Influence of Alternative Lifestyles on Antibiotic Use during Pregnancy, Lactation and in Children
Abstract
:1. Introduction
2. Results
2.1. Demographic, Lifestyle and Health-Related Characteristics
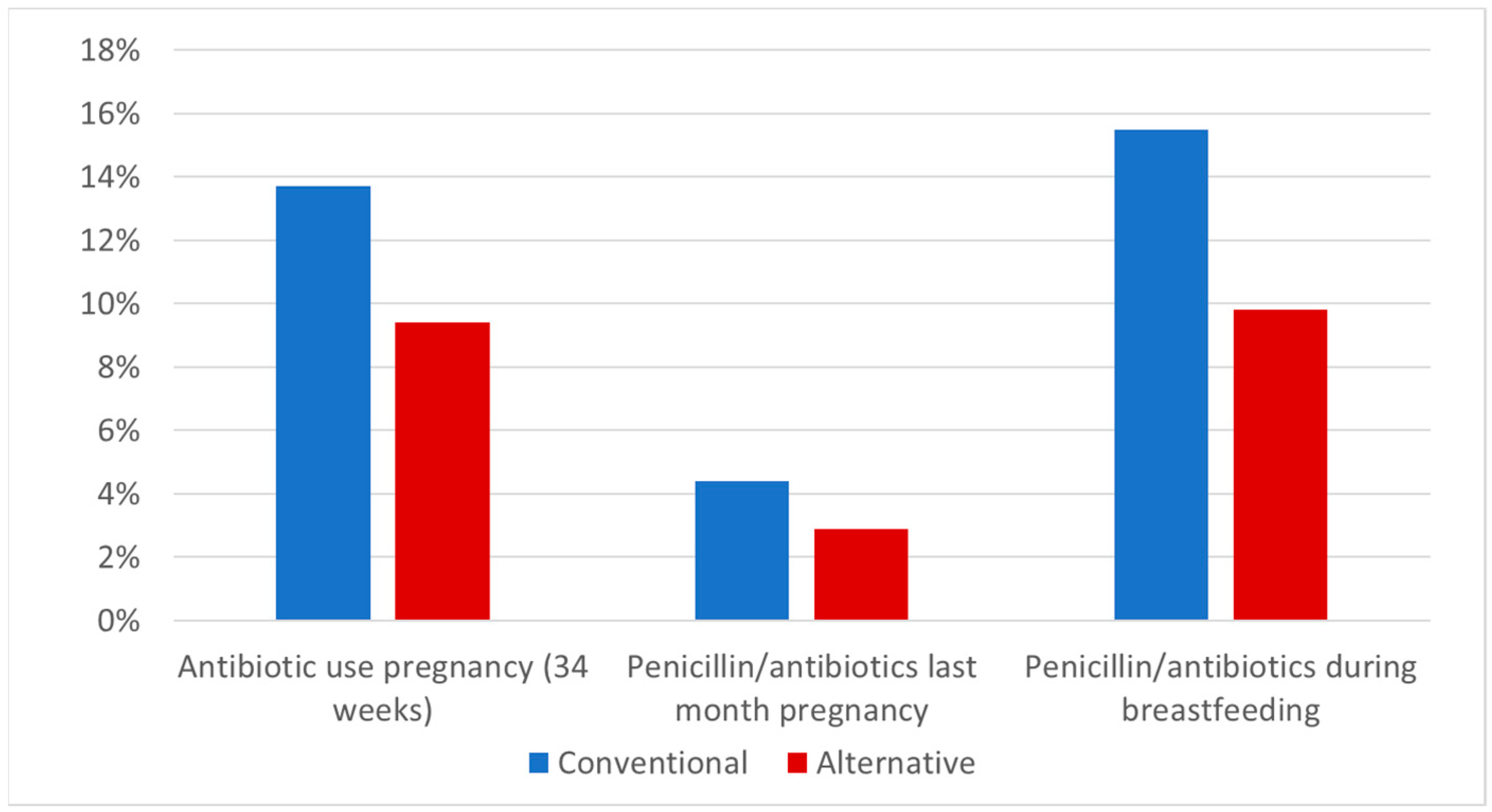
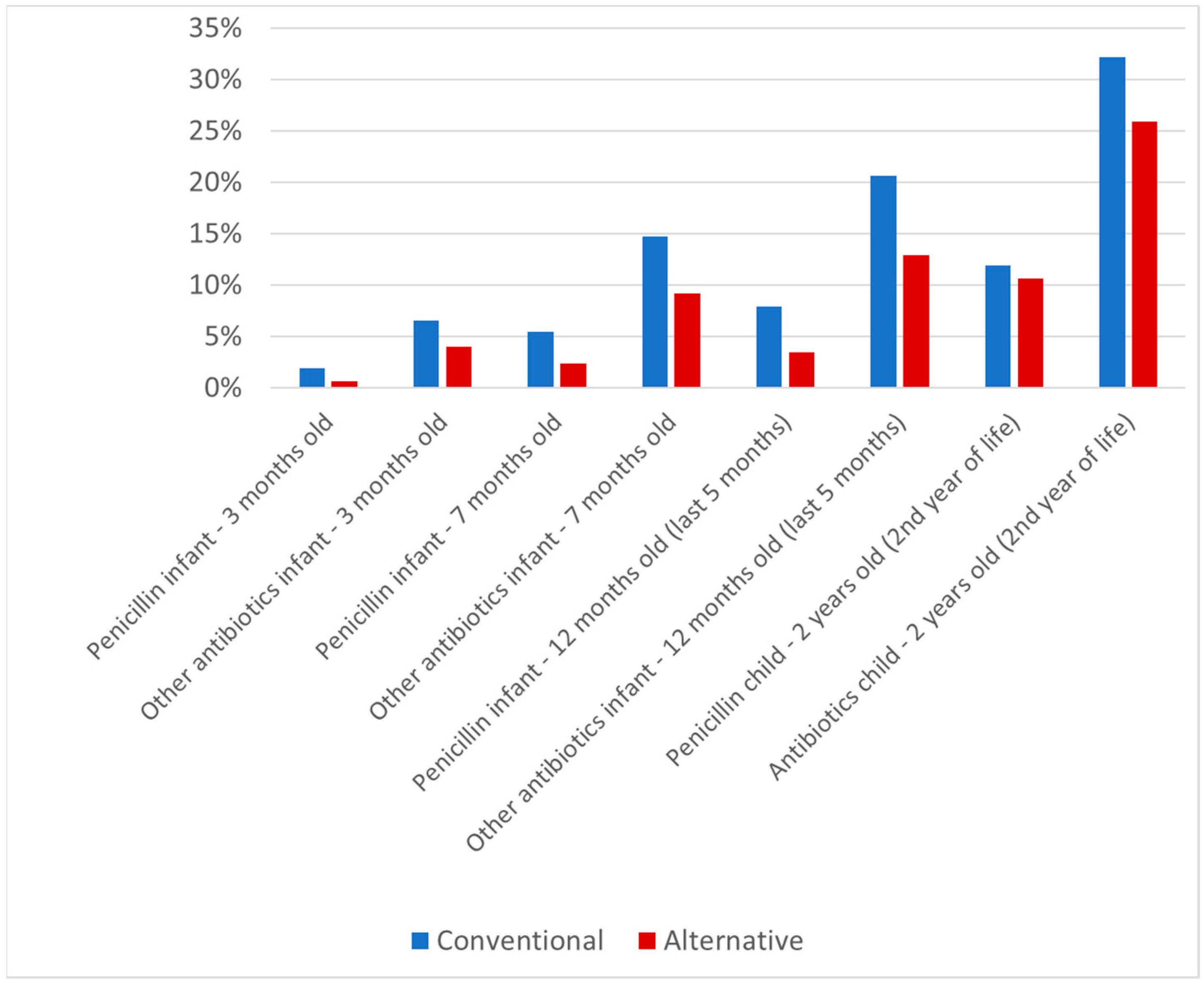
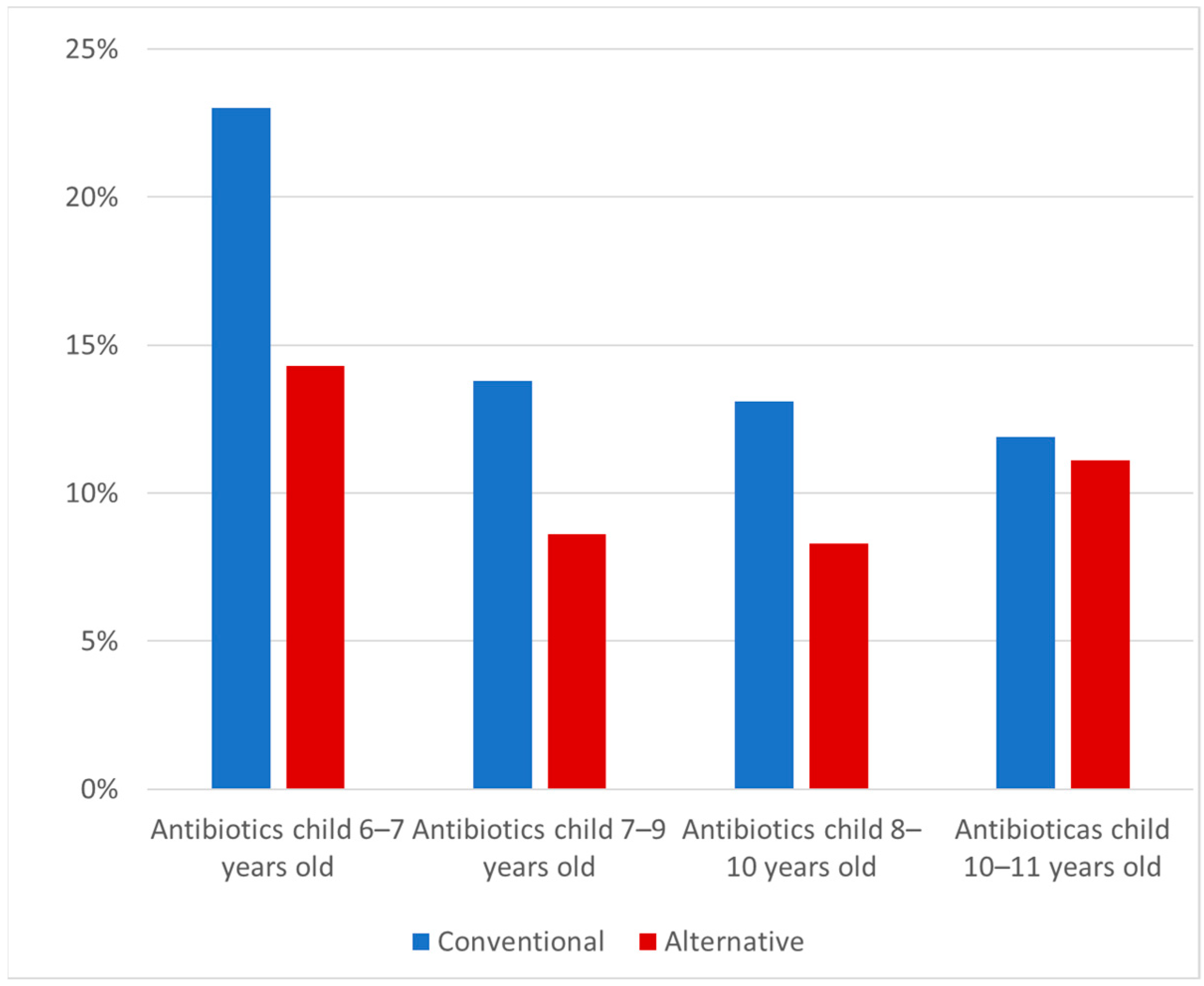
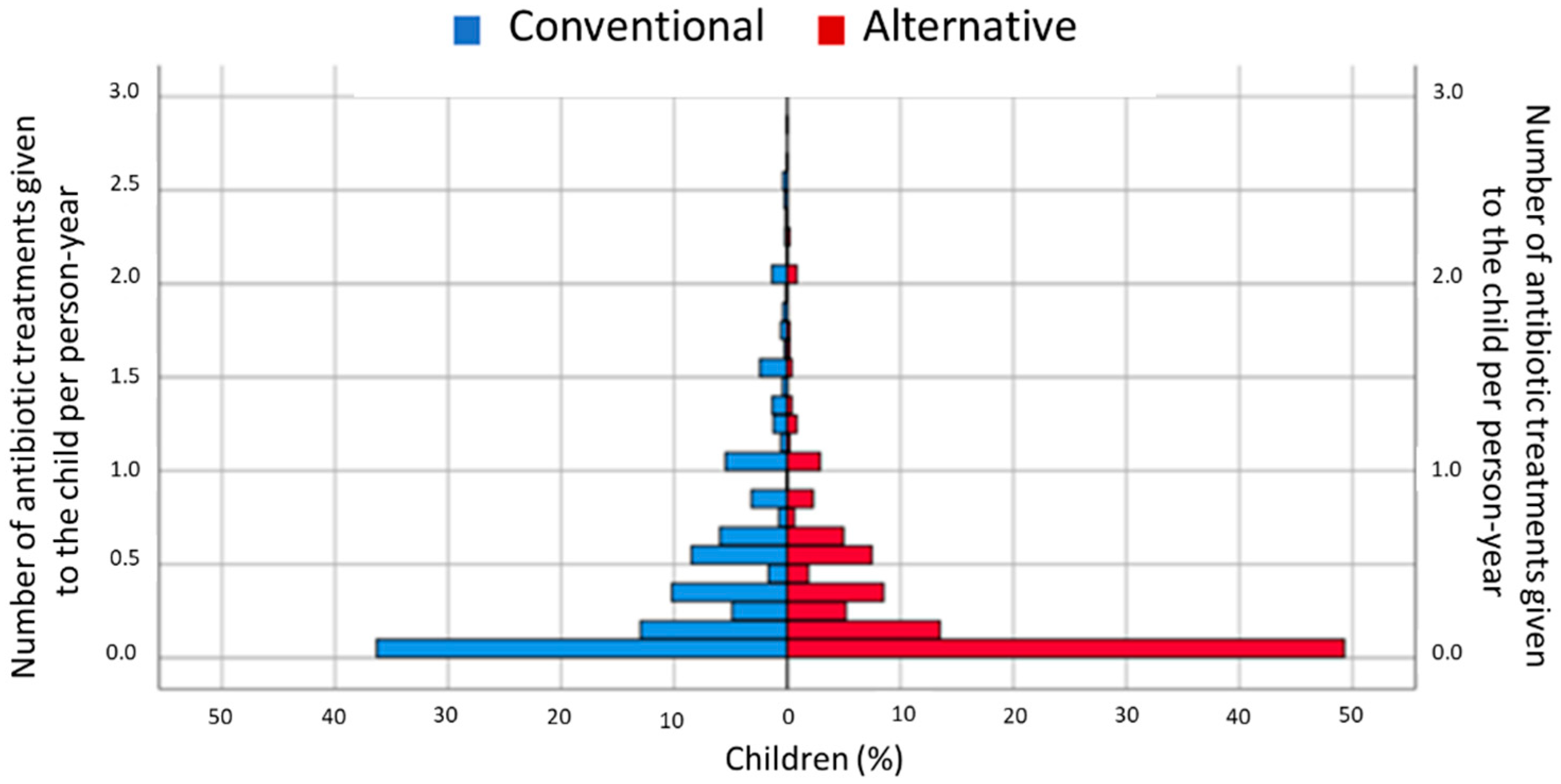
2.2. Antibiotic Use
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data on Antibiotic Use
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antibiotic Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance#:~:text=Bacteria%2C%20not%20humans%20or%20animals,hospital%20stays%2C%20and%20increased%20mortality (accessed on 7 May 2021).
- Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob. Chemother. 2018, 73 (Suppl. 2), 19–26. [Google Scholar] [CrossRef]
- Conroy, S.; Impicciatore, C.P.; Mohn, A.; Arnell, H.; Rane, A.; Knoeppel, C.; Seyberth, H.; Pandolfini, C.; Raffaelli, M.P.; Rocchi, F.; et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ 2000, 320, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goosens, H.; ARPEC Group. The worldwide antibiotic resistance and prescribing in European children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Vernacchio, L.; Kelly, J.P.; Kaufman, D.W.; Mitchell, A.A. Medication use among children <12 years of age in the United States: Results from the slone survey. Pediatrics 2009, 124, 446–454. [Google Scholar] [CrossRef]
- Amadeo, B.; Zarb, P.; Muller, A.; Drapier, N.; Vankerckhoven, V.; Rogues, A.-M.; Davey, P.; Goosens, H.; ESAC III Hospital Care Group. European Surveillance of Antibiotic Consumption (ESAC) point prevalence survey 2008: Paediatric antimicrobial prescribing in 32 hospitals of 21 European countries. J. Antimicrob. Chemother. 2010, 65, 2247–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, Y.; Sharland, M.; Jackson, C.; Wong, I.C.; Magrini, N.; Bielicki, J.A. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2019, 19, 67–75. [Google Scholar] [CrossRef]
- Bailey, L.C.; Forrest, C.B.; Zhang, P.; Richards, T.M.; Livshits, A.; DeRusso, P.A. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014, 168, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, A.; Virta, L.J.; Sankilampi, U.; Dunkel, L.; Saxen, H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 2015, 135, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Simões-Wust, A.P.; Kummeling, I.; Mommers, M.; Huber, M.A.S.; Rist, L.; van de Vijver, L.P.L.; Dagnelie, P.C.; Thijs, C. Influence of alternative lifestyles on self-reported body weight and health characteristics in women. Eur. J. Public Health 2013, 24, 321–327. [Google Scholar] [CrossRef] [Green Version]
- OECD. Antimicrobial Resistance-Policy Insights. 2016. Available online: https://www.oecd.org/health/health-systems/AMR-Policy-Insights-November2016.pdf (accessed on 29 May 2021).
- Akkerman, A.E.; Kuyvenhoven, M.M.; van der Wouden, J.C.; Verheij, T.J.M. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. J. Antimicrob. Chemother. 2005, 56, 930–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, C.; Peters, P.W.; Miller, R.K. Drugs during Pregnancy and Lactation: Treatment Options and Risk Assessment; Academic Press: Munich, Germany, 2014. [Google Scholar]
- Hviid, A.; Svanstrom, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Rousounidis, A.; Papaevangelou, V.; Hadjipanayis, A.; Panagakou, S.; Theodoridou, M.; Syrogiannopoulos, G.; Hadijchristodoulou, C. Descriptive study on parents’ knowledge, attitudes and practices on antibiotic use and misuse in children with upper respiratory tract infections in Cyprus. Int. J. Environ. Res. Public Health 2011, 8, 3246–3262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, J.Y. Infant benefits of breastfeeding, UpToDate. 2021. Available online: https://www.uptodate.com/contents/infant-benefits-of-breastfeeding# (accessed on 8 July 2021).
- Kummeling, I.; Thijs, C.; Penders, J.; Snijders, B.E.P.; Foekje, S.; Reimerink, J.; Koopmans, M.; Dagnelie, P.C.; Huber, M.; Jansen, M.C.J.; et al. Etiology of atopy in infancy: The KOALA birth cohort study. Pediatr Allergy Immunol. 2005, 16, 679–684. [Google Scholar] [CrossRef]
