The Efficiency of Pd Addition and Sr Substitution on La1−xSrxMnO3 to Remove Ventilation Air Methane in a Catalytic Flow Reversal Reactor
Abstract
:1. Introduction
2. Materials and Methods
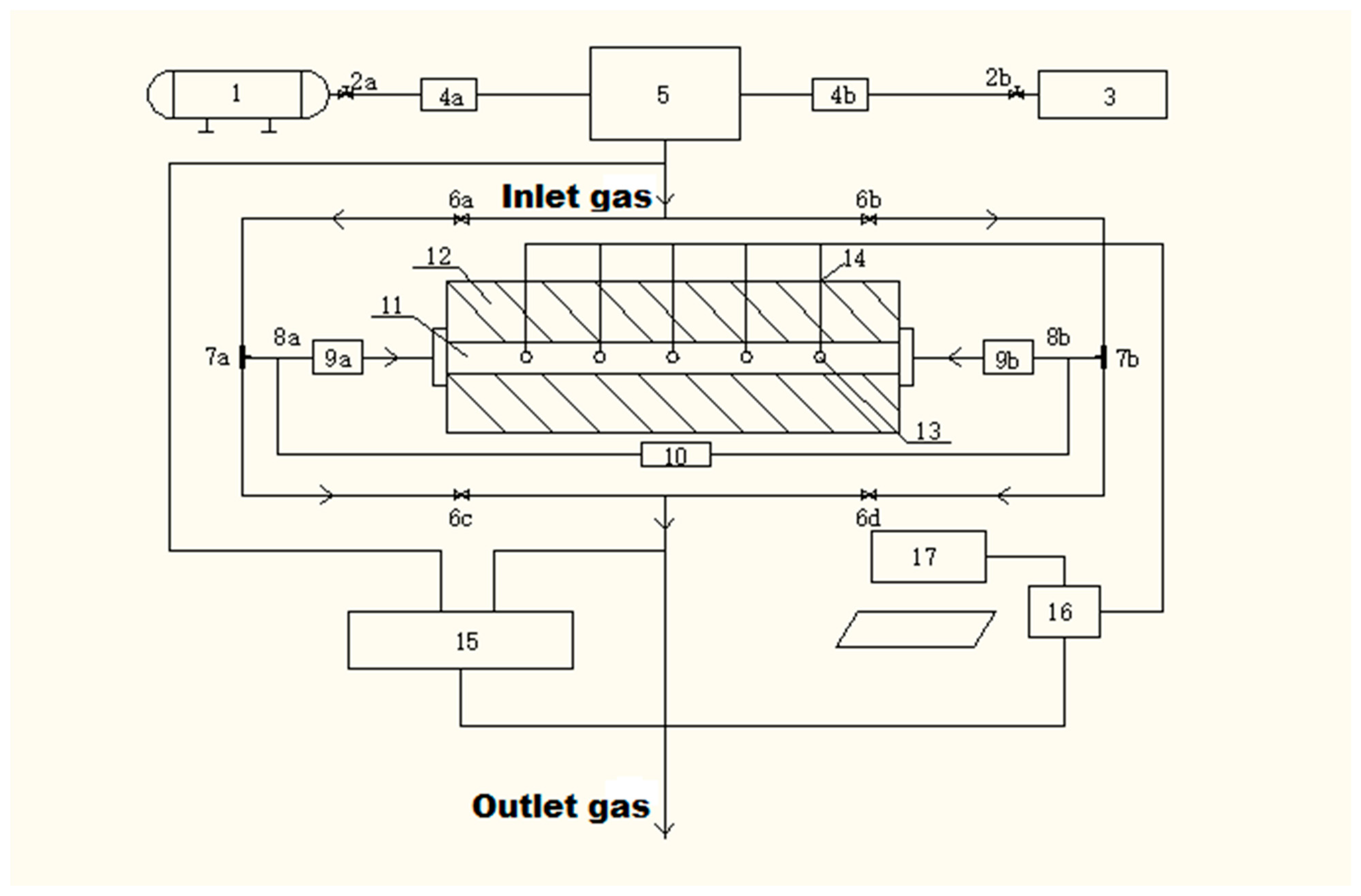
2.1. Introduction of the Experimental Station
2.2. Preparation of the Catalysts
2.2.1. Acid Treatment of the Cordierite
2.2.2. Coating of γ-Al2O3
2.2.3. Impregnation of Catalytically Active Ingredient
2.3. Coating Adhesion Test
2.4. Brunauer–Emmett–Teller (BET) Surface Area Analysis
2.5. Data Analysis
3. Results and Discussion
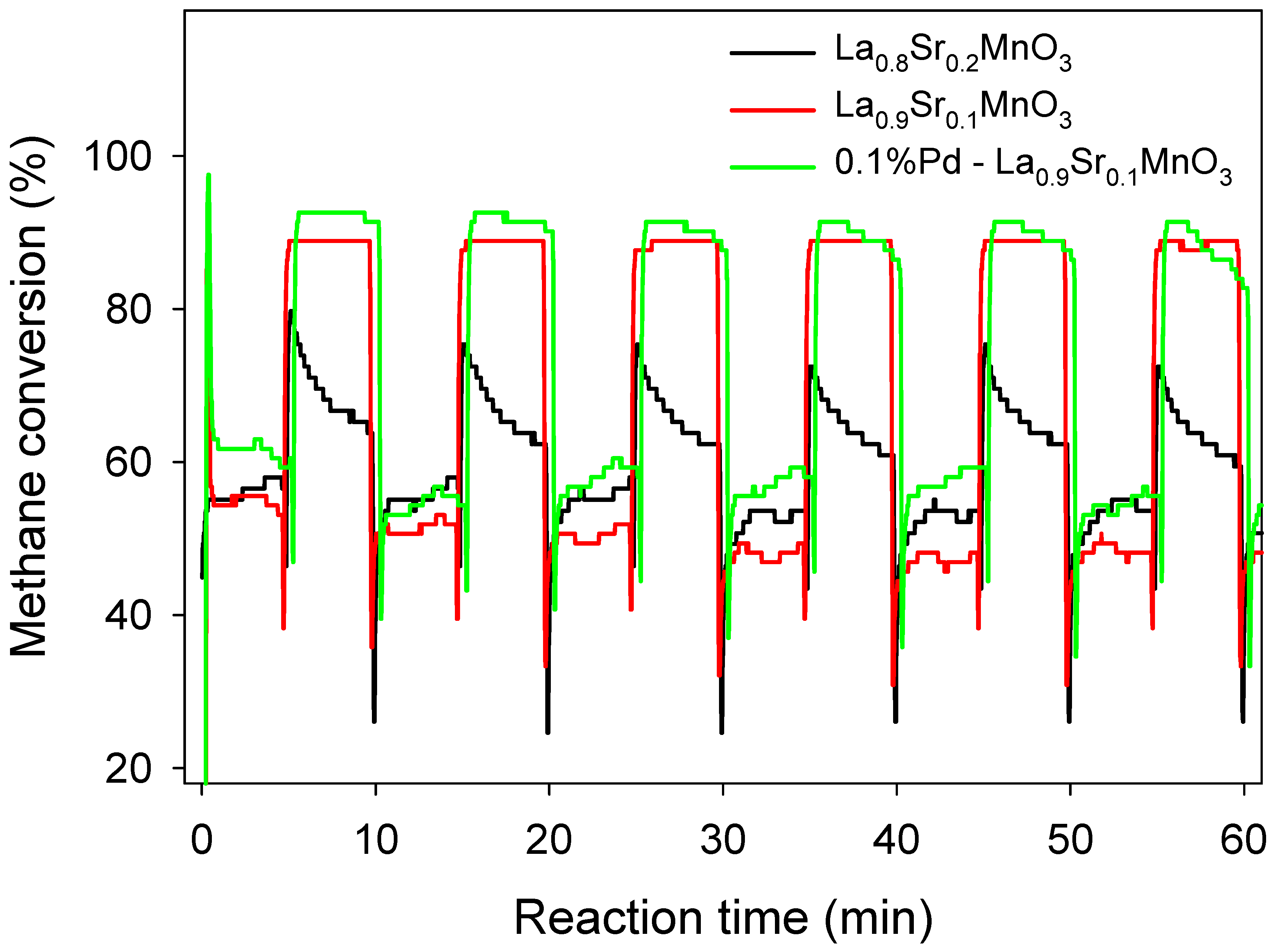
3.1. Comparison of the Conversion of Methane with or without Catalyst
3.2. Effect of Methane Inlet Concentration on Methane Conversion
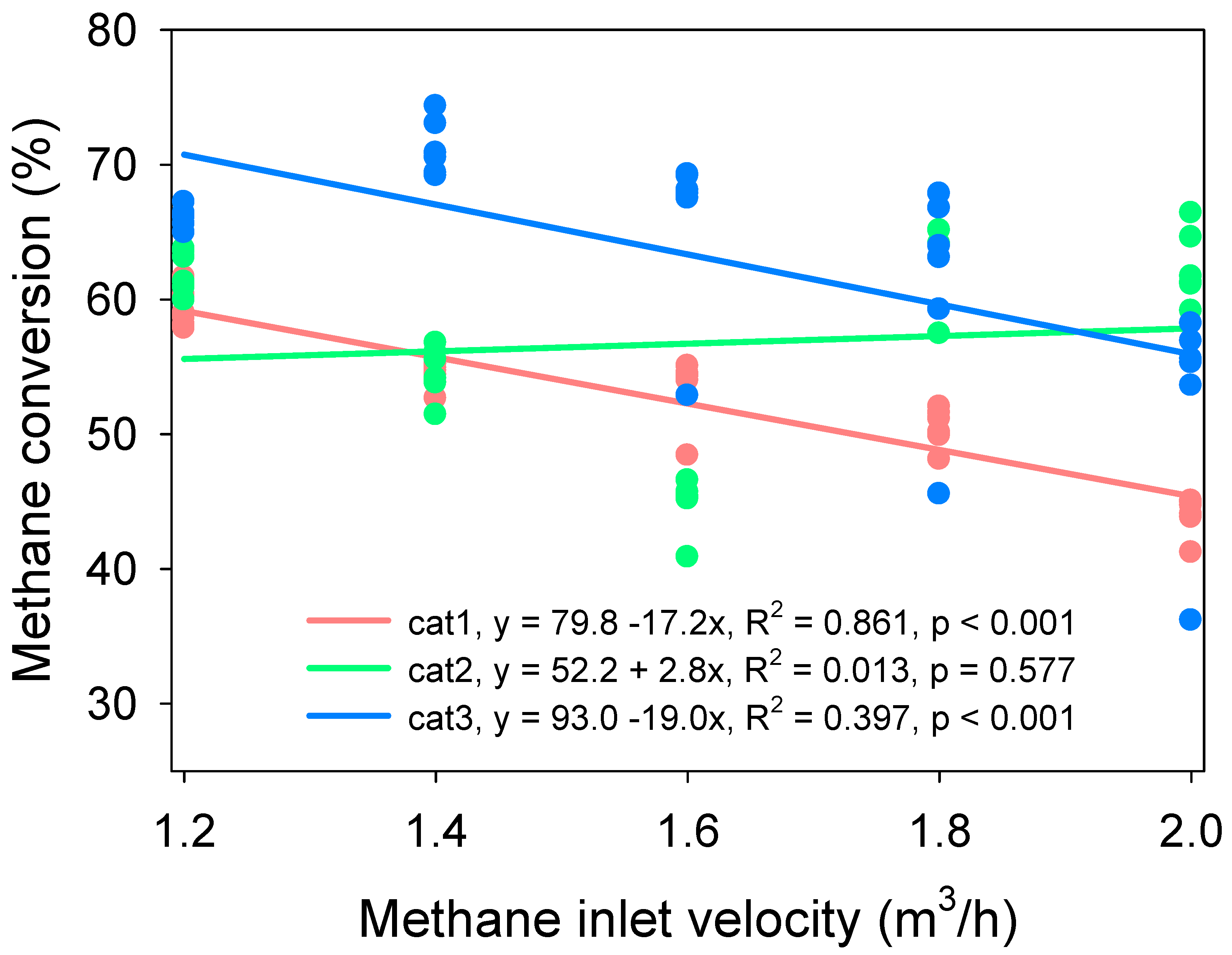
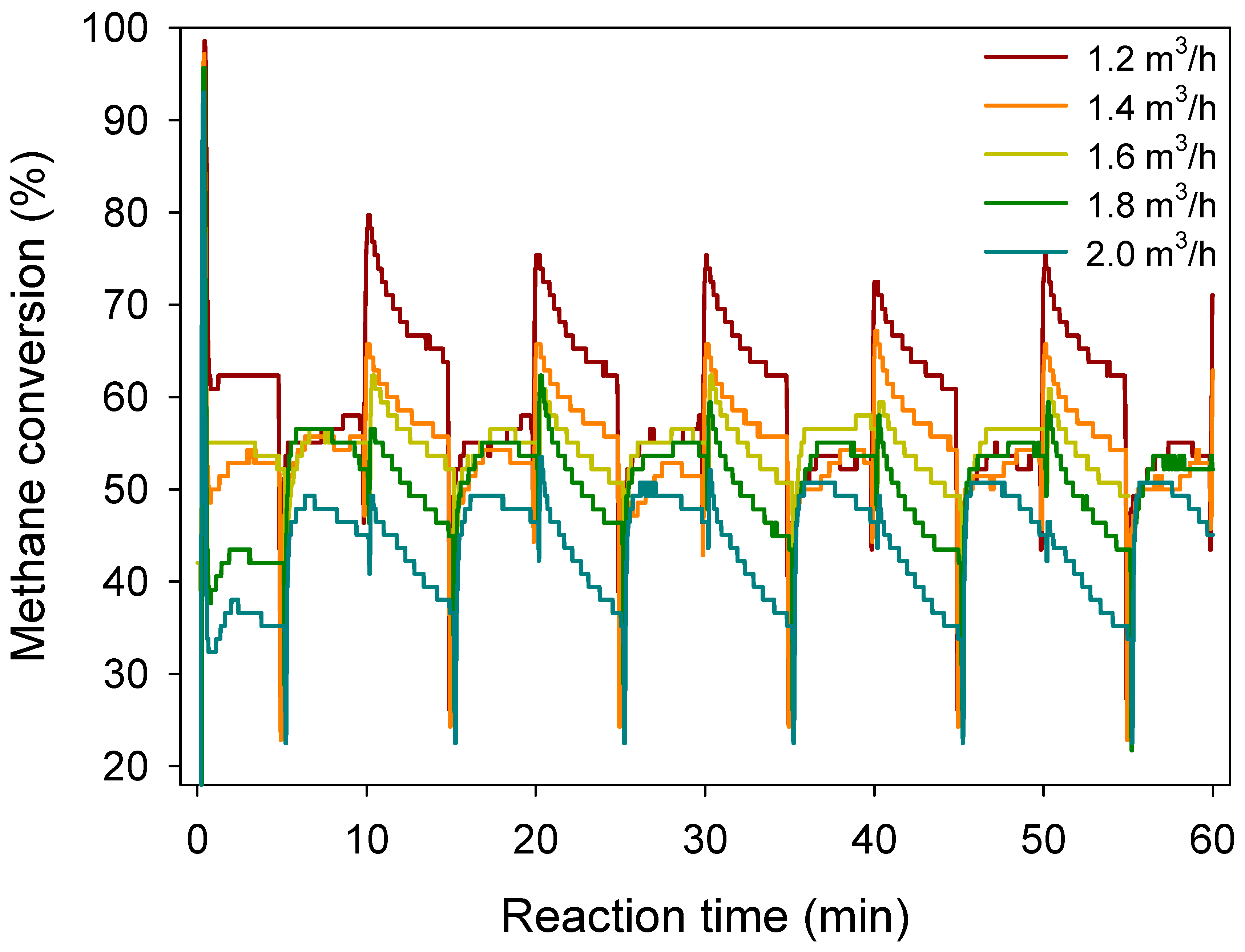
3.3. Effect of Methane Inlet Velocity on Methane Conversion
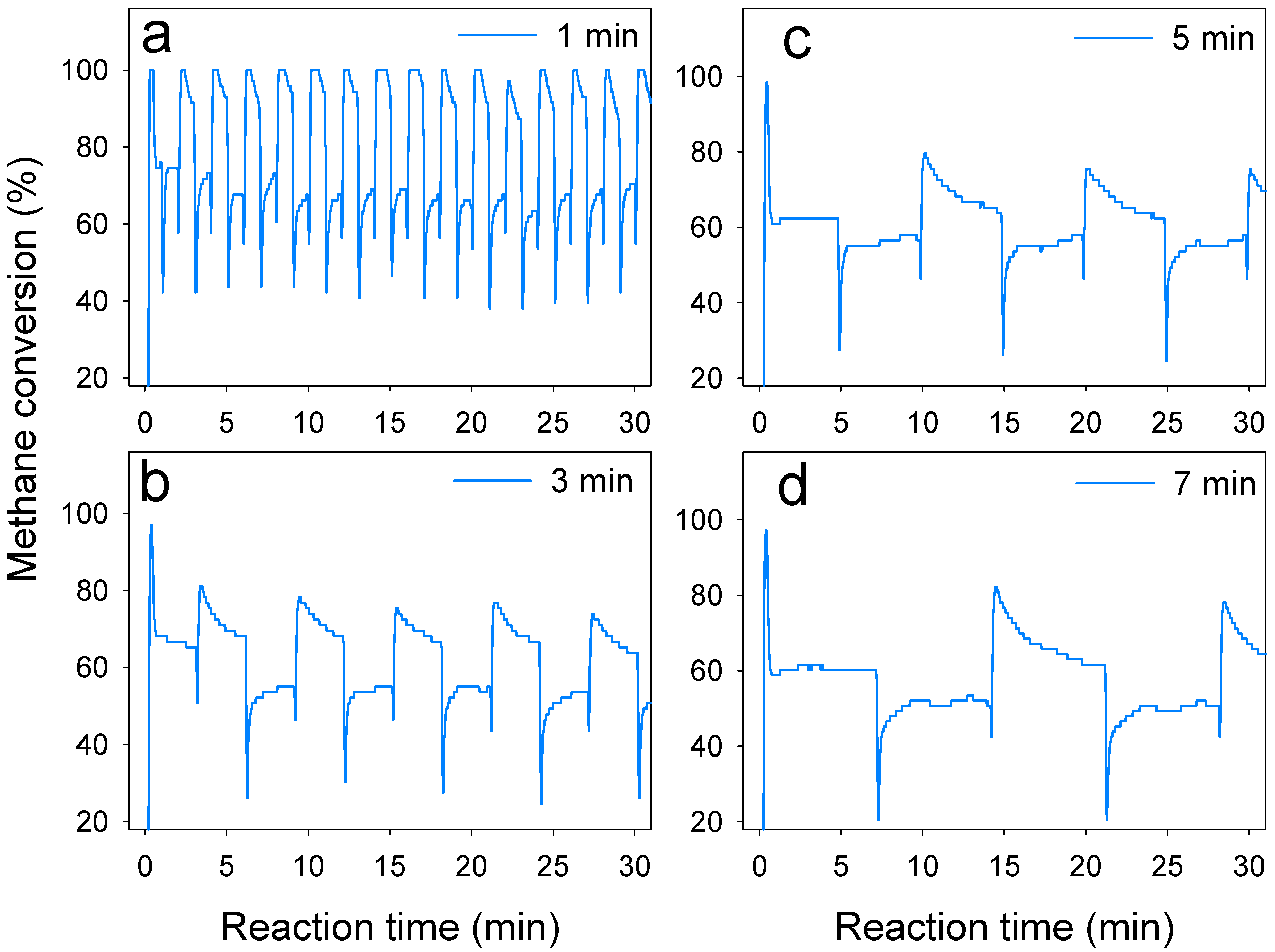
3.4. Effect of Switch Frequency on Methane Conversion
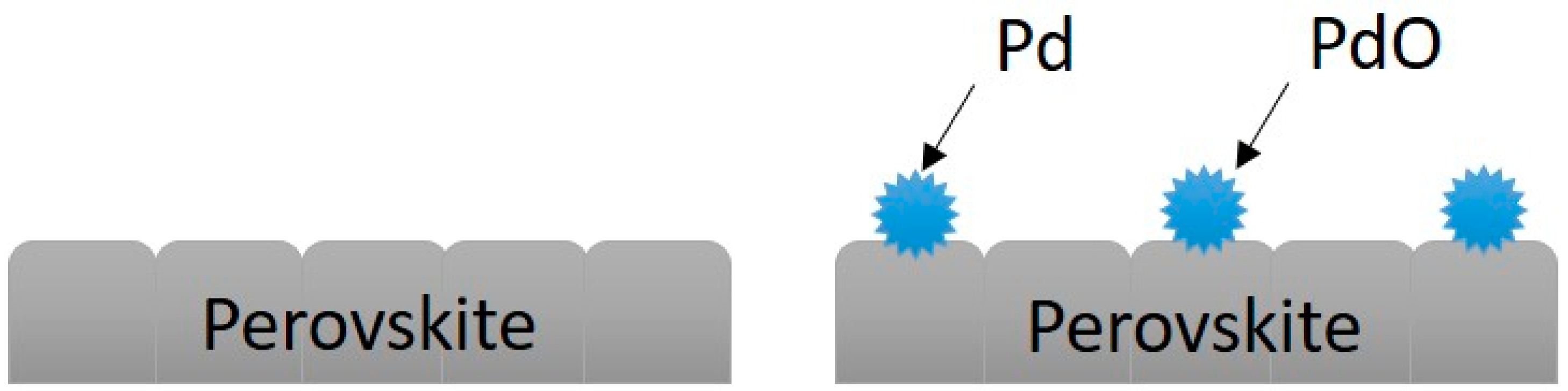
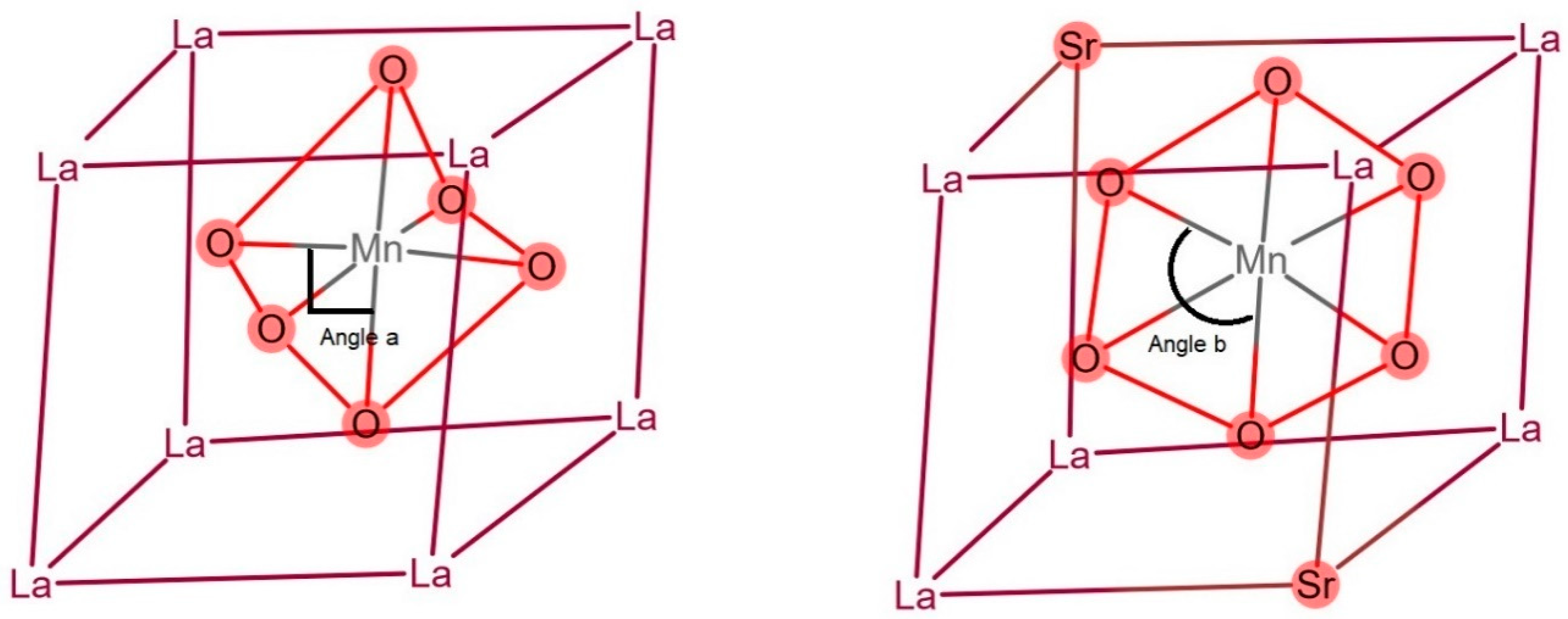
3.5. Catalysis Characteristics and Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kholod, N.; Evans, M.; Pilcher, R.C.; Roshchanka, V.; Ruiz, F.; Coté, M.; Collings, R. Global Methane Emissions from Coal Mining to Continue Growing Even with Declining Coal Production. J. Clean. Prod. 2020, 256, 120489. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Global Non-CO2 Greenhouse Gas Emission Projections & Mitigation Potential: 2015–2050: Download the Report. Available online: https://www.epa.gov/global-mitigation-non-co2-greenhouse-gases/global-non-co2-greenhouse-gas-emission-projections (accessed on 30 October 2021).
- Su, S.; Beath, A.; Guo, H.; Mallett, C. An Assessment of Mine Methane Mitigation and Utilisation Technologies. Prog. Energy Combust. Sci. 2005, 31, 123–170. [Google Scholar] [CrossRef]
- Su, S.; Agnew, J. Catalytic Combustion of Coal Mine Ventilation Air Methane. Fuel 2006, 85, 1201–1210. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Ran, J. Experiment and Modeling of Low-Concentration Methane Catalytic Combustion in a Fluidized Bed Reactor. Appl. Therm. Eng. 2016, 93, 660–667. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Procedures for Heat Recovery in the Catalytic Combustion of Lean Methane–Air Mixtures in a Reverse Flow Reactor. Chem. Eng. J. 2009, 147, 356–365. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Z.; Zhang, Y.; Wu, Z.; Wang, H.; Li, S.; Shi, R.; Dong, M.; Fan, W.; Wang, J. A Control Strategy of Flow Reversal with Hot Gas Withdrawal for Heat Recovery and Its Application in Mitigation and Utilization of Ventilation Air Methane in a Reverse Flow Reactor. Chem. Eng. J. 2013, 228, 243–255. [Google Scholar] [CrossRef]
