa, T.; Asai, D.; Fukuchi, K.; Takemura, H.; Kanamoto, T.; et al. Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue. Medicines 2021, 8, 33. https://doi.org/10.3390/medicines8070033
Sakagami H, Furukawa T, Satoh K, Amano S, Iijima Y, Koshikawa T, Asai D, Fukuchi K, Takemura H, Kanamoto T, et al. Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue. Medicines. 2021; 8(7):33. https://doi.org/10.3390/medicines8070033
Chicago/Turabian StyleSakagami, Hiroshi, Toshiko Furukawa, Keitaro Satoh, Shigeru Amano, Yosuke Iijima, Takuro Koshikawa, Daisuke Asai, Kunihiko Fukuchi, Hiromu Takemura, Taisei Kanamoto, and et al. 2021. "Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue" Medicines 8, no. 7: 33. https://doi.org/10.3390/medicines8070033
APA StyleSakagami, H., Furukawa, T., Satoh, K., Amano, S., Iijima, Y., Koshikawa, T., Asai, D., Fukuchi, K., Takemura, H., Kanamoto, T., & Yokose, S. (2021). Re-Evaluation of Chemotherapeutic Potential of Pyoktanin Blue. Medicines, 8(7), 33. https://doi.org/10.3390/medicines8070033