- Davey, P.; Pagliari, C.; Hayes, A. The patient’s role in the spread and control of bacterial resistance to antibiotics. Clin. Microbiol. Infect. 2002, 8 (Suppl. 2), 43–68. [Google Scholar] [CrossRef] [Green Version]
- Butler, C.C.; Rollnick, S.; Pill, R.; Maggs-Rapport, F.; Stott, N. Understanding the culture of prescribing: Qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ 1998, 317, 637–642. [Google Scholar] [CrossRef] [Green Version]
- de Bont, E.G.; Loonen, N.; Hendrix, D.A.S.; Lepot, J.M.M.; Dinant, G.-J.; Cals, J.W.L. Childhood fever: A qualitative study on parents’ expectations and experiences during general practice out-of-hours care consultations. BMC Fam. Pract. 2015, 16, 131. [Google Scholar] [CrossRef] [Green Version]
- de Bont, E.G.; Loonen, N.; Hendrix, A.S.; Lepot, J.M.M.; Dinant, G.-J.; Cals, J.W.L. Childhood fever: A qualitative study on GPs’ experiences during out-of-hours care. Fam. Pract. 2015, 32, 449–455. [Google Scholar] [CrossRef] [Green Version]
- de Bont, E.; Dinant, G.-J.; Elshout, G.; van Well, G.; Francis, N.A.; Winkens, B.; Cals, J.W.L. Booklet for childhood fever in out-of-hours primary care: A cluster-randomized controlled trial. Ann. Fam. Med. 2018, 16, 314–321. [Google Scholar] [CrossRef]
- Kienle, G.S.; Albonico, H.-U.; Baars, E.; Hamre, H.J.; Zimmermann, P.; Kiene, H. Anthroposophic medicine: An integrative medical system originating in europe. Glob. Adv. Health Med. 2013, 2, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Kienle, G.; Kiene, H.; Albonico, H.-U. Anthroposophic Medicine-Effectiveness, Utility, Costs, Safety; Schattauer: Stuttgart, Germany, 2006. [Google Scholar]
- Hamre, H.J.; Glockmann, A.; Scharwz, R.; Riley, D.S.; Baars, E.W.; Kiene, H.; Kienle, G.S. Antibiotic use in children with acute respiratory or ear infections: Prospective observational comparison of anthroposophic and conventional treatment under routine primary care conditions. Evid. Based Complement Alternat. Med. 2014, 2014, 243801. [Google Scholar] [CrossRef] [Green Version]
- Spurling, G.K.; Del Mar, C.B.; Dooley, L.; Foxlee, R.; Farley, R. Delayed antibiotic prescriptions for respiratory infections. Cochr. Database Syst. Rev. 2017, 9, CD004417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoialã, E.L.; Stanciu, G.D.; Bild, V.; Ababei, D.C.; Gavrilovici, C. From evidence to clinical guidelines in antibiotic treatment in acute otitis media in children. Antibiotics 2021, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Szoke, H.; Marodi, M.; Vagedes, J.; Szekely, B.; Magyarosi, I.; Bedo, A.; Fellegi, V.; Somogyvari, K.; Moricz, P. The P.E.A.N.U.T. Method: Update on an integrative system approach for the treatment of chronic otitis media with effusion and adenoid hypertrophy in children. Antibiotics 2021, 10, 134. [Google Scholar] [CrossRef]
- Hamre, H.J.; Witt, C.M.; Glockmann, A.; Ziegler, R.; Willich, S.N.; Kiene, H. Anthroposophic medical therapy in chronic disease: A four-year prospective cohort study. BMC Complement Altern. Med. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baars, E.W.; Belt-van-Zoen, E.; Breitkreuz, T.; Martin, D.; Matthes, H.; von Schoen-Angerer, T.; Soldner, G.; Vagedes, J.; van Wietmarschen, H.; Patjin, O.; et al. The contribution of complementary and alternative medicine to reduce antibiotic use: A narrative review of health concepts, prevention, and treatment strategies. Evid. Based Complement Alternat. Med. 2019, 2019, 5365608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and Government of the United Kingdom: London, UK, 2016. [Google Scholar]
