The Biogas Potential of Oxytree Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Dry Mass and Organics and Elemental Composition
2.2. Determining the Quantity and Quality of Generated Gases
3. Results
3.1. Initial Parameters of Substrate
3.2. Biogas and Methane Efficiency
4. Discussion
5. Conclusions
- Considering the climatic conditions and growth requirements of Oxytree, it is one of the best species to grow as an energy crop plantation. Its leaves, which are usually waste material, regardless of harvest, are a valuable source of chemical energy as biogas (liquid biofuel).
- Its elemental composition and physico-chemical characteristics are very similar (often even greater) to other fast-growing or energetic plants.
- The collection of leaves is quite convenient because they fall at the same time and they are large and heavy (they are in close proximity to the plant in large clusters).
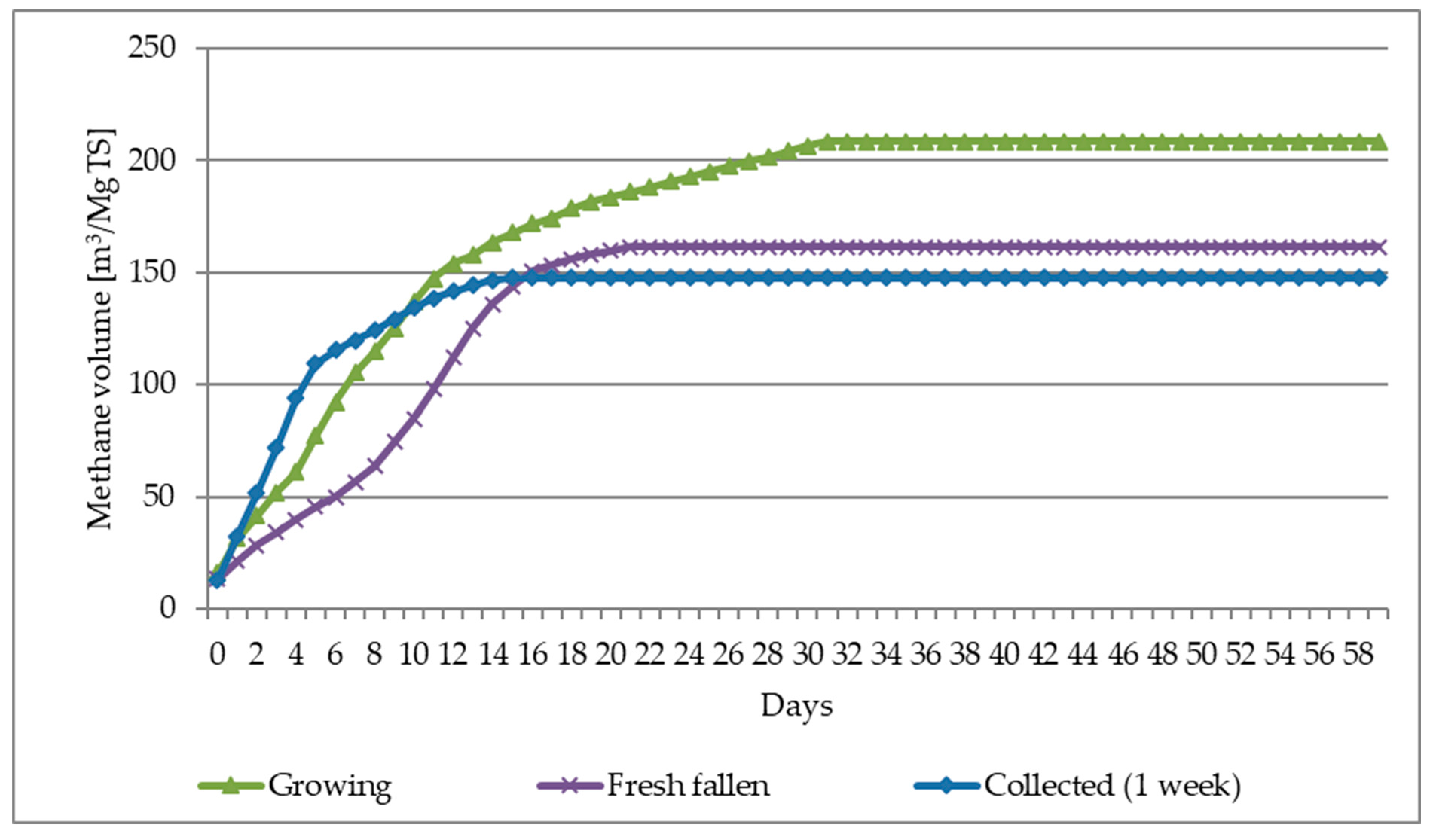
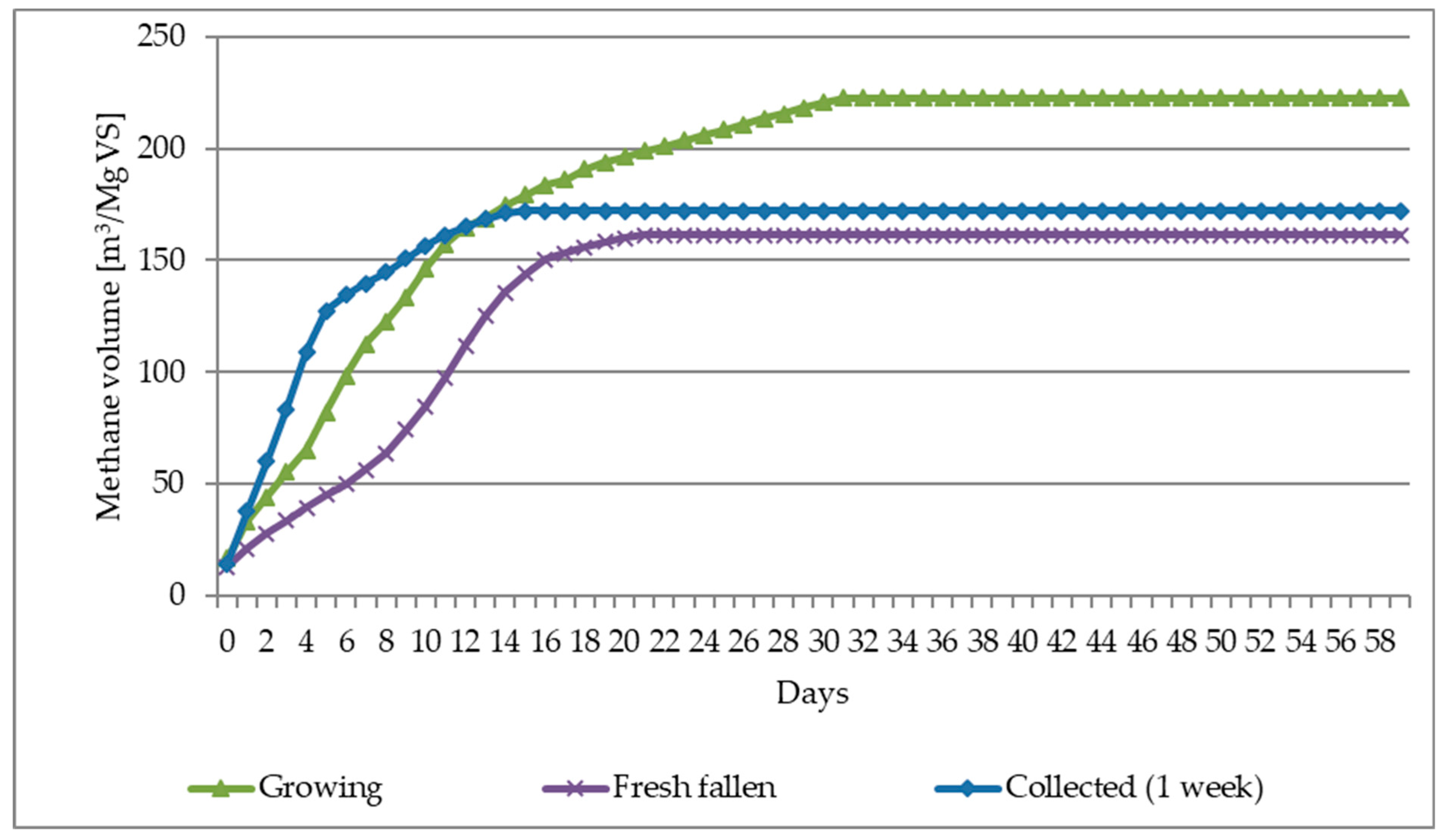
- The average biogas yield from all leaf-harvesting methods was approximately 386 m3/Mg of dry organic mass (VS), and for the most effective biogas harvesting—“Growing” (fresh leaves)—even 430 m3/Mg VS.
- The average methane yield from all methods of leaves harvesting was about 194 m3/Mg of dry organic mass (VS), and for the most effective biogas harvest—“Growing” (fresh leaves), it was even 223 m3/Mg VS.