- Gosiewski, K.; Matros, Y.S.; Warmuzinski, K.; Jaschik, M.; Tanczyk, M. Homogeneous vs. Catalytic Combustion of Lean Methane—Air Mixtures in Reverse-Flow Reactors. Chem. Eng. Sci. 2008, 63, 5010–5019. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or Thermal Reversed Flow Combustion of Coal Mine Ventilation Air Methane: What Is Better Choice and When? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Qin, Z.; Zhu, H.; Wu, J.; Wang, R.; Lei, L.; Chen, J.; Dong, M.; Fan, W.; et al. Demonstration of Mitigation and Utilization of Ventilation Air Methane in a Pilot Scale Catalytic Reverse Flow Reactor. Fuel Process. Technol. 2017, 160, 102–108. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Oliveira, E.C.; Fraga, M.A.; Pastore, H.O. Performance of Pd Supported on Mesoporous Molecular Sieves on Methane Combustion. Catal. Commun. 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Fan, X.; Wang, F.; Zhu, T.; He, H. Effects of Ce on Catalytic Combustion of Methane over Pd-Pt/Al2O3 Catalyst. J. Environ. Sci. China 2012, 24, 507–511. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic Combustion of Methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Xiong, H.; Lester, K.; Ressler, T.; Schlögl, R.; Allard, L.F.; Datye, A.K. Metastable Pd ↔ PdO Structures During High Temperature Methane Oxidation. Catal. Lett. 2017, 147, 1095–1103. [Google Scholar] [CrossRef]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Niskala Koivikko, N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224. [Google Scholar] [CrossRef]
- Tang, W.; Liu, G.; Li, D.; Liu, H.; Wu, X.; Han, N.; Chen, Y. Design and Synthesis of Porous Non-Noble Metal Oxides for Catalytic Removal of VOCs. Sci. China Chem. 2015, 58, 1359–1366. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of γ-MnO2 as a VOC Removal Catalyst: Comparison with a Noble Metal Catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Milt, V.G.; Spretz, R.; Ulla, M.A.; Lombardo, E.A.; Fierro, J.L.G. The Nature of Active Sites for the Oxidation of Methane on La-Based Perovskites. Catal. Lett. 1996, 42, 57–63. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Structure and Reactivity of Perovskite-Type Oxides. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 36, pp. 237–328. [Google Scholar] [CrossRef]
- Cimino, S.; Pirone, R.; Russo, G. Thermal Stability of Perovskite-Based Monolithic Reactors in the Catalytic Combustion of Methane. Ind. Eng. Chem. Res. 2001, 40, 80–85. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic Combustion of Methane over Various Perovskite-Type Oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W. Metallic Monolith Supported LaMnO3 Perovskite-Based Catalysts in Methane Combustion. Catal. Lett. 2007, 115, 122–132. [Google Scholar] [CrossRef]