| Characteristics | Alternative Lifestyle Group (N = 491) | Conventional Lifestyle Group (N = 2343) | |||
|---|---|---|---|---|---|
| n/N or n | % or Mean ± S.D. | n/N or N | % or Mean ± S.D. | ||
| Age of mother | 491 | 33.7 ± 4.3 | 2340 | 31.7 ± 3.6 | |
| Highest educational attainment | WO Academic education | 132/491 | 27.0% | 256/2340 | 11.1% |
| HBO Higher vocational education | 225/491 | 46.0% | 728/2340 | 31.5% | |
| MBO Vocational secondary education | 52/491 | 10.6% | 706/2340 | 30.6% | |
| VWO—high school Pre-university education | 18/491 | 3.7% | 57/2340 | 2.5% | |
| HAVO—high school Senior general secondary education | 33/491 | 6.8% | 194/2340 | 8.4% | |
| MAVO—high school Pre-vocational secondary education | 8/491 | 1.6% | 145/2340 | 6.3% | |
| LBO—high school Lower vocational secondary education | 8/491 | 1.6% | 123/2340 | 5.3% | |
| Elementary school | 2/491 | 0.4% | 3/2340 | 0.1% | |
| Other | 11/491 | 2.3% | 97/2340 | 4.2% | |
| Missing values | 2/491 | 0.4% | 34/2340 | 1.5% | |
| Adhered to living rules during the last month | 156/491 | 32.6% | 51/2340 | 2.2% | |
| Adhered to… | |||||
| ...Vegetarianism | 83/491 | 16.9% | 36/2343 | 1.5% | |
| …Veganism | 1/491 | 0.2% | 1/2343 | 0.0% | |
| …Macrobiotics | 6/491 | 1.2% | 1/2343 | 0.0% | |
| …Anthroposophic lifestyle | 70/491 | 14.3% | 3/2343 | 0.1% | |
| …Life reform movement | 19/491 | 3.9% | 4/2343 | 0.2% | |
| …Islam | 0/491 | 0.0% | 10/2343 | 0.4% | |
| …Buddhism | 3/491 | 0.6% | 0/2343 | 0.0% | |
| …Judaism | 3/491 | 0.6% | 0/2343 | 0.0% | |
| …Hinduism | 0/491 | 0.0% | 1/2343 | 0.0% | |
| …Other | 31/491 | 6.3% | 6/2343 | 0.3% | |
| Intentional choice of some organic products | |||||
| Consumed organic products | 428/490 | 87.3% | 485/2333 | 20.8% | |
| Did not consume organic products | 52/490 | 10.6% | 1471/2333 | 63.1% | |
| Did not know/did not care | 10/428 | 2.0% | 377/2333 | 16.2% | |
| Intentional choice of biodynamic products | |||||
| Consumed biodynamic products | 357/488 | 73.2% | 167/2327 | 7.2% | |
| Did not consume biodynamic products | 131/488 | 26.8% | 2120/2327 | 92.8% | |
| Characteristics | Alternative Lifestyle Group (N = 491) | Conventional Lifestyle Group (N = 2343) | |||
|---|---|---|---|---|---|
| n/N or n | % or Mean ± S.D. | n/N or N | % or Mean ± S.D. | p-Value | |
| Candida infection in the mouth during the pregnancy | 6/490 | 1.2% | 13/2339 | 0.6% | 0.095 |
| Candida infection in the genital area during the pregnancy | 111/488 | 22.7% | 330/2340 | 14.1% | <0.001 |
| Aphthae during the pregnancy | 63/489 | 12.9% | 186/2338 | 8.0% | 0.001 |
| Lip cold sores during the pregnancy | 58/487 | 11.9% | 287/2338 | 12.3% | 0.446 |
| Common cold after third month | 315/490 | 64.3% | 1502/2339 | 64.2% | 0.510 |
| Fever after third month | 46/490 | 9.4% | 222/2338 | 9.5% | 0.510 |
| Flu after third month | 52/491 | 10.6% | 252/2337 | 10.8% | 0.488 |
| Diarrhea after third month | 97/490 | 19.8% | 551/2334 | 23.6% | 0.037 |
| Urinary tract infections after third month | 28/489 | 5.7% | 187/2338 | 8.0% | 0.048 |
| Duration of pregnancy (weeks) | 480 | 39.6 ± 1.4 | 2332 | 39.5 ± 1.5 | 0.128 |
| Birth weight (g) | 482 | 3582 ± 506 | 2330 | 3487 ± 512 | <0.001 |
| During Pregnancy AB (%) Total | During Lactation AB (%) Total | |
|---|---|---|
| Antibiotic use | ||
| Conventional group | 321 (13.8%) 2327 | 252 (15.5%) 1629 |