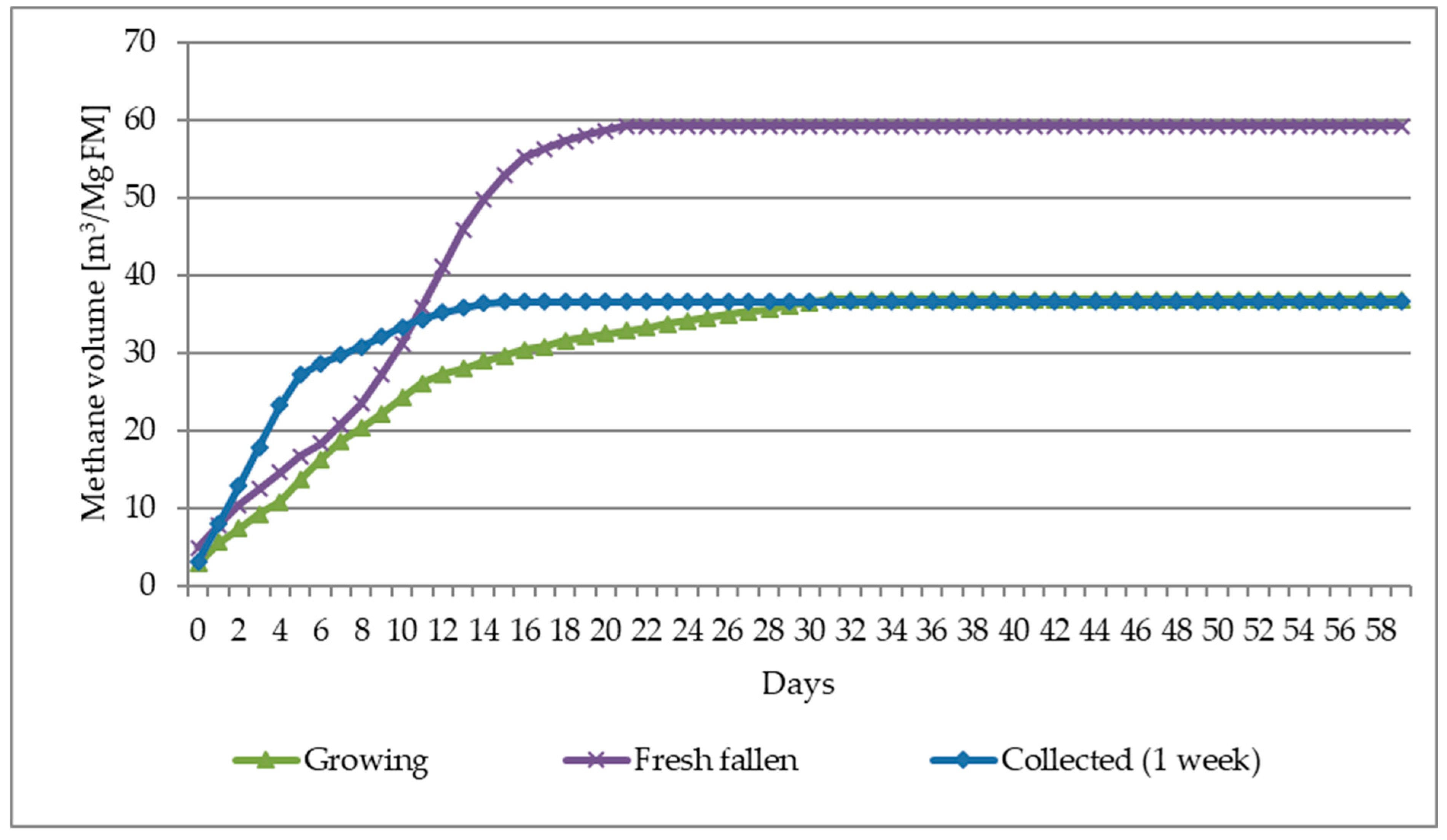
- The average biogas yield from all methods of leaf harvesting in terms of fresh mass (FM) was approximately 44 m3/Mg, and for the most biogas-effective harvest—“Fresh fallen” (recently fallen leaves—maximum 1 day)—even 59 m3/Mg FM.
- The average methane yield from all methods of leaves harvesting in terms of fresh mass (FM) was approximately 89 m3/Mg, and for the most biogas-effective harvest—“Fresh fallen” (recently fallen leaves—maximum 1 day)—even 123 m3/Mg FM.
- The use of Oxytree leaves in anaerobic digestion processes contributes to reducing the carbon footprint of Oxytree crops productions.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. Energy. Our World Data. 2022. Available online: https://ourworldindata.org/energy (accessed on 23 October 2022).
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Our World Data. 2020. Available online: https://ourworldindata.org/greenhouse-gas-emissions (accessed on 23 October 2022).
- OECD. Energy: The Next Fifty Years; OECD: Paris, France, 1999; Available online: https://www.oecd.org/futures/17738498.pdf (accessed on 23 October 2022).
