Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy
Abstract
1. Introduction
2. Results
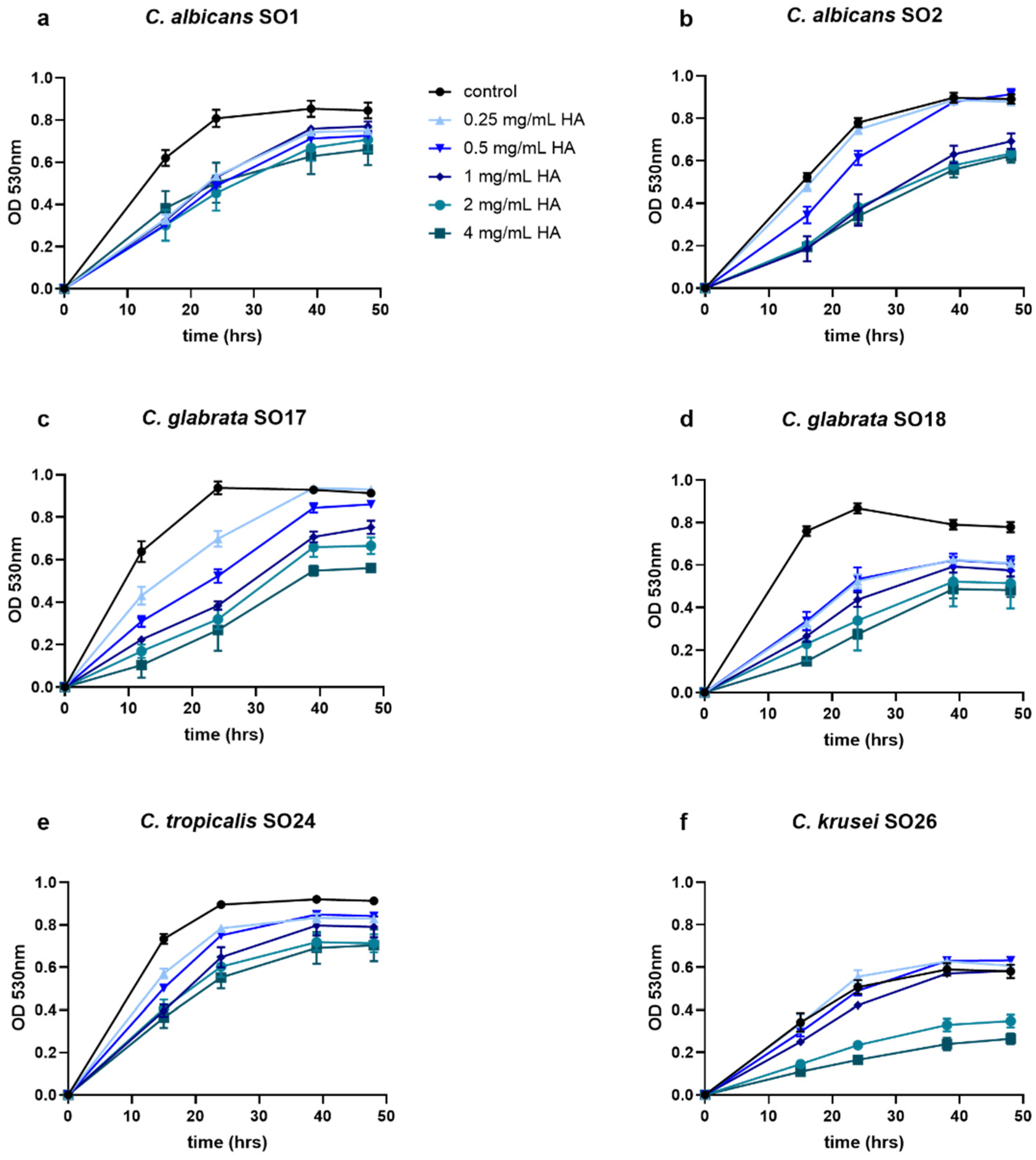
2.1. HA Reduced Candida Growth
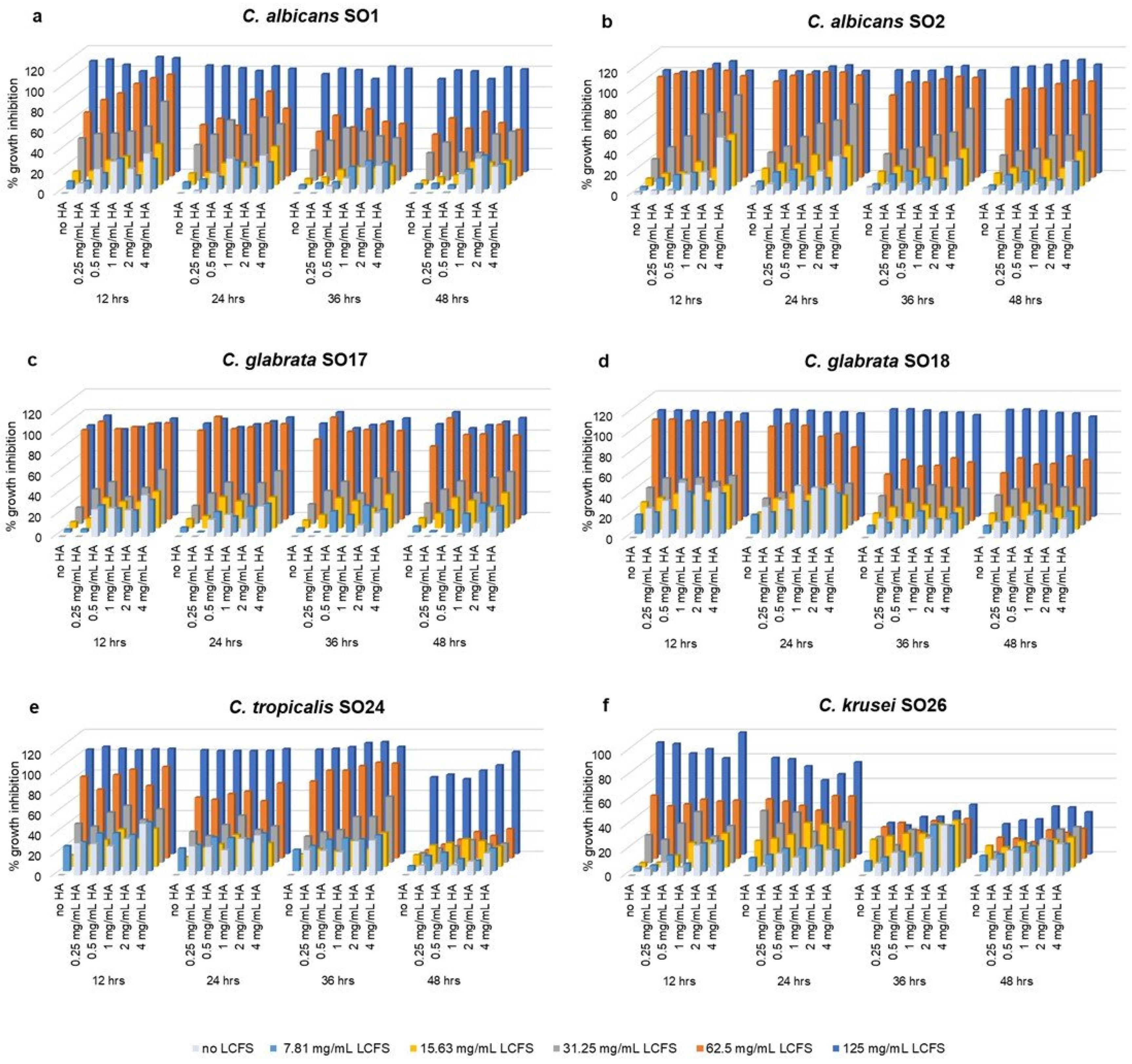
2.2. Anti-Candida Effect of Combined HA-LCFS
2.3. Characterization of Vaginal Matrices
2.4. Vaginal Matrices Reduce C. albicans Growth
3. Discussion
4. Materials and Methods
4.1. Candida Strains and Culture Conditions
4.2. Lactobacillus Cultivation and Preparation of LCFS
4.3. HA Susceptibility Assays
4.4. HA-LCFS Susceptibility Assays
- FIC (HA) = MIC of the combination/MIC of HA alone
- FIC (LCFS) = MIC of the combination/MIC of LCFS alone
- FICI values ≤ 0.5 indicated a synergic effect, while antagonism was defined as a FICI value > 4, addition was defined as a FICI value in the range 0.5–1. A FICI value between 1 and 4 was considered indifferent.
