Association of Slowly Digestible Starch Intake with Reduction of Postprandial Glycemic Response: An Update Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
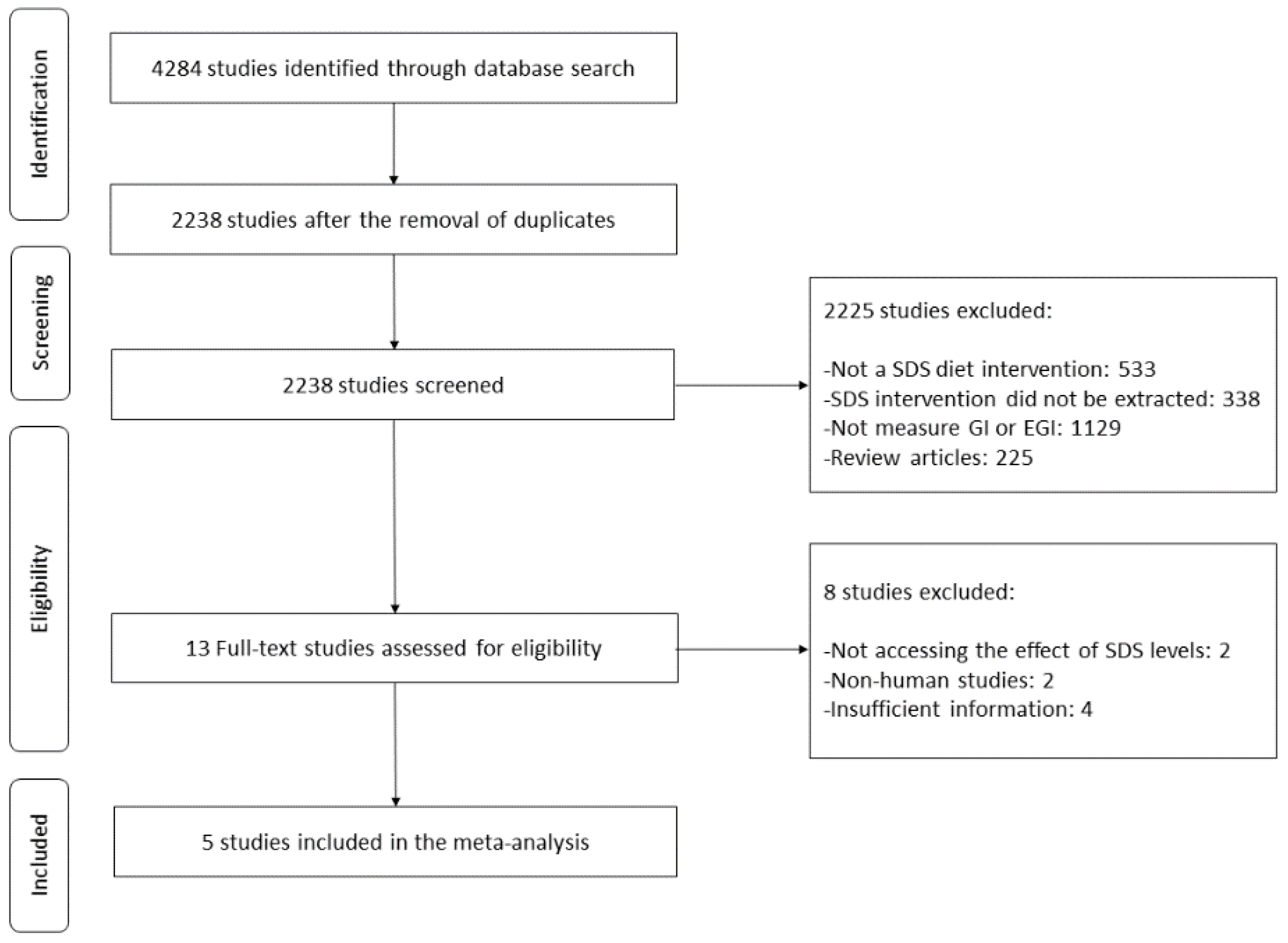
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
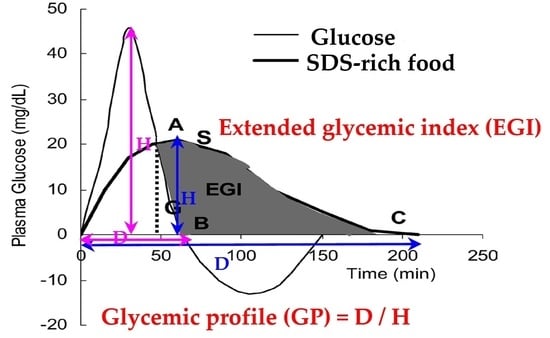
2.4. Statistical Analysis
3. Results
3.1. Research Data
3.2. Study Characteristics and Quality Assessment
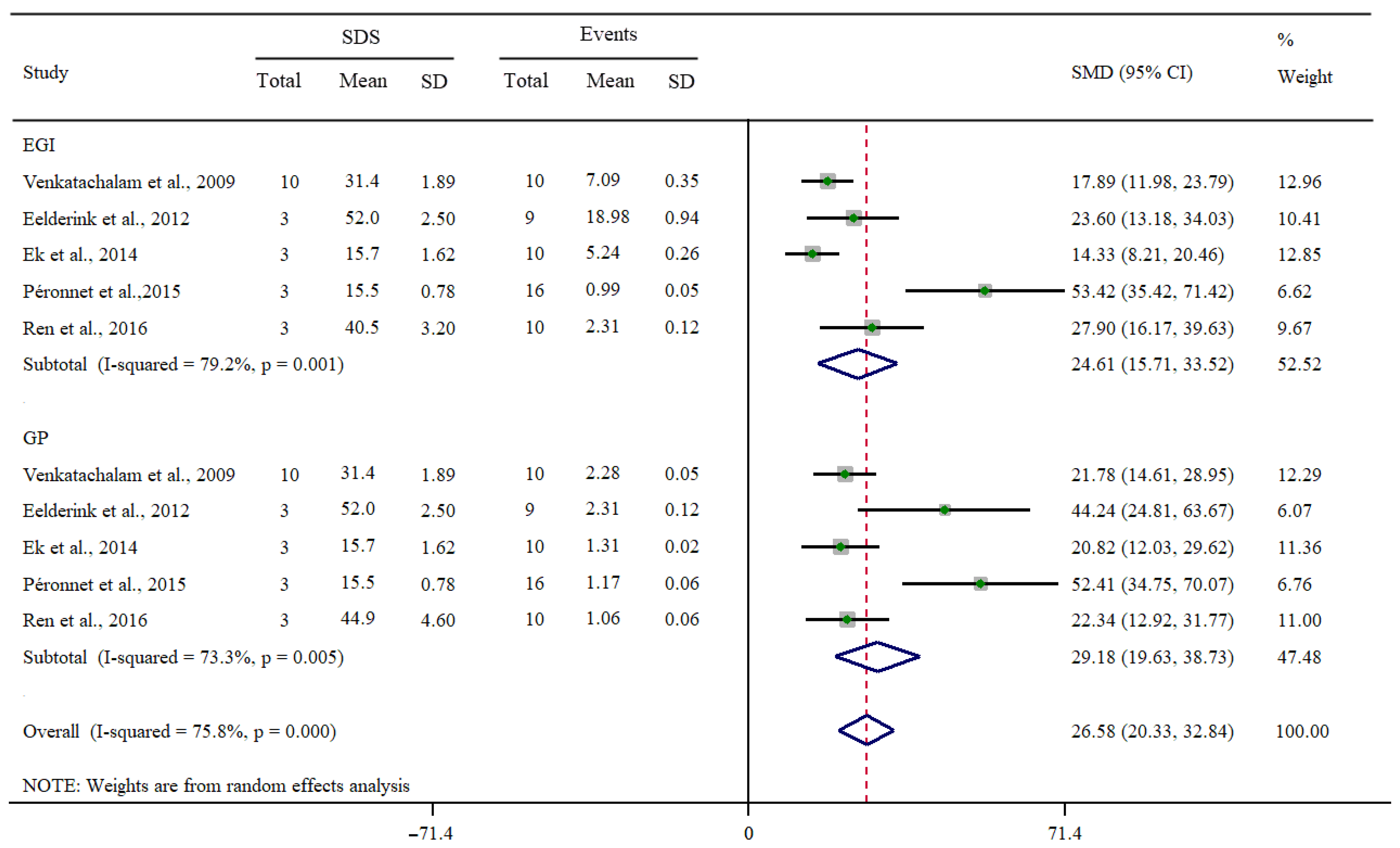
3.3. Associations of SDS Intake with EGI or GP
3.4. Subgroup and Sensitivity Analyses
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas (10th Edition), 2021. Available online: https://diabetesatlas.org (accessed on 15 December 2021).
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycaemic index, glycaemic load and glycaemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycaemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Hamaker, B.R. Food matrix effects for modulating starch bioavailability. Annu. Rev. Food Sci. Technol. 2021, 12, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Vinoy, S.; Laville, M.; Feskens, E.J. Slow-release carbohydrates: Growing evidence on metabolic responses and public health interest. Summary of the symposium held at the 12th European Nutrition Conference (FENS 2015). Food Nutr. Res. 2016, 60, 31662. [Google Scholar] [CrossRef]
- Wachters-Hagedoorn, R.E.; Priebe, M.G.; Heimweg, J.A.; Heiner, A.M.; Englyst, K.N.; Holst, J.J.; Stellaard, F.; Vonk, R.J. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr. 2006, 136, 1511–1516. [Google Scholar] [CrossRef]
- Ells, L.J.; Seal, C.J.; Kettlitz, B.; Bal, W.; Mathers, J.C. Postprandial glycaemic, lipaemic and haemostatic responses to ingestion of rapidly and slowly digested starches in healthy young women. Br. J. Nutr. 2005, 94, 948–955. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Kushnick, M.R.; Zhang, G.; Hamaker, B.R. Starch-entrapped biopolymer microspheres as a novel approach to vary blood glucose profiles. J. Am. Coll. Nutr. 2009, 28, 583–590. [Google Scholar] [CrossRef]
- Rosén, L.A.; Silva, L.O.; Andersson, U.K.; Holm, C.; Ostman, E.M.; Björck, I.M. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutr. J. 2009, 8, 42. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.C.O.; Vieira, A.F.; Moritz, C.E.J.; Reischak-Oliveira, A. Effects of fructose consumption on postprandial TAG: An update on systematic reviews with meta-analysis. Br. J. Nutr. 2018, 120, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.; Shen, Q. In vitro starch digestibility and in vivo glycaemic response of foxtail millet and its products. Food Funct. 2016, 7, 372–379. [Google Scholar] [CrossRef]
- Péronnet, F.; Meynier, A.; Sauvinet, V.; Normand, S.; Bourdon, E.; Mignault, D.; Stpierre, D.H.; Laville, M.; Rabasa-Lhoret, R.; Vinoy, S. Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: Effect of starch digestibility. Eur. J. Clin. Nutr. 2015, 69, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ek, K.L.; Wang, S.; Copeland, L.; Brand-Miller, J.C. Discovery of a low-glycaemic index potato and relationship with starch digestion in vitro. Br. J. Nutr. 2014, 111, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Eelderink, C.; Schepers, M.; Preston, T.; Vonk, R.J.; Oudhuis, L.; Priebe, M.G. Slowly and rapidly digestible starchy foods can elicit a similar glycaemic response because of differential tissue glucose uptake in healthy men. Am. J. Clin. Nutr. 2012, 96, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Hasjim, J.; Lavau, G.C.; Gidley, M.J.; Gilbert, R.G. In vivo and in vitro starch digestion: Are current in vitro techniques adequate? Biomacromolecules 2010, 11, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, B.; Jin, Z.; BeMiller, J.N. Microbial starch-converting enzymes: Recent insights and perspectives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1238–1260. [Google Scholar] [CrossRef]