| Alternative group | 46 (9.4%) 488 | 44 (9.8%) 447 |
| Univariable model | OR (95%CI) | OR (95%CI) |
| Conventional group | 1.0 (reference) | 1.0 (reference) |
| Alternative group | 0.65 (0.47–0.90) | 0.60 (0.42–0.84) |
| Multivariable models | ||
| Conventional group | 1.0 (reference) | 1.0 (reference) |
| Alternative group | 0.67 (0.48–0.94) | 0.63 (0.44–0.90) |
| High education | 1.0 (reference) | 1.0 (reference) |
| Mid-level education | 1.13 (0.88–1.45) | 0.81 (0.61–1.08) |
| Low education | 1.59 (1.17–2.18) | 0.98 (0.66–1.45) |
| Home delivery | - | 1.0 (reference) |
| Natural hospital birth | - | 1.12 (0.84–1.50) |
| Instrumental hospital * | - | 1.43 (1.02–2.00) |
| No older siblings | - | 1.0 (reference) |
| One or more siblings | - | 0.91 (0.61–1.21) |
| Breastfeeding <1 month | - | 1.0 (reference) |
| Breastfeeding 1 month | - | 1.22 (0.70–2.11) |
| Breastfeeding 2 months | - | 1.00 (0.60–1.66) |
| Breastfeeding 3 months | - | 0.95 (0.60–1.49) |
| Maternal age (per year) | 1.03 (0.99–1.06) | 0.99 (0.96–1.03) |
| Age Period (Children) | ||||
|---|---|---|---|---|
| 0–3 months AB (%) total | 4–7 months AB (%) total | 8–12 months AB (%) total | 2nd year AB (%) total | |
| Antibiotic use | ||||
| Conventional group | 169 (7.8%) 2161 | 397 (18.8%) 2117 | 548 (26.3%) 2085 | 519 (25.9%) 2003 |
| Alternative group | 20 (4.2%) 473 | 49 (10.4%) 472 | 71 (15.2%) 467 | 86 (19.6%) 439 |
| Univariable model | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) |
| Conventional group | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Alternative group | 0.52 (0.32–0.84) | 0.50 (0.37–0.69) | 0.50 (0.38–0.66) | 0.70 (0.54–0.90) |
| Multivariable models | ||||
| Conventional group | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Alternative group | 0.60 (0.36–0.99) | 0.63 (0.45–0.88) | 0.59 (0.44–0.79) | 0.72 (0.54–0.95) |
| High education | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Mid-level education | 0.99 (0.70–1.40) | 0.84 (0.66–1.06) | 0.88 (0.71–1.08) | 1.05 (0.85–1.29) |
| Low education | 1.31 (0.86–2.02) | 0.90 (0.66–1.24) | 0.92 (0.69–1.23) | 1.04 (0.78–1.39) |
| Home delivery | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Natural hospital birth | 1.40 (0.99–1.97) | 1.13 (0.89–1.43) | 1.34 (1.08–1.66) | 1.16 (0.94–1.43) |
| Instrumental hospital | 1.44 (0.94–2.19) | 1.14 (0.85–1.53) | 1.62 (1.25–2.09) | 1.08 (0.83–1.41) |
| No older siblings | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| One or more siblings | 1.57 (1.11–2.23) | 1.45 (1.14–1.83) | 1.33 (1.07–1.64) | 0.90 (0.73–1.10) |
| Breastfeeding (per month) | 0.92 (0.84–1.02) | 0.92 (0.88–0.95) | 0.95 (0.93–0.98) | 0.99 (0.97–1.02) |
| Maternal age (per year) | 0.98 (0.94–1.02) | 0.98 (0.95–1.01) | 1.01 (0.98–1.04) | 1.01 (0.99–1.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eras, P.; Simões-Wüst, A.P.; Thijs, C. Influence of Alternative Lifestyles on Antibiotic Use during Pregnancy, Lactation and in Children. Antibiotics 2021, 10, 837. https://doi.org/10.3390/antibiotics10070837
Eras P, Simões-Wüst AP, Thijs C. Influence of Alternative Lifestyles on Antibiotic Use during Pregnancy, Lactation and in Children. Antibiotics. 2021; 10(7):837. https://doi.org/10.3390/antibiotics10070837
Chicago/Turabian StyleEras, Pien, Ana Paula Simões-Wüst, and Carel Thijs. 2021. "Influence of Alternative Lifestyles on Antibiotic Use during Pregnancy, Lactation and in Children" Antibiotics 10, no. 7: 837. https://doi.org/10.3390/antibiotics10070837