- Zhang, H.M.; Teraoka, Y.; Yamazoe, N. Preparation of Supported La1−xSrxMnO3 Catalysts by the Citrate Process. Appl. Catal. 1988, 41, 137–146. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowskib, S.T.; Li, P.K.C.; Awdry, S. Evaluating the Effective Diffusivity of Methane in the Washcoat of a Honeycomb Monolith. Appl. Catal. B Environ. 2000, 25, 93–104. [Google Scholar] [CrossRef]
- Al-Harbi, O.A.; Özgür, C.; Khan, M.M. Fabrication and Characterization of Single Phase Cordierite Honeycomb Monolith with Porous Wall from Natural Raw Materials as Catalyst Support. Ceram. Int. 2015, 41 Pt A, 3526–3532. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, F.; Niu, X.; Zhu, Y. Preparation of Pd Supported on La(Sr)-Mn-O Perovskite by Microwave Irradiation Method and Its Catalytic Performances for the Methane Combustion. Sci. Rep. 2016, 6, 19511. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Casaletto, M.P.; Lisi, L.; Russo, G. Pd–LaMnO3 as Dual Site Catalysts for Methane Combustion. Appl. Catal. Gen. 2007, 327, 238–246. [Google Scholar] [CrossRef]
- Eyssler, A.; Mandaliev, P.; Winkler, A.; Hug, P.; Safonova, O.; Figi, R.; Weidenkaff, A.; Ferri, D. The Effect of the State of Pd on Methane Combustion in Pd-Doped LaFeO3. J. Phys. Chem. C 2010, 114, 4584–4594. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, R. Catalytic Activity for Methane Combustion of the Perovskite-Type La1−xSrxCoO3−δ Oxide Prepared by the Urea Decomposition Method. Appl. Catal. B Environ. 2010, 98, 147–153. [Google Scholar] [CrossRef]
- Budhi, Y.W.; Hoebink, J.H.B.J.; Schouten, J.C. Reverse Flow Operation with Reactor Side Feeding: Analysis, Modeling, and Simulation. Ind. Eng. Chem. Res. 2004, 43, 6955–6963. [Google Scholar] [CrossRef]
- Zhikai, L.; Zhangfeng, Q.; Yagang, Z.; Zhiwei, W.; Hui, W.; Shuna, L.; Mei, D.; Weibin, F.; Jianguo, W. A Logic-Based Controller for the Mitigation of Ventilation Air Methane in a Catalytic Flow Reversal Reactor. Front. Chem. Sci. Eng. 2013, 7, 347–356. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G. Dual-Site Pd/Perovskite Monolithic Catalysts for Methane Catalytic Combustion. Ind. Eng. Chem. Res. 2004, 43, 6670–6679. [Google Scholar] [CrossRef]
- Civera, A.; Negro, G.; Speccia, S.; Saracco, G.; Soecchia, V. Optimal compositional and structural design of a LaMnO3/ZrO2/Pd-based catalyst for methane combustion. Catal. Today 2005, 100, 275–281. [Google Scholar] [CrossRef]
- Petrović, S.; Karanović, L.; Stefanov, P.K.; Zdujić, M.; Terlecki-Baričević, A. Catalytic Combustion of Methane over Pd Containing Perovskite Type Oxides. Appl. Catal. B Environ. 2005, 58, 133–141. [Google Scholar] [CrossRef]
- Ciambelli, P.; Cimino, S.; De Rossi, S.; Faticanti, M.; Lisi, L.; Minelli, G.; Pettiti, I.; Porta, P.; Russo, G.; Turco, M. AMnO3 (A = La, Nd, Sm) and Sm1−xSrxMnO3 Perovskites as Combustion Catalysts: Structural, Redox and Catalytic Properties. Appl. Catal. B Environ. 2000, 24, 243–253. [Google Scholar] [CrossRef]
- McCarty, J.G.; Wise, H. Perovskite Catalysts for Methane Combustion. Catal. Today 1990, 8, 231–248. [Google Scholar] [CrossRef]
- Miniajluk, N.; Trawczyński, J.; Zawadzki, M.; Tylus, W. LaMnO3 (La0.8Sr0.2MnO3) Perovskites for Lean Methane Combustion: Effect of Synthesis Method. Adv. Mater. Phys. Chem. 2018, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Zwinkels, M.F.M.; Järås, S.G.; Menon, P.G.; Griffin, T.A. Catalytic Materials for High-Temperature Combustion. Catal. Rev. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Quinlan, M.A.; Wise, H.; McCarty, J.G. Basic Research on Natural Gas Combustion Phenomena-Catalytic Combustion. Final Report, 15 January 1986–14 January 1989; PB-90-106493/XAB; SRI International: Menlo Park, CA, USA, 1989. [Google Scholar]
- McBride, K.; Cook, J.; Gray, S.; Felton, S.; Stella, L.; Poulidi, D. Evaluation of La1−xSrxMnO3 (0 ≤ x < 0.4) Synthesised via a Modified Sol–Gel Method as Mediators for Magnetic Fluid Hyperthermia. Cryst. Eng. Comm. 2016, 18, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(Electro)Catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]




| Treatment | Catalysts | Surface Area (m2/g) |
|---|---|---|
| cat1 | La0.8Sr0.2MnO3 | 16.86 |
| cat2 | La0.9Sr0.1MnO3 | 17.58 |
| cat3 | 0.1% Pd-La0.9Sr0.1MnO3 | 20.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, T. The Efficiency of Pd Addition and Sr Substitution on La1−xSrxMnO3 to Remove Ventilation Air Methane in a Catalytic Flow Reversal Reactor. Atmosphere 2022, 13, 54. https://doi.org/10.3390/atmos13010054
Wang Y, Zhu T. The Efficiency of Pd Addition and Sr Substitution on La1−xSrxMnO3 to Remove Ventilation Air Methane in a Catalytic Flow Reversal Reactor. Atmosphere. 2022; 13(1):54. https://doi.org/10.3390/atmos13010054
Chicago/Turabian StyleWang, Yanxia, and Tao Zhu. 2022. "The Efficiency of Pd Addition and Sr Substitution on La1−xSrxMnO3 to Remove Ventilation Air Methane in a Catalytic Flow Reversal Reactor" Atmosphere 13, no. 1: 54. https://doi.org/10.3390/atmos13010054
APA StyleWang, Y., & Zhu, T. (2022). The Efficiency of Pd Addition and Sr Substitution on La1−xSrxMnO3 to Remove Ventilation Air Methane in a Catalytic Flow Reversal Reactor. Atmosphere, 13(1), 54. https://doi.org/10.3390/atmos13010054