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Fuel Properties of Torrefied Biomass from Pruning of Oxytree. Data 2019, 4, 55. [Google Scholar] [CrossRef]
- Rozakis, S.; Juvančič, L.; Kovacs, B. Bioeconomy for Resilient Post-COVID Economies. Energies 2022, 15, 2958. [Google Scholar] [CrossRef]
- Verner, V.; Mazancová, J.; Jelínek, M.; Phung, L.D.; Van Dung, D.; Banout, J.; Roubík, H. Economics and Perception of Small-Scale Biogas Plant Benefits Installed among Peri-Urban and Rural Areas in Central Vietnam. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize Straw as a Valuable Energetic Material for Biogas Plant Feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, J.; Zaborowicz, M.; Dach, J.; Czekała, W.; Przybył, J. Biological Waste Management in the Case of a Pandemic Emergency and Other Natural Disasters. Determination of Bioenergy Production from Floricultural Waste and Modeling of Methane Production Using Deep Neural Modeling Methods. Energies 2020, 13, 3014. [Google Scholar] [CrossRef]
- Czekała, W. Agricultural Biogas Plants as a Chance for the Development of the Agri-Food Sector. J. Ecol. Eng. 2018, 19, 179–183. [Google Scholar] [CrossRef]
- Czekała, W.; Bartnikowska, S.; Dach, J.; Janczak, D.; Smurzyńska, A.; Kozłowski, K.; Bugała, A.; Lewicki, A.; Cieślik, M.; Typańska, D.; et al. The Energy Value and Economic Efficiency of Solid Biofuels Produced from Digestate and Sawdust. Energy 2018, 159, 1118–1122. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing Acidogenesis and Methanogenesis Metabolism in Thermophilic Anaerobic Digestion of Food Waste under a High Loading Rate. Sci. Total Environ. 2022, 824, 153867. [Google Scholar] [CrossRef]
- Ren, L.; Hou, Z.; Gao, Y.; Fu, X.; Yu, D.; Lin, M.; Dong, R.; Qiao, W. Maintaining the Long Term Stability of Anaerobic Digestion of Maize Straw in a Continuous Plug Flow Reactor by Verifying the Key Role of Trace Elements. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Kupryaniuk, K.; Oniszczuk, T.; Combrzyński, M.; Wójtowicz, A.; Mitrus, M. Effect of Extrusion-Cooking Conditions on the Physical Properties of Jerusalem Artichoke Straw. Int. Agrophys. 2020, 34, 441–449. [Google Scholar] [CrossRef]
- Sytnyk, S.; Lovynska, V.; Kharytonov, M.; Rula, I.; Poliakh, V.; Roubík, H. Thermal Analysis of Aboveground Biomass of the Two Species Cultivated in Artificial Forest Plantations in Marginal Lands of Ukraine. Int. J. Environ. Stud. 2021, 1–10. [Google Scholar] [CrossRef]
- Pochwatka, P.; Kowalczyk-Juśko, A.; Sołowiej, P.; Wawrzyniak, A.; Dach, J. Biogas Plant Exploitation in a Middle-Sized Dairy Farm in Poland: Energetic and Economic Aspects. Energies 2020, 13, 6058. [Google Scholar] [CrossRef]
- Marks, S.; Dach, J.; Morales, F.J.F.; Mazurkiewicz, J.; Pochwatka, P.; Gierz, Ł. New Trends in Substrates and Biogas Systems in Poland. J. Ecol. Eng. 2020, 21, 19–25. [Google Scholar] [CrossRef]
- Czekała, W.; Janczak, D.; Cieślik, M.; Mazurkiewicz, J.; Pulka, J. Food Waste Management Using Hermetia Illucens Insect. J. Ecol. Eng. 2020, 21, 214–216. [Google Scholar] [CrossRef]
- Majlingova, A.; Lieskovsky, M.; Zachar, M. Technical University in Zvolen. 181. Available online: https://www.tuzvo.sk/sites/default/files/Fire%2525252520and%2525252520energetic%2525252520properties%2525252520of%2525252520selected%2525252520fast%2525252520growing%2525252520species%2525252520and%2525252520energy%2525252520crop%2525252520species_scientific.pdf (accessed on 23 October 2022).
- Icka, P.; Damo, R.; Icka, E. Paulownia Tomentosa, a Fast Growing Timber. Ann. Valahia Univ. Targoviste Agric. 2016, 10, 14. [Google Scholar] [CrossRef]
- A Study of Selected Features of Shan Tong Variety of Plantation Paulownia. Available online: https://wulsannals.com/resources/html/article/details?id=212736&language=en (accessed on 5 November 2022).
- Huang, H.; Szumacher-Strabel, M.; Patra, A.K.; Ślusarczyk, S.; Lechniak, D.; Vazirigohar, M.; Varadyova, Z.; Kozłowska, M.; Cieślak, A. Chemical and Phytochemical Composition, in Vitro Ruminal Fermentation, Methane Production, and Nutrient Degradability of Fresh and Ensiled Paulownia Hybrid Leaves. Anim. Feed Sci. Technol. 2021, 279, 115038. [Google Scholar] [CrossRef]
- Bodnár, A.; Pajor, F.; Steier, J.; Kispál, T.; Póti, P. Nutritive Value of Paulownia (Paulownia Spp.) Hybrid Tree Leaves. Hung. Agric. Res. 2014, 23, 27–32. [Google Scholar]
- Jakubowski, M. Cultivation Potential and Uses of Paulownia Wood: A Review. Forests 2022, 13, 668. [Google Scholar] [CrossRef]
- Pikoń, K.; Bogacka, M. Contemporary Problems of Power Engineering and Environmental Protection 2020; Department of Technologies and Installations for Waste Management: Gliwice, Poland, 2021; ISBN 978-83-950087-9-5. [Google Scholar]
- Lachowicz, H.; Giedrowicz, A. Charakterystyka jakości technicznej drewna paulowni COTE-2. Sylwan 2020, 164, 414–423. [Google Scholar] [CrossRef]
- Vityi, A.; Marosvölgyi, B. New Tree Species for Agroforestry and Energy Purposes. In Proceedings of the 2014 International Conference on Energy, Environment, Ecosystems and Development II (EEED ‘14)/2014 International Conference on Biology and Biomedicine II (BIO ‘14), Prague, Czech Republic, 2–4 April 2014. [Google Scholar]
- Huseinovic, S.; Osmanović, Z.; Bektić, S.; Ahmetbegović, S. Paulownia Elongata Sy Hu in Function of Improving the Quality of the Environment. Period. Eng. Nat. Sci. PEN 2017, 5, 117–123. [Google Scholar] [CrossRef]
- Oxytree—Strona Główna. Available online: https://oxytree.pl/ (accessed on 4 November 2022).
- Microbial Diversity of Paulownia Spp. Leaves—A New Source of Green Manure: BioResources. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 4 November 2022).
- Drzewiecka, K.; Gąsecka, M.; Magdziak, Z.; Budzyńska, S.; Szostek, M.; Niedzielski, P.; Budka, A.; Roszyk, E.; Doczekalska, B.; Górska, M.; et al. The Possibility of Using Paulownia elongata S. Y. Hu × Paulownia fortunei Hybrid for Phytoextraction of Toxic Elements from Post-Industrial Wastes with Biochar. Plants 2021, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, J.; Novosadová, K.; Pokorný, R. The Estimate of the Required Amount of Water on the Growth of Five Species of Paulownia at the First Year of Cultivation in a Central Europe. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Popova, T.P.; Baykov, B.D. Antimicrobial Activity of Aqueous Extracts of Leaves and Silage from Paulownia Elongata. Am. J. Biol. Chem. Pharm. Sci. 2013, 1, 8–15. [Google Scholar]
- Dżugan, M.; Miłek, M.; Grabek-Lejko, D.; Hęclik, J.; Jacek, B.; Litwińczuk, W. Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia Spp. Clones. Agronomy 2021, 11, 2001. [Google Scholar] [CrossRef]
- Home. Available online: https://www.mels-project.eu/ (accessed on 1 November 2022).
- Paulownia Trees Best Cultivars for Timber Bellissia & Paulemia. Paulownia Trees. Available online: https://paulowniatrees.eu/products/paulownia-planting-material/ (accessed on 23 October 2022).
- Ates, S.; Ni, Y.; Akgul, M.; Tozluoglu, A. Characterization and Evaluation of Paulownia Elongota as a Raw Material for Paper Production. Afr. J. Biotechnol. 2008, 7, 4153–4158. [Google Scholar] [CrossRef]
- Yadav, N.K.; Vaidya, B.N.; Henderson, K.; Lee, J.F.; Stewart, W.M.; Dhekney, S.A.; Joshee, N. A Review of Paulownia Biotechnology: A Short Rotation, Fast Growing Multipurpose Bioenergy Tree. Am. J. Plant Sci. 2013, 4, 2070–2082. [Google Scholar] [CrossRef]
- He, T.; Vaidya, B.N.; Perry, Z.D.; Parajuli, P.; Joshee, N. Paulownia as a Medicinal Tree: Traditional Uses and Current Advances. Eur. J. Med. Plants 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Adach, W.; Żuchowski, J.; Moniuszko-Szajwaj, B.; Szumacher-Strabel, M.; Stochmal, A.; Olas, B.; Cieslak, A. In Vitro Antiplatelet Activity of Extract and Its Fractions of Paulownia Clone in Vitro 112 Leaves. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 137, 111301. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Moniuszko-Szajwaj, B.; Zuchowski, J.; Pecio, Ł.; Kontek, B.; Szumacher-Strabel, M.; Olas, B.; Cieslak, A. Qualitative and Quantitative Analysis of Secondary Metabolites in Morphological Parts of Paulownia Clon In Vitro 112® and Their Anticoagulant Properties in Whole Human Blood. Molecules 2022, 27, 980. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagheer, A.A.; Abd El-Hack, M.E.; Alagawany, M.; Naiel, M.A.; Mahgoub, S.A.; Badr, M.M.; Hussein, E.O.S.; Alowaimer, A.N.; Swelum, A.A. Paulownia Leaves as A New Feed Resource: Chemical Composition and Effects on Growth, Carcasses, Digestibility, Blood Biochemistry, and Intestinal Bacterial Populations of Growing Rabbits. Animals 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; Sahfi, M.E.; Elnesr, S.S.; Alqaisi, O.; El-Kassas, S.; Al-wajeeh, A.S.; Taha, A.E.; Abd E-Hack, M.E. Phytochemical Characteristics of Paulownia Trees Wastes and Its Use as Unconventional Feedstuff in Animal Feed. Anim. Biotechnol. 2022, 33, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Özelçam, H.; İpçak, H.H.; Özüretmen, S.; Canbolat, Ö. Feed Value of Dried and Ensiled Paulownia (Paulownia Spp.) Leaves and Their Relationship to Rumen Fermentation, in Vitro Digestibility, and Gas Production Characteristics. Rev. Bras. Zootec. 2021, 50, e20210057. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Du, L.; Wu, Y.; Liu, Z.; Han, L. The Potential of Paulownia Fortunei Seedlings for the Phytoremediation of Manganese Slag Amended with Spent Mushroom Compost. Ecotoxicol. Environ. Saf. 2020, 196, 110538. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Meng, H.; Wu, Z.; Zhao, J. Study on Gas Products Distributions During Fast Co-Pyrolysis of Paulownia Wood and PET at High Temperature. Energy Procedia 2017, 105, 391–397. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Slow Pyrolysis of Paulownia Wood: Effects of Pyrolysis Parameters on Product Yields and Bio-Oil Characterization. J. Anal. Appl. Pyrolysis 2015, 114, 68–78. [Google Scholar] [CrossRef]
- Palma, A.; Loaiza, J.M.; Díaz, M.J.; García, J.C.; Giráldez, I.; López, F. Tagasaste, Leucaena and Paulownia: Three Industrial Crops for Energy and Hemicelluloses Production. Biotechnol. Biofuels 2021, 14, 89. [Google Scholar] [CrossRef]
- Domínguez, E.; del Río, P.G.; Romaní, A.; Garrote, G.; Domingues, L. Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes 2021, 9, 173. [Google Scholar] [CrossRef]
- Yavorov, N.; Petrin, S.; Valchev, I.; Nenkova, S. Potential of Fast Growing Poplar, Willow and Paulownia for Bioenergy Production. Bulg. Chem. Commun. 2015, 47, 5–9. [Google Scholar]
- Zhang, Q.; Jin, P.; Li, Y.; Zhang, Z.; Zhang, H.; Ru, G.; Jiang, D.; Jing, Y.; Zhang, X. Analysis of the Characteristics of Paulownia Lignocellulose and Hydrogen Production Potential via Photo Fermentation. Bioresour. Technol. 2022, 344, 126361. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Nadeem, F.; Jabeen, F.; Rani Singhania, R.; Yaqub Qazi, U.; Kumar Patel, A.; Javaid, R.; Zhang, Q. Enhancing Biohydrogen Production from Lignocellulosic Biomass of Paulownia Waste by Charge Facilitation in Zn Doped SnO2 Nanocatalysts. Bioresour. Technol. 2022, 355, 127299. [Google Scholar] [CrossRef]
- Yi, W.; Nadeem, F.; Xu, G.; Zhang, Q.; Joshee, N.; Tahir, N. Modifying Crystallinity, and Thermo-Optical Characteristics of Paulownia Biomass through Ultrafine Grinding and Evaluation of Biohydrogen Production Potential. J. Clean. Prod. 2020, 269, 122386. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Y.; Wang, J. Biohydrogen Production from Co-Fermentation of Fallen Leaves and Sewage Sludge. Bioresour. Technol. 2019, 285, 121342. [Google Scholar] [CrossRef] [PubMed]
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant–Challenges and Barriers. Proceedings 2018, 2, 614. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E. Sewage Sludge and Fat Rich Materials Co-Digestion—Performance and Energy Potential. J. Clean. Prod. 2018, 198, 1076–1089. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, Y.; Wang, J. Co-Fermentation of Sewage Sludge and Lignocellulosic Biomass for Production of Medium-Chain Fatty Acids. Bioresour. Technol. 2022, 361, 127665. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, J. Production of Medium-Chain Fatty Acids by Co-Fermentation of Antibiotic Fermentation Residue with Fallen Ginkgo Leaves. Bioresour. Technol. 2022, 360, 127607. [Google Scholar] [CrossRef]
- Kujawa, S.; Mazurkiewicz, J.; Czekała, W. Using Convolutional Neural Networks to Classify the Maturity of Compost Based on Sewage Sludge and Rapeseed Straw. J. Clean. Prod. 2020, 258, 120814. [Google Scholar] [CrossRef]
- Żukowska, G.; Mazurkiewicz, J.; Myszura, M.; Czekała, W. Heat Energy and Gas Emissions during Composting of Sewage Sludge. Energies 2019, 12, 4782. [Google Scholar] [CrossRef]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M.; Borgulat, A. The Impact of Sewage Sludge Processing on the Safety of Its Use. Sci. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef]
- Cárdenas-Talero, J.L.; Silva-Leal, J.A.; Pérez-Vidal, A.; Torres-Lozada, P. The Influence of Municipal Wastewater Treatment Technologies on the Biological Stabilization of Sewage Sludge: A Systematic Review. Sustainability 2022, 14, 5910. [Google Scholar] [CrossRef]
- Demonstration Des Quantitativen Nachweises von Salmonellen—VDLUFA. Available online: https://www.vdlufa.de/schulungen-2/schulungen-2013/demonstration-des-quantitativen-nachweises-von-salmonellen/ (accessed on 30 October 2022).
- KTBL: Ktbl.De. Available online: https://www.ktbl.de/ (accessed on 30 October 2022).
- Biogas from Waste and Renewable Resources—Resources • SuSanA. Available online: https://www.susana.org/en/knowledge-hub/resources-and-publications/library/details/3038# (accessed on 7 November 2022).
- Mazurkiewicz, J. Energy and Economic Balance between Manure Stored and Used as a Substrate for Biogas Production. Energies 2022, 15, 413. [Google Scholar] [CrossRef]
- Dubrovskis, V.; Plume, I.; Kazulis, V.; Celms, A.; Kotelenecs, V.; Zabarovskis, E. Biogas Production Potential from Agricultural Biomass and Organic Residues in Latvia. Renewable Energy and Energy Efficiency. In Proceedings of the International Scientific Conference: Renewable Energy and Energy Efficiency, Jelgava, Latvia, 28–30 May 2012; Latvia University of Agriculture: Jelgava, Latvia, 2012; pp. 115–120. Available online: https://www.tf.llu.lv/conference/proceedings2012/Papers/100_Dubrovskis_V.pdf (accessed on 7 November 2022).
- Dach, J.; Mazurkiewicz, J.; Janczak, D.; Pulka, J.; Pochwatka, P.; Kowalczyk-Juśko, A. Cow Manure Anaerobic Digestion or Composting–Energetic and Economic Analysis. In Proceedings of the 2020 4th International Conference on Green Energy and Applications (ICGEA), Singapore, 7–9 March 2020; pp. 143–147. [Google Scholar]
- Zhang, S.; Wang, Y.; Liu, S. Process Optimization for the Anaerobic Digestion of Poplar (Populus L.) Leaves. Bioengineered 2020, 11, 439–448. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Kafle, G.K. Importance of “Weak-Base” Poplar Wastes to Process Performance and Methane Yield in Solid-State Anaerobic Digestion. J. Environ. Manag. 2017, 193, 423–429. [Google Scholar] [CrossRef]
- Yao, Y.; He, M.; Ren, Y.; Ma, L.; Luo, Y.; Sheng, H.; Xiang, Y.; Zhang, H.; Li, Q.; An, L. Anaerobic Digestion of Poplar Processing Residues for Methane Production after Alkaline Treatment. Bioresour. Technol. 2013, 134, 347–352. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Enhancing the Solid-State Anaerobic Digestion of Fallen Leaves through Simultaneous Alkaline Treatment. Bioresour. Technol. 2011, 102, 8828–8834. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of Inoculum Sources on the Anaerobic Digestion of Rice Straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of Seven Chemical Pretreatments of Corn Straw for Improving Methane Yield by Anaerobic Digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- Witaszek, K.; Pilarski, K.; Niedbała, G.; Pilarska, A.A.; Herkowiak, M. Energy Efficiency of Comminution and Extrusion of Maize Substrates Subjected to Methane Fermentation. Energies 2020, 13, 1887. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Studies on Iranian Cultivated Paulownia—A Potential Source of Fibrous Raw Material for Paperindustry. Eur. J. Wood Wood Prod. 2009, 67, 323–327. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarski, K.; Olesienkiewicz, A. A Comparison of the Influence of Kraft Lignin and the Kraft Lignin/Silica System as Cell Carriers on the Stability and Efficiency of the Anaerobic Digestion Process. Energies 2020, 13, 5803. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutiérrez, A.; Nieto, L.; Jiménez-Barbero, J.; Martínez, Á.T.; del Río, J.C. Isolation and Structural Characterization of the Milled-Wood Lignin from Paulownia Fortunei Wood. Ind. Crops Prod. 2009, 30, 137–143. [Google Scholar] [CrossRef]
- Witaszek, K.; Herkowiak, M.; Pilarska, A.A.; Czekała, W. Methods of Handling the Cup Plant (Silphium perfoliatum L.) for Energy Production. Energies 2022, 15, 1897. [Google Scholar] [CrossRef]
- Pavliukh, L.; Boichenko, S.; Onopa, V.; Tykhenko, O.; Topilnytskyy, P.; Romanchuk, V.; Samsin, I. Resource Potential for Biogas Production in Ukraine. Chem. Chem. Technol. 2019, 13, 101–106. [Google Scholar] [CrossRef]
- Klimek, K.E.; Wrzesińska-Jedrusiak, E.; Kapłan, M.; Łaska-Zieja, B. Management of biomass of selected grape leaves varieties in the process of methane fermentation. J. Water Land Dev. 2022, 55, 17–27. [Google Scholar] [CrossRef]
- Technical Possibilities of Biogas Production from Olive and Date Waste in Jordan: BioResources. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 19 November 2022).
- Rouf, M.A.; Islam, M.S.; Rabeya, T.; Mondal, A. Anaerobic Digestion of Mixed Dried Fallen Leaves by Mixing with Cow Dung. Bangladesh J. Sci. Ind. Res. 2015, 50, 163–168. [Google Scholar] [CrossRef][Green Version]
- Xu, S.; Bi, G.; Liu, X.; Yu, Q.; Li, D.; Yuan, H.; Chen, Y.; Xie, J. Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects. Fermentation 2022, 8, 399. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Co-Fermentation of Sewage Sludge with Ryegrass for Enhancing Hydrogen Production: Performance Evaluation and Kinetic Analysis. Bioresour. Technol. 2017, 243, 1027–1036. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Principle and Application of Different Pretreatment Methods for Enriching Hydrogen-Producing Bacteria from Mixed Cultures. Int. J. Hydrogen Energy 2017, 42, 4804–4823. [Google Scholar] [CrossRef]
- De Jesús Vargas-Soplín, A.; Prochnow, A.; Herrmann, C.; Tscheuschner, B.; Kreidenweis, U. The Potential for Biogas Production from Autumn Tree Leaves to Supply Energy and Reduce Greenhouse Gas Emissions—A Case Study from the City of Berlin. Resour. Conserv. Recycl. 2022, 187, 106598. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Samuelsson, J.; Christensen, T.H.; Scheutz, C. Quantification of Greenhouse Gas Emissions from Windrow Composting of Garden Waste. J. Environ. Qual. 2010, 39, 713–724. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Christensen, T.H.; Scheutz, C. Mass Balances and Life-Cycle Inventory for a Garden Waste Windrow Composting Plant (Aarhus, Denmark). Waste Manag. Res. 2010, 28, 1010–1020. [Google Scholar] [CrossRef]





| Leaves/Parameters-> | TS | SD; VC | VS | SD; VC | C | N | C/N | pH |
|---|---|---|---|---|---|---|---|---|
| Unit | % FM | % | % TS | % | % TS | % TS | - | - |
| Growing | 17.66 | 0.53; 2.97 | 93.60 | 0.46; 0.49 | 50.90 | 2.19 | 23.20 | 5.38 |
| Fresh fallen | 36.67 | 0.89; 2.42 | 86.11 | 1.07; 1.25 | 47.02 | 2.19 | 21.45 | 5.42 |
| Collected (1 week) | 24.78 | 0.58; 2.32 | 85.80 | 0.15; 0.17 | 46.01 | 2.20 | 20.96 | 5.39 |
| Leaves | Growing | Fresh Fallen | Collected (1 Week) |
|---|---|---|---|
| Elements | Units—ppm | ||
| Ag | 3829 | 4416 | 4043 |
| Bal | 937,610,750 | 936,310,750 | 936,856,063 |
| Ca | 19,800,084 | 20,248,988 | 20,401,322 |
| Cd | 12,186 | 11,738 | 13,297 |
| Cl | 453,866 | 500,118 | 662,751 |
| Fe | 265,755 | 300,924 | 318,112 |
| K | 32,943,508 | 33,162,426 | 32,103,723 |
| Mo | 5433 | 5992 | 5976 |
| Nb | 7853 | 8004 | 8122 |
| P | 1,795,822 | 1,917,396 | 2,027,264 |
| Pd | 2935 | 3587 | 3454 |
| Rb | 7706 | 7847 | 7931 |
| Re | <LOD | <LOD | <LOD |
| S | 4,735,686 | 4,629,216 | 4,630,707 |
| Sb | 12,475 | 12,895 | 13,065 |
| Se | <LOD | <LOD | <LOD |
| Si | 2,228,237 | 2,768,440 | 2,727,961 |
| Sr | 14,286 | 14,164 | 13,514 |
| Th | 1531 | 1688 | 1513 |
| Ti | 56,956 | 53,306 | 65,129 |
| U | 1418 | <LOD | <LOD |
| Leaves/Parameters-> | CH4 ratio | CH4 | Biogas | CH4 | Biogas | CH4 | Biogas |
|---|---|---|---|---|---|---|---|
| Unit | [%] | [m3/Mg FM] | [m3/Mg TS] | [m3/Mg VS] | |||
| Growing | 51.86 | 36.81 | 70.99 | 208.45 | 402.07 | 222.70 | 429.46 |
| Fresh fallen | 48.09 | 59.19 | 123.09 | 161.42 | 335.66 | 187.46 | 389.81 |
| Collected (1 week) | 50.66 | 36.56 | 72.18 | 147.56 | 291.31 | 171.98 | 339.51 |
| Mean value | 50.20 | 44.19 | 88.75 | 172.48 | 343.01 | 194.05 | 386.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurkiewicz, J. The Biogas Potential of Oxytree Leaves. Energies 2022, 15, 8872. https://doi.org/10.3390/en15238872
Mazurkiewicz J. The Biogas Potential of Oxytree Leaves. Energies. 2022; 15(23):8872. https://doi.org/10.3390/en15238872
Chicago/Turabian StyleMazurkiewicz, Jakub. 2022. "The Biogas Potential of Oxytree Leaves" Energies 15, no. 23: 8872. https://doi.org/10.3390/en15238872
APA StyleMazurkiewicz, J. (2022). The Biogas Potential of Oxytree Leaves. Energies, 15(23), 8872. https://doi.org/10.3390/en15238872