4.5. Preparation and Characterization of Vaginal Matrices
4.6. Vaginal Matrices Anti-Candida Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Coll. Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Gómez-Serranillos, M.P. Natural Products for Vulvovaginal Candidiasis Treatment: Evidence from Clinical Trials. Curr. Top. Med. Chem. 2018, 18, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Refeld, A.G.; Bogdanova, A.A.; Prazdnova, E.V.; Popov, I.V.; Kutsevalova, O.Y.; Ermakov, A.M.; Bren, A.B.; Rudoy, D.V.; Chistyakov, V.A.; et al. Mechanisms of Candida Resistance to Antimycotics and Promising Ways to Overcome It: The Role of Probiotics. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Cermelli, C.; Cuoghi, A.; Scuri, M.; Bettua, C.; Neglia, R.G.; Ardizzoni, A.; Blasi, E.; Iannitti, T.; Palmieri, B. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol. J. 2011. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Neglia, R.G.; Baschieri, M.C.; Cermelli, C.; Caratozzolo, M.; Righi, E.; Palmieri, B.; Blasi, E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J. Mater. Sci. Mater. Med. 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Foschi, C.; Frisco, G.; Calonghi, N.; Cerchiara, T.; et al. Liposomes containing biosurfactants isolated from Lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus aureus strains. Eur. J. Pharm. Biopharm. 2019, 139. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Palomino, R.A.Ñ.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of vaginal lactobacilli and characterization of anti-candida activity. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moncla, B.J.; Chappell, C.A.; Debo, B.M.; Meyn, L.A. The Effects of Hormones and Vaginal Microflora on the Glycome of the Female Genital Tract: Cervical-Vaginal Fluid. PLoS ONE 2016, 11, e0158687. [Google Scholar] [CrossRef] [PubMed]
- Stevan, M.; Fusato, E.; Armanini, D.; Bertoloni, G.; De Seta, F.; Leli, C.; Rassu, M. In vitro effects of glycyrrhetinic acid and hyaluronic acid on the growth of vulvovaginal Candida albicans and other yeasts. Microbiol. Med. 2017, 32. [Google Scholar] [CrossRef]
- Bohbot, J.-M.; de Belilovsky, C.; Brami, G.; Mares, P. Efficacy of a medical device containing liposomal hyaluronic acid against vulvo-vaginal dryness. Gynecol. Obstet. Fertil. 2015, 43, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Hefzy, E.M.; Khalil, M.A.F.; Ibrahim Amin, A.A.; Ashour, H.M.; Abdelaliem, Y.F. Bacteriocin-like inhibitory substances from probiotics as therapeutic agents for Candida vulvovaginitis. Antibiotics 2021, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Giordani, B.; Parolin, C.; De Gregorio, P.R.; Foschi, C.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Luppi, B. Lactobacillus crispatus BC1 biosurfactant delivered by hyalurosomes: An advanced strategy to counteract candida biofilm. Antibiotics 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Parolin, C.; Abruzzo, A.; Luppi, B.; Protti, M.; Mercolini, L.; Silva, J.A.; Giordani, B.; Marangoni, A.; Nader-Macías, M.E.F.; et al. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb. