Chloroform Fraction of Prasiola japonica Ethanolic Extract Alleviates UPM 1648a-Induced Lung Injury by Suppressing NF-κB Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
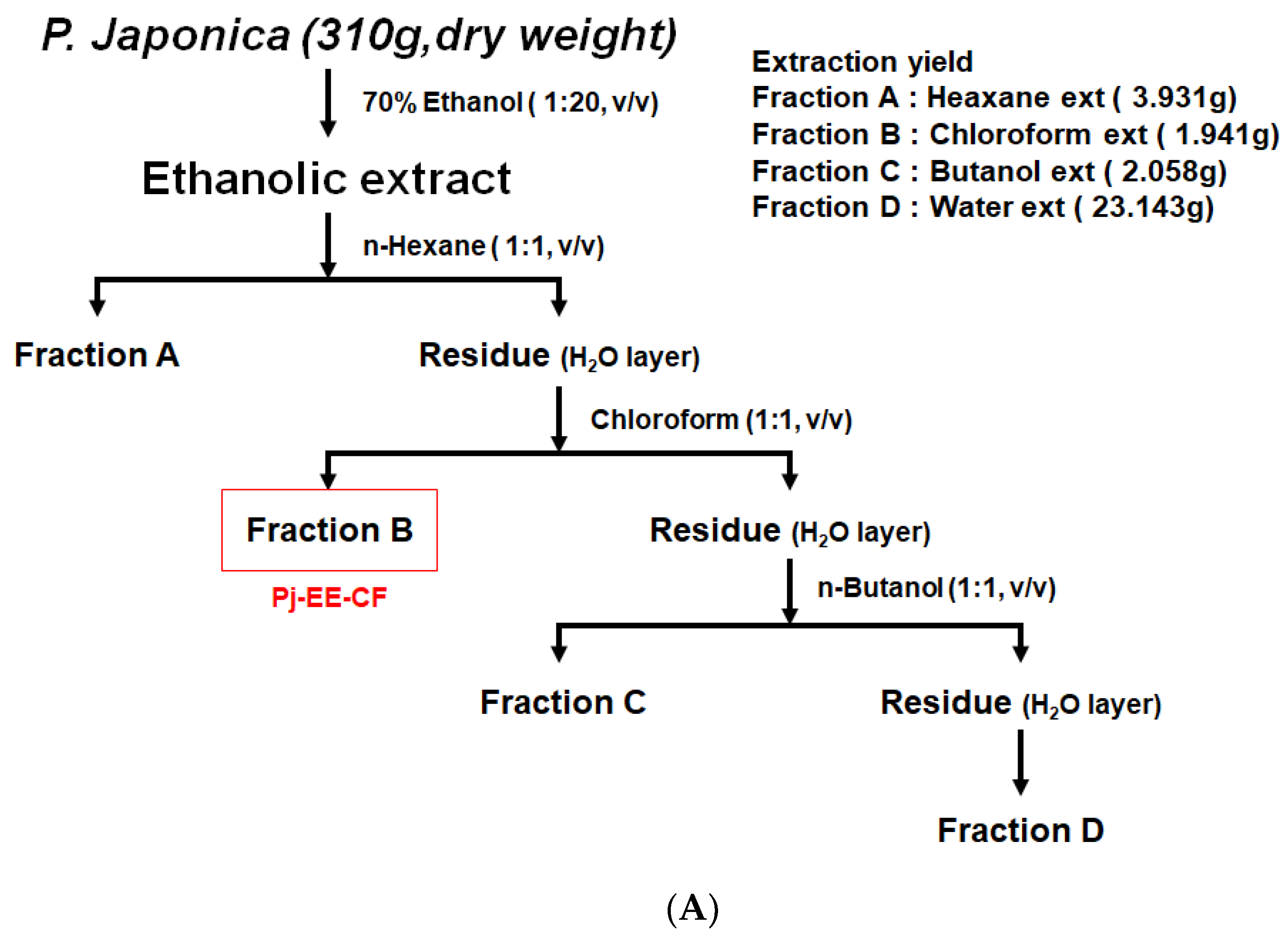
2.2. Pj-EE and Fraction Preparation
2.3. Cell Culture and Cell Viability Assay
2.4. Animals
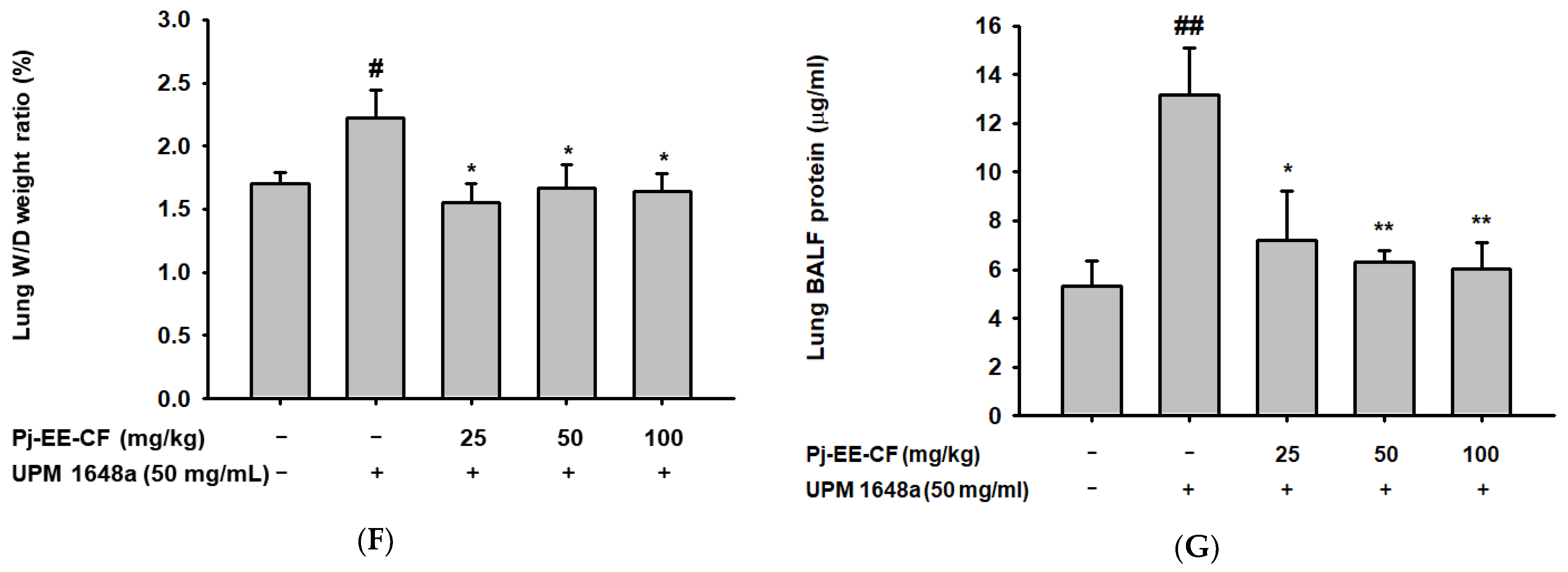
2.5. Lung Wet-to-Dry Weight Ratio and Protein Concentration Ratio Measurement
2.6. Histological Analysis of Lung Tissue
2.7. ELISA in BALF and Lung Tissue Lysate
2.8. Whole-Cell Lysate Preparation and Western Blotting Analysis
2.9. Luciferase Reporter Gene Activity
2.10. Cellular ROS Assay
2.11. Statistical Analysis
3. Results
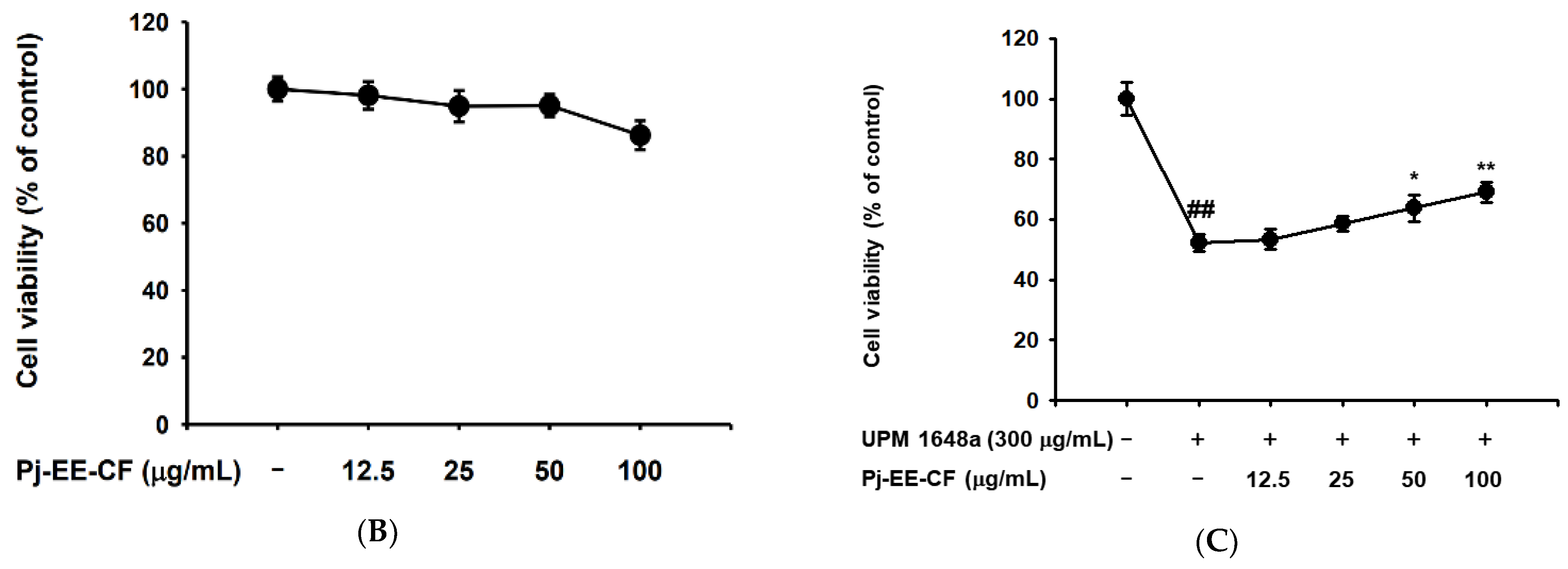
3.1. Pj-EE-CF Protects UPM 1648a-Exposed Human Bronchial Epithelium Cells
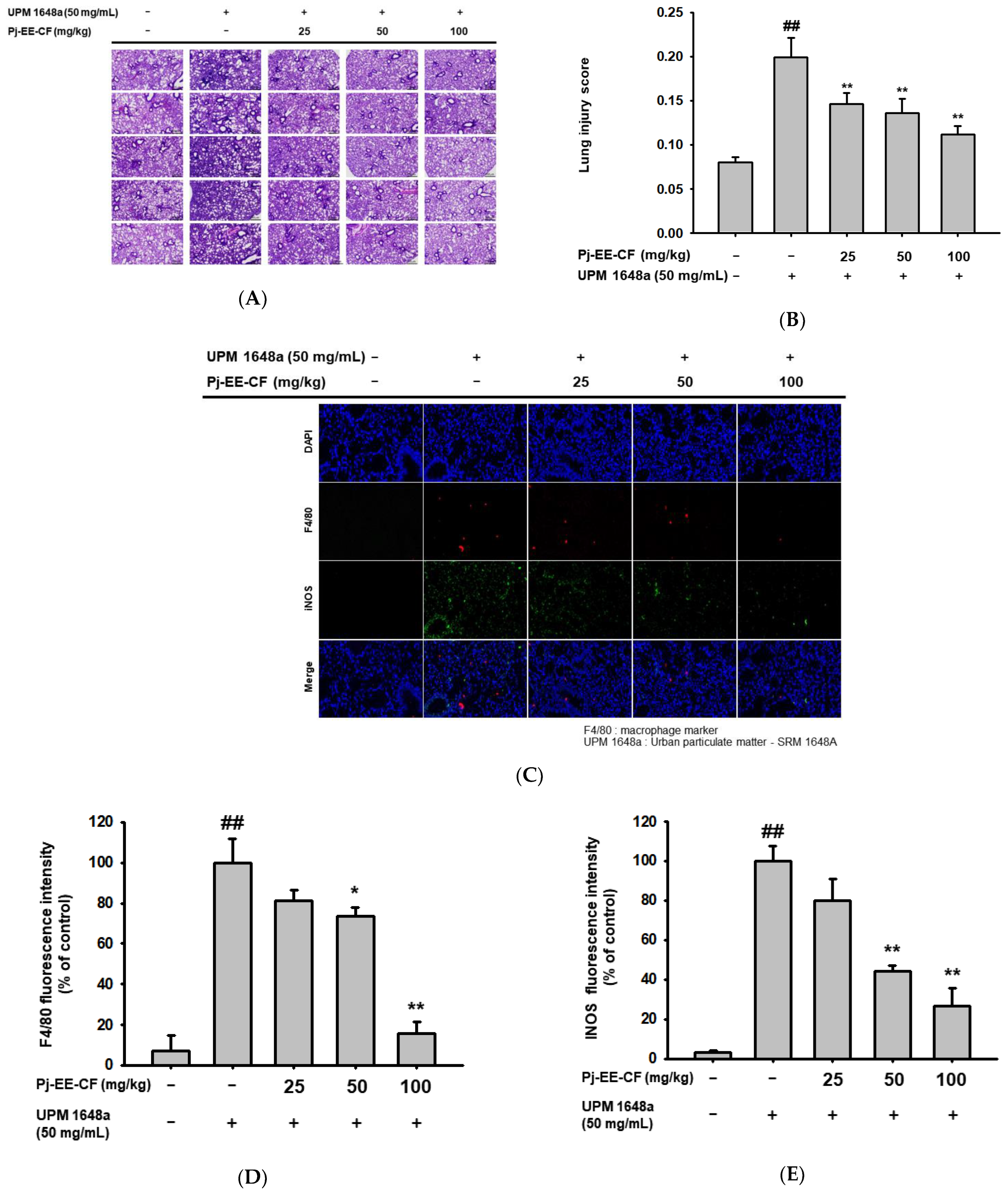
3.2. Pj-EE-CF Alleviates Pathological Changes of Lung Tissues in UPM 1648a-Stimulated Mice
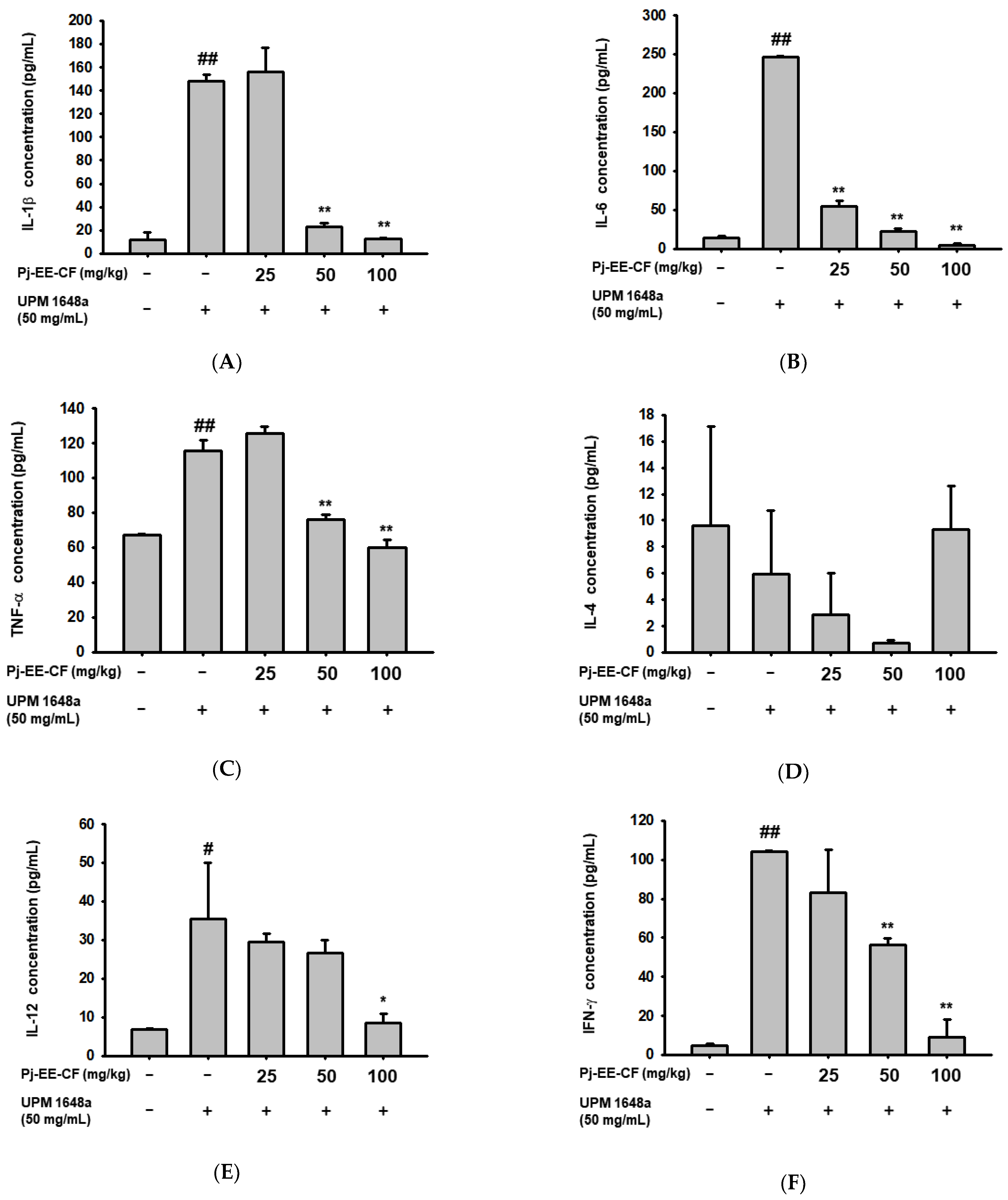
3.3. Pj-EE-CF Suppresses UPM 1648a-Induced Cytokine Levels in BALF
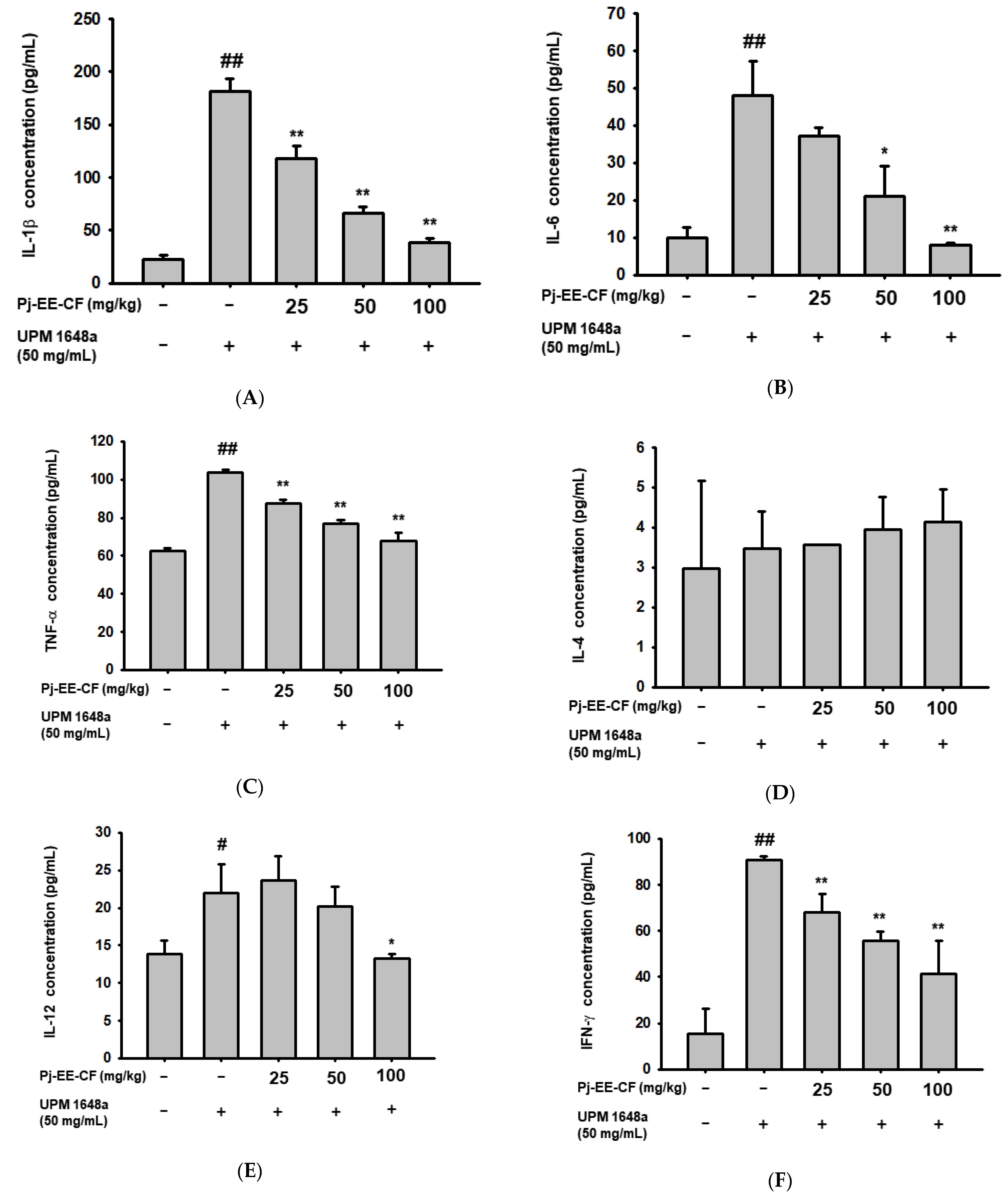
3.4. Pj-EE-CF Decreases UPM 1648a-Induced Cytokine Production in Lung Tissues
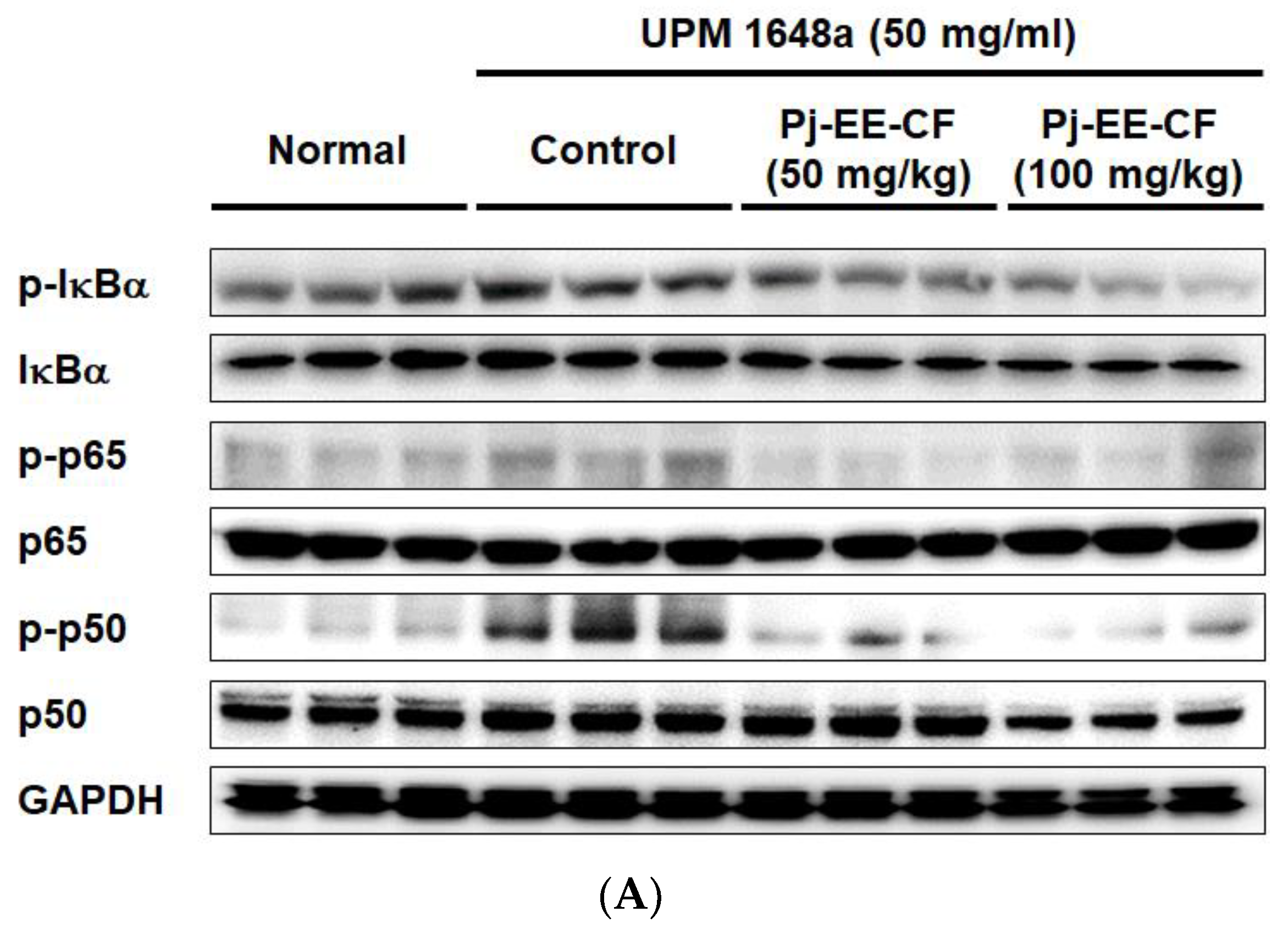
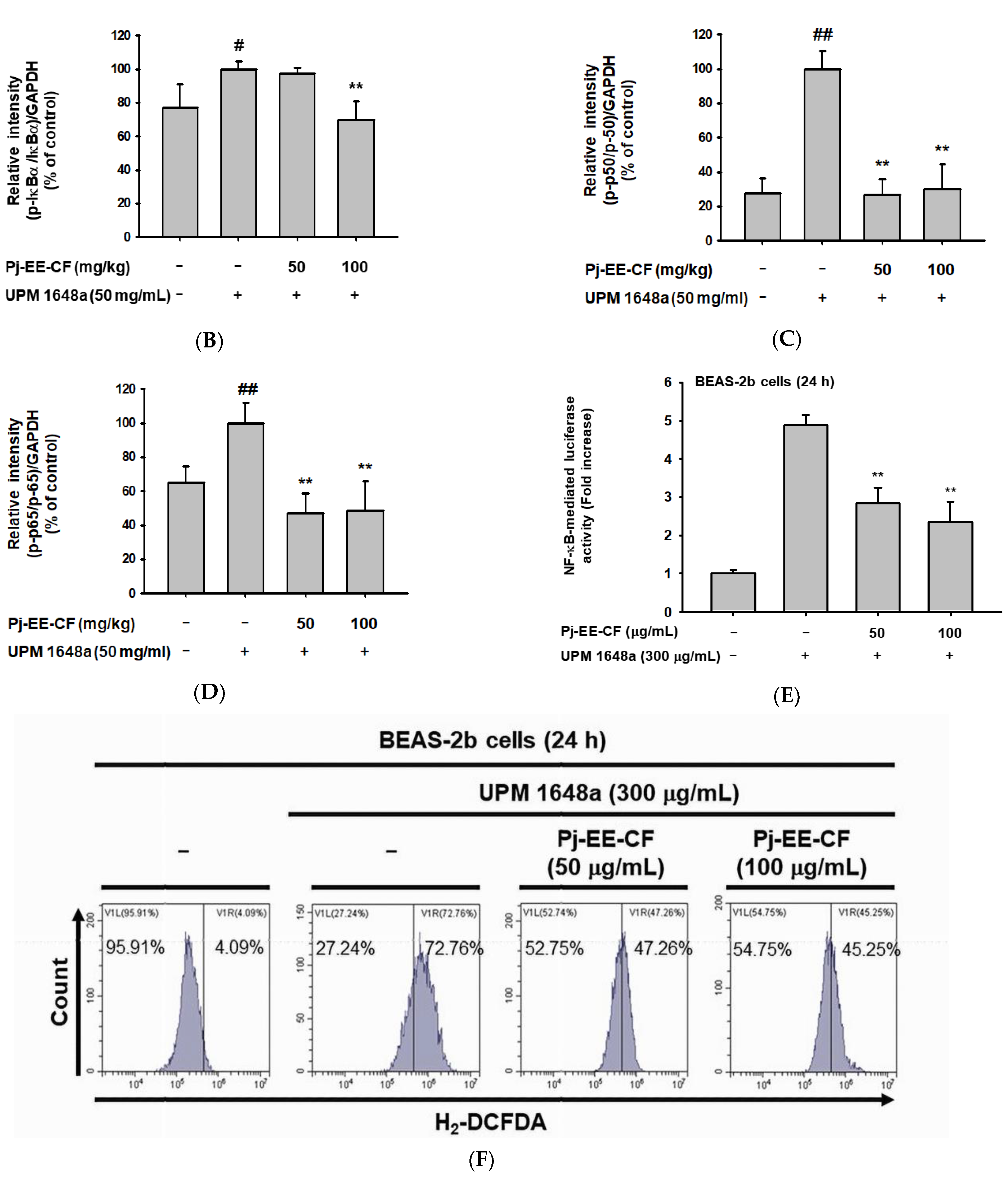
3.5. Pj-EE-CF Suppresses NF-κB and Exerts Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aditya, T.; Bitu, G.; Mercy Eleanor, G. The role of algae in pharmaceutical development. Res. Rev. J. Pharm. Nanotechnol. 2016, 4, 82–89. [Google Scholar]
- Moniz, M.B.; Rindi, F.; Guiry, M.D. Phylogeny and taxonomy of prasiolales (trebouxiophyceae, chlorophyta) from Tasmania, including Rosenvingiella tasmanica sp. Nov. Phycologia 2012, 51, 86–97. [Google Scholar] [CrossRef]
- Park, M.; Kim, W.; Chung, I.; Lee, E. Study on the Prasiola sp. In Korea. I. Ecological and morphological studies on the Prasiola sp. In the Samchuck-Chodang. Korean J. Bot. 1970, 13, 71–80. [Google Scholar]
- Kim, M.S.; Jun, M.-S.; Kim, C.A.; Yoon, J.; Kim, J.H.; Cho, G.Y. Morphology and phylogenetic position of a freshwater Prasiola species (Prasiolales, Chlorophyta) in Korea. Algae 2015, 30, 197–205. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Choi, E.; Kim, S.; Kim, D.S.; Kim, J.H.; Chang, S.; Choi, J.S.; Park, K.J.; Roh, K.-B.; Lee, J. Oxidative stress-protective and anti-melanogenic effects of loliolide and ethanol extract from fresh water green algae, Prasiola japonica. Int. J. Mol. Sci. 2018, 19, 2825. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Yi, Y.S.; Lee, J.; Park, S.H.; Kim, S.; Hossain, M.A.; Jang, S.; Choi, Y.I.; Park, K.J.; Kim, D.S.; et al. Anti-apoptotic and anti-inflammatory activities of edible fresh water algae Prasiola japonica in UVB-irradiated skin keratinocytes. Am. J. Chin. Med. 2019, 47, 1853–1868. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, S.H.; Lim, H.Y.; Jang, S.G.; Park, K.J.; Kim, D.S.; Kim, J.H.; Cho, J.Y. In vivo anti-inflammatory effects of Prasiola japonica ethanol extract. J. Funct. Foods 2021, 80, 104440. [Google Scholar] [CrossRef]
- Rahmawati, L.; Park, S.H.; Kim, D.S.; Lee, H.P.; Aziz, N.; Lee, C.Y.; Kim, S.A.; Jang, S.G.; Kim, D.S.; Cho, J.Y. Anti-inflammatory activities of the ethanol extract of Prasiola japonica, an edible freshwater green algae, and its various solvent fractions in lps-induced macrophages and carrageenan-induced paw edema via the ap-1 pathway. Molecules 2021, 27, 194. [Google Scholar] [CrossRef]
- Baek, K.S.; Yi, Y.S.; Son, Y.J.; Yoo, S.; Sung, N.Y.; Kim, Y.; Hong, S.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J. Ginseng Res. 2016, 40, 437–444. [Google Scholar] [CrossRef]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Hong, Y.C. Decrease in ambient fine particulate matter during COVID-19 crisis and corresponding health benefits in Seoul, Korea. Int. J. Environ. Res. Public Health 2020, 17, 5279. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Smith, C.B.; Madden, M.C. Diesel exhaust particles and airway inflammation. Curr. Opin. Pulm. Med. 2012, 18, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, X.; Zhao, S.; Yang, Z.; Yuan, W.; Han, H.; Zhang, B.; Zhou, L.; Zheng, S.; Li, M.D. Landscape analysis of lncrnas shows that ddx11-as1 promotes cell-cycle progression in liver cancer through the parp1/p53 axis. Cancer Lett. 2021, 520, 282–294. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate matter air pollution: Effects on the cardiovascular system. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Tong, S. Air pollution and disease burden. Lancet Planet. Health 2019, 3, e49–e50. [Google Scholar] [CrossRef]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef]
- Ziou, M.; Tham, R.; Wheeler, A.J.; Zosky, G.R.; Stephens, N.; Johnston, F.H. Outdoor particulate matter exposure and upper respiratory tract infections in children and adolescents: A systematic review and meta-analysis. Environ. Res. 2022, 210, 112969. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Migliaccio, C.T.; Kobos, E.; King, Q.O.; Porter, V.; Jessop, F.; Ward, T. Adverse effects of wood smoke PM2.5 exposure on macrophage functions. Inhal. Toxicol. 2013, 25, 67–76. [Google Scholar] [CrossRef]
- Psoter, K.J.; De Roos, A.J.; Mayer, J.D.; Kaufman, J.D.; Wakefield, J.; Rosenfeld, M. Fine particulate matter exposure and initial pseudomonas aeruginosa acquisition in cystic fibrosis. Ann. Am. Thorac. Soc. 2015, 12, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Ezzati, M.; Hall, L.; Dickson, I.; Kirwan, M.; Png, K.M.; Mudway, I.S.; Grigg, J. Adhesion of Streptococcus pneumoniae to human airway epithelial cells exposed to urban particulate matter. J. Allergy Clin. Immunol. 2011, 127, 1236–1242.e1232. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Zhou, J.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir. Res. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Barrier, M.; Begorre, M.A.; Baudrimont, I.; Dubois, M.; Freund-Michel, V.; Marthan, R.; Savineau, J.P.; Muller, B.; Courtois, A. Involvement of heme oxygenase-1 in particulate matter-induced impairment of no-dependent relaxation in rat intralobar pulmonary arteries. Toxicol. In Vitro 2016, 32, 205–211. [Google Scholar] [CrossRef]
- Beauchef, G.; Favre-Mercuret, M.; Blanc, B.; Fitoussi, R.; Vié, K.; Compagnone, N. Effect of red panax ginseng on mitochondrial dynamics and bioenergetics in hacat cells exposed to urban pollutants. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 84–95. [Google Scholar]
- Nowak, B.; Majka, G.; Srottek, M.; Skalkowska, A.; Marcinkiewicz, J. The effect of inhaled air particulate matter srm 1648a on the development of mild collagen-induced arthritis in dba/j mice. Arch. Immunol. Ther. Exp. 2022, 70, 17. [Google Scholar] [CrossRef]
- Gawda, A.; Majka, G.; Nowak, B.; Srottek, M.; Walczewska, M.; Marcinkiewicz, J. Air particulate matter srm 1648a primes macrophages to hyperinflammatory response after lps stimulation. Inflamm. Res. 2018, 67, 765–776. [Google Scholar] [CrossRef]
- Lorz, L.R.; Kim, D.; Kim, M.Y.; Cho, J.Y. Panax ginseng-derived fraction biogf1k reduces atopic dermatitis responses via suppression of mitogen-activated protein kinase signaling pathway. J. Ginseng Res. 2020, 44, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lorz, L.R.; Lee, J.; Cho, J.Y. In vitro photoprotective, anti-inflammatory, moisturizing, and antimelanogenic effects of a methanolic extract of chrysophyllum lucentifolium cronquist. Plants 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Kim, D.S.; Park, S.H.; Shin, C.Y.; Woo, J.J.; Kim, J.W.; An, R.B.; Lee, C.; Cho, J.Y. Antioxidant Capacity of Potentilla paradoxa> Nutt. and Its Beneficial Effects Related to Anti-Aging in HaCaT and B16F10 Cells. Plants 2022, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Hwang, S.H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Jang, W.Y.; Lee, H.P.; Kim, S.A.; Huang, L.; Yoon, J.H.; Shin, C.Y.; Mitra, A.; Kim, H.G.; Cho, J.Y. Angiopteris cochinchinensis de vriese ameliorates lps-induced acute lung injury via src inhibition. Plants 2022, 11, 1306. [Google Scholar] [CrossRef]
- Mitra, A.; Rahmawati, L.; Lee, H.P.; Kim, S.A.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean red ginseng water extract inhibits cadmium-induced lung injury via suppressing mapk/erk1/2/ap-1 pathway. J. Ginseng Res. 2022, 46, 690–699. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; Acute Lung Injury in Animals Study Group. An official american thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, E.; Hong, Y.H.; Kim, H.; Jang, Y.J.; Lee, J.S.; Choung, E.S.; Woo, B.Y.; Hong, Y.D.; Lee, S.; et al. Syk/nf-kappab-targeted anti-inflammatory activity of Melicope accedens (Blume) T.G. Hartley methanol extract. J. Ethnopharmacol. 2021, 271, 113887. [Google Scholar] [CrossRef]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Park, S.K.; Yeon, S.H.; Choi, M.R.; Choi, S.H.; Lee, S.B.; Rha, K.S.; Kim, Y.M. Urban particulate matters may affect endoplasmic reticulum stress and tight junction disruption in nasal epithelial cells. Am. J. Rhinol. Allergy 2021, 35, 817–829. [Google Scholar] [CrossRef]
- Van Vyve, T.; Chanez, P.; Bernard, A.; Bousquet, J.; Godard, P.; Lauwerijs, R.; Sibille, Y. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J. Allergy Clin. Immunol. 1995, 95, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. Nf-κb signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.C.; Huxford, T.; Ghosh, G.J.S.I. Nf-κb, iκb, and ikk: Integral components of immune system signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and nf-kappab signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Frontini, F.; Qi, W.; Hariharan, A.; Ronner, M.; Wipplinger, M.; Blanquart, C.; Rehrauer, H.; Fonteneau, J.F.; Felley-Bosco, E. Endogenous retrovirus expression activates type-i interferon signaling in an experimental mouse model of mesothelioma development. Cancer Lett. 2021, 507, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Casal-Mourino, A.; Ruano-Ravina, A.; Torres-Duran, M.; Parente-Lamelas, I.; Provencio-Pulla, M.; Castro-Anon, O.; Vidal-Garcia, I.; Pena-Alvarez, C.; Abal-Arca, J.; Pineiro-Lamas, M.; et al. Lung cancer survival in never-smokers and exposure to residential radon: Results of the lcrins study. Cancer Lett. 2020, 487, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. Pm 2.5. Proc. Natl. Acad. Sci. USA 2013, 110, 8756. [Google Scholar] [CrossRef]
- Xu, X.C.; Wu, Y.F.; Zhou, J.S.; Chen, H.P.; Wang, Y.; Li, Z.Y.; Zhao, Y.; Shen, H.H.; Chen, Z.H. Autophagy inhibitors suppress environmental particulate matter-induced airway inflammation. Toxicol. Lett. 2017, 280, 206–212. [Google Scholar] [CrossRef]
- Xia, Y.; Dolgor, S.; Jiang, S.; Fan, R.; Wang, Y.; Wang, Y.; Tang, J.; Zhang, Y.; He, R.L.; Yu, B.; et al. Yiqifumai lyophilized injection attenuates particulate matter-induced acute lung injury in mice via tlr4-mtor-autophagy pathway. Biomed. Pharmacother. 2018, 108, 906–913. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Integrative analysis of mrnas, mirnas and lncrnas in urban particulate matter srm 1648a-treated ea.Hy926 human endothelial cells. Chemosphere 2019, 233, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.H.; Li, J.R.; Zheng, T.H.; Yao, F.B.; Gao, B.; Xue, T.C. Neutrophils: Driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021, 522, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ho, L.; Tergaonkar, V. Sorf-encoded micropeptides: New players in inflammation, metabolism, and precision medicine. Cancer Lett. 2021, 500, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Wang, B.; Chen, H.; Ho, K.F.; Cao, J.; Hai, G.; Jalaludin, B.; Herbert, C.; Thomas, P.S.; Saad, S.; et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L424–L430. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; He, H.Q.; Nie, Y.J.; Ding, Y.H.; Sun, L.; Qian, F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol. Sin. 2018, 39, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Q.; Jin, Y.; Qiao, H.J.E.; Pathology, M. Associations of tumor necrosis factor-α polymorphisms with the risk of asthma: A meta-analysis. Exp. Mol. Pathol. 2018, 105, 411–416. [Google Scholar] [CrossRef]
- Lai, W.-Y.; Wang, J.-W.; Huang, B.-T.; Lin, E.P.-Y.; Yang, P.-C.J.T. A novel tnf-α-targeting aptamer for tnf-α-mediated acute lung injury and acute liver failure. Theranostics 2019, 9, 1741. [Google Scholar] [CrossRef]
- Leija-Martínez, J.J.; Huang, F.; Del-Río-Navarro, B.E.; Sanchéz-Muñoz, F.; Muñoz-Hernández, O.; Giacoman-Martínez, A.; Hall-Mondragon, M.S.; Espinosa-Velazquez, D.J.M.h. Il-17a and tnf-α as potential biomarkers for acute respiratory distress syndrome and mortality in patients with obesity and COVID-19. Med. Hypotheses 2020, 144, 109935. [Google Scholar] [CrossRef]
- Lee, S.G.; An, J.H.; Kim, D.H.; Yoon, M.S.; Lee, H.J. A case of interstitial lung disease and autoimmune thyroiditis associated with ustekinumab. Acta Derm. Venereol. 2019, 99, 331–332. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of nuclear factor-kappab (nf-κb) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Q.; Tang, H.; Zou, X.; Guo, X.; Hu, Y.; Zhou, K.; Liu, R. Ltb4 promotes acute lung injury via upregulating the plcepsilon-1/tlr4/nf-kappab pathway in one-lung ventilation. Dis. Markers 2022, 2022, 1839341. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhu, G.; Zhu, M.; Song, J.; Cai, H.; Song, Y.; Wang, J.; Jin, M. Edaravone Attenuated Particulate Matter-Induced Lung Inflammation by Inhibiting ROS-NF-κB Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 6908884. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.-F.; Lin, C.-S.; Hung, S.-C.; Yang, K.-Y. Mesenchymal stem cell-conditioned medium induces neutrophil apoptosis associated with inhibition of the nf-κb pathway in endotoxin-induced acute lung injury. Int. J. Mol. Sci. 2019, 20, 2208. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Park, S.H.; Han, B.S.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Park, S.J.; Kim, J.; Kim, J.W.; Cho, J.Y.; et al. Negative cellular effects of urban particulate matter on human keratinocytes are mediated by p38 mapk and nf-kappab-dependent expression of trpv 1. Int. J. Mol. Sci. 2018, 19, 2660. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Kandori, S.; Tanuma, K.; Nitta, S.; Chihara, I.; Shiga, M.; Hoshi, A.; Negoro, H.; Kojima, T.; Mathis, B.J.; et al. Pld1 promotes tumor invasion by regulation of mmp-13 expression via nf-kappab signaling in bladder cancer. Cancer Lett. 2021, 511, 15–25. [Google Scholar] [CrossRef]
- Sanjeewa, K.; Kim, H.-S.; Lee, H.-G.; Jayawardena, T.U.; Nagahawatta, D.; Yang, H.-W.; Udayanga, D.; Kim, J.-I.; Jeon, Y.-J. 3-hydroxy-5, 6-epoxy-β-ionone isolated from invasive harmful brown seaweed Sargassum horneri protects mh-s mouse lung cells from urban particulate matter-induced inflammation. Appl. Sci. 2021, 11, 10929. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Kobayashi, M.; Arashidani, K.; Yoshida, S.; Nishikawa, M.; Takano, H.; Sun, G.; Shibamoto, T. Differences in allergic inflammatory responses between urban pm2. 5 and fine particle derived from desert-dust in murine lungs. Toxicol Appl. Pharmacol. 2016, 297, 41–55. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Y.H.; Zhang, J.Z.; Tang, J.H.; Fan, R.P.; Li, F.; Yu, B.Y.; Kou, J.P.; Zhang, Y.Y. Ruscogenin attenuates particulate matter-induced acute lung injury in mice via protecting pulmonary endothelial barrier and inhibiting tlr4 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 726–734. [Google Scholar] [CrossRef]
- Ma, D.; Zhan, D.; Fu, Y.; Wei, S.; Lal, B.; Wang, J.; Li, Y.; Lopez-Bertoni, H.; Yalcin, F.; Dzaye, O.; et al. Mutant idh1 promotes phagocytic function of microglia/macrophages in gliomas by downregulating icam1. Cancer Lett. 2021, 517, 35–45. [Google Scholar] [CrossRef]
- Lu, H.; Fu, C.; Kong, S.; Wang, X.; Sun, L.; Lin, Z.; Luo, P.; Jin, H. Maltol prevents the progression of osteoarthritis by targeting pi3k/akt/nf-kappab pathway: In vitro and in vivo studies. J. Cell. Mol. Med. 2021, 25, 499–509. [Google Scholar] [CrossRef]