- Meynier, A.; Goux, A.; Atkinson, F.; Brack, O.; Vinoy, S. Postprandial glycaemic response: How is it influenced by characteristics of cereal products? Br. J. Nutr. 2015, 113, 1931–1939. [Google Scholar] [CrossRef]
- Miao, M.; BeMiller, J.N. Enzymatic approaches for structuring starch to improve functionality. Annu. Rev. Food Sci. Technol. 2023, 14. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Bello-Pérez, L.A.; Lin, H.M.; Kim, C.Y.; Hamaker, B.R. Importance of location of digestion and colonic fermentation of starch related to its quality. Cereal Chem. 2013, 90, 335–343. [Google Scholar] [CrossRef]
- Zhang, G.; Hasek, L.Y.; Lee, B.H.; Hamaker, B.R. Gut feedback mechanisms and food intake: A physiological approach to slow carbohydrate bioavailability. Food Funct. 2015, 6, 1072–1089. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Daly, M.E.; Thomas, L.C.; Bal, W.; Birkett, A.M.; Jeffcoat, R.; Mathers, J.C. Postprandial carbohydrate metabolism in healthy subjects and those with type 2 diabetes fed starches with slow and rapid hydrolysis rates determined in vitro. Br. J. Nutr. 2003, 90, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Lambert-Porcheron, S.; Normand, S.; Blond, E.; Sothier, M.; Roth, H.; Meynier, A.; Vinoy, S.; Laville, M.; Nazare, J. Modulation of starch digestibility in breakfast cereals consumed by subjects with metabolic risk: Impact on markers of oxidative stress and inflammation during fasting and the postprandial period. Mol. Nutr. Food Res. 2017, 61, 1700212. [Google Scholar] [CrossRef]
- Vinoy, S.; Meynier, A.; Goux, A.; Jourdan-Salloum, N.; Normand, S.; Rabasa-Lhoret, R.; Brack, O.; Nazare, J.-A.; Péronnet, F.; Laville, M. The Effect of a breakfast rich in slowly digestible starch on glucose metabolism: A statistical meta-analysis of randomized controlled trials. Nutrients 2017, 9, 318. [Google Scholar] [CrossRef]
- Vinoy, S.; Normand, S.; Meynier, A.; Sothier, M.; Louche-Pelissier, C.; Peyrat, J.; Maitrepierre, C.; Nazare, J.-A.; Brand-Miller, J.; Laville, M. Cereal processing influences postprandial glucose metabolism as well as the GI effect. J. Am. Coll. Nutr. 2013, 32, 79–91. [Google Scholar] [CrossRef]
- Harbis, A.; Perdreau, S.; Vincent-Baudry, S.; Charbonnier, M.; Bernard, M.C.; Raccah, D.; Senft, M.; Lorec, A.-M.; Defoort, C.; Portugal, H.; et al. Glycaemic and insulinemic meal responses modulate postprandial hepatic and intestinal lipoprotein accumulation in obese, insulin-resistant subjects. Am. J. Clin. Nutr. 2004, 80, 896–902. [Google Scholar] [CrossRef]
- Sands, A.L.; Leidy, H.J.; Hamaker, B.R.; Maguire, P.; Campbell, W.W. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr. Res. 2009, 29, 383–390. [Google Scholar] [CrossRef]
- Nazare, J.A.; de Rougemont, A.; Normand, S.; Sauvinet, V.; Sothier, M.; Vinoy, S.; Désage, M.; Laville, M. Effect of postprandial modulation of glucose availability: Short-and long-term analysis. Br. J. Nutr. 2010, 103, 1461–1470. [Google Scholar] [CrossRef]
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef]
- Gourineni, V.; Stewart, M.L.; Skorge, R.; Sekula, B.C. Slowly digestible carbohydrate for balanced energy: In vitro and in vivo evidence. Nutrients 2017, 9, 1230. [Google Scholar] [CrossRef]
| Study (Year) | Study Design | Age (Mean ± SD) | BMI (Mean ± SD) | Geographic location | Population | SDS Content (Mean ± SD) | Adjustment Period | Index (Mean ± SD) | |
|---|---|---|---|---|---|---|---|---|---|
| EGI | GP | ||||||||
| Venkatachalam et al. (2009) [10] | crossover | 21 ± 0.5 | 23 ± 0.6 | North America | Human (7M, 3F) | 31.4 ± 1.89 | fasted for >9 h | 7.09 ± 0.35 | 2.28 ± 0.05 |
| Eelderink et al. (2012) [17] | crossover | 27 ± 5.1 | 22.3 ± 2.0 | Europe | Human (9M) | 52.0 ± 2.50 | fasted overnight, limited physical activity | 18.98 ± 0.94 | 2.31 ± 0.12 |
| Ek et al. (2014) [16] | crossover | 26 | 20.8 | Oceania | Human (12M, 15F) | 15.7 ± 1.62 | fasted for 10–12 h, physical activity | 5.24 ± 0.26 | 1.31 ± 0.02 |
| Péronnet et al. (2015) [15] | crossover | 18–40 | 20–25 | North America | Human (16F) | 15.5 ± 0.78 | energy intake, physical activity, taking an oral contraceptive | 0.99 ± 0.05 | 1.17 ± 0.06 |
| Ren et al. (2016) [14] | crossover | 23 ± 4.0 | 26.15 ± 2.6 | Asia | Human (3M, 7F) | 40.5 ± 3.20 | fasted for 10–12 h | 2.31 ± 0.12 | 1.06 ± 0.06 |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants & Personnel | Blinding of Outcome Assessments | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Venkatachalam et al. (2009) [10] | Un | Un | L | L | L | L | L |
| Eelderink et al. (2012) [17] | Un | Un | L | Un | Un | L | L |
| Ek et al. (2014) [16] | Un | Un | L | L | L | Un | L |
| Péronnet et al. (2015) [15] | L | Un | L | Un | Un | Un | L |
| Ren et al. (2016) [14] | L | Un | Un | Un | L | Un | L |
| EGI | GP | |||||
|---|---|---|---|---|---|---|
| SMD (95% CI) | PH | I2, % | SMD (95% CI) | PH | I2, % | |
| Total | 24.01 (21.72, 26.29) | <0.01 | 78.6 | 28.57 (26.18, 30.96) | <0.01 | 69.5 |
| M | 17.57 (16.16, 18.98) | 0.31 | 10.5 | 23.98 (21.66, 26.31) | 0.03 | 37.0 |
| F | 31.40 (27.07, 35.74) | <0.01 | 86.4 | 32.31 (28.35, 36.26) | <0.01 | 77.9 |
| Group | Std. Eff. | Coef. | Std. Err. | t | P > |t| | [95% CI] |
|---|---|---|---|---|---|---|
| EGI | slope | 6.56249 | 7.982013 | 0.82 | 0.56 | −94.86, 107.98 |
| bias | 3.14865 | 2.29361 | 1.37 | 0.40 | −25.99, 32.29 | |
| GP | slope | 21.53624 | 4.868787 | 4.42 | 0.14 | −40.33, 83.40 |
| bias | 0.02540 | 1.150894 | 0.02 | 0.99 | −14.60, 14.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, X.; Xiang, X.; Miao, M. Association of Slowly Digestible Starch Intake with Reduction of Postprandial Glycemic Response: An Update Meta-Analysis. Foods 2023, 12, 89. https://doi.org/10.3390/foods12010089
Wang Y, Zhou X, Xiang X, Miao M. Association of Slowly Digestible Starch Intake with Reduction of Postprandial Glycemic Response: An Update Meta-Analysis. Foods. 2023; 12(1):89. https://doi.org/10.3390/foods12010089
Chicago/Turabian StyleWang, Yanli, Xiao Zhou, Xuesong Xiang, and Ming Miao. 2023. "Association of Slowly Digestible Starch Intake with Reduction of Postprandial Glycemic Response: An Update Meta-Analysis" Foods 12, no. 1: 89. https://doi.org/10.3390/foods12010089
APA StyleWang, Y., Zhou, X., Xiang, X., & Miao, M. (2023). Association of Slowly Digestible Starch Intake with Reduction of Postprandial Glycemic Response: An Update Meta-Analysis. Foods, 12(1), 89. https://doi.org/10.3390/foods12010089