- Kim, B.-H.; Cho, I.-A.; Kang, K.-R.; Lee, S.-Y.; Jung, S.-Y.; Kim, J.-S.; Kim, S.-G. Bavachin counteracts receptor activator of nuclear factor-κb-induced osteoclastogenesis though the suppression of nuclear factor-κb signaling pathway in raw264. 7 cells. Oral Biol. Res. 2018, 42, 130–139. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin inhibits il-1β-induced inflammation in chondrocytes via suppression of nf-κb signaling and attenuates osteoarthritis in mice. Front. Pharmacol. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed]
| Measurement Criteria | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| A. Neutrophil infiltration into the interstitial space | Not found | 1 to 5 | More than 5 |
| B. Neutrophil infiltration into the alveolar space | Not found | 1 to 5 | More than 5 |
| C. Number of hyaline membranes | Not found | 3 | More than 3 |
| D. Septal thickening of the alveolar wall | More than 2× | 2 to 4× | More than 4× |
| Score = [(20 × A) + (14 × B) + (7 × C) + (2 × D)]/(field number × 100) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Kim, J.H.; Song, M.; Lee, H.P.; Yoon, J.H.; Kim, D.S.; Jang, S.G.; Kim, D.S.; Cho, J.Y. Chloroform Fraction of Prasiola japonica Ethanolic Extract Alleviates UPM 1648a-Induced Lung Injury by Suppressing NF-κB Signaling. Foods 2023, 12, 88. https://doi.org/10.3390/foods12010088
Park SH, Kim JH, Song M, Lee HP, Yoon JH, Kim DS, Jang SG, Kim DS, Cho JY. Chloroform Fraction of Prasiola japonica Ethanolic Extract Alleviates UPM 1648a-Induced Lung Injury by Suppressing NF-κB Signaling. Foods. 2023; 12(1):88. https://doi.org/10.3390/foods12010088
Chicago/Turabian StylePark, Sang Hee, Ji Hye Kim, Minkyung Song, Hwa Pyoung Lee, Ji Hye Yoon, Dong Seon Kim, Seok Gu Jang, Dong Sam Kim, and Jae Youl Cho. 2023. "Chloroform Fraction of Prasiola japonica Ethanolic Extract Alleviates UPM 1648a-Induced Lung Injury by Suppressing NF-κB Signaling" Foods 12, no. 1: 88. https://doi.org/10.3390/foods12010088
APA StylePark, S. H., Kim, J. H., Song, M., Lee, H. P., Yoon, J. H., Kim, D. S., Jang, S. G., Kim, D. S., & Cho, J. Y. (2023). Chloroform Fraction of Prasiola japonica Ethanolic Extract Alleviates UPM 1648a-Induced Lung Injury by Suppressing NF-κB Signaling. Foods, 12(1), 88. https://doi.org/10.3390/foods12010088







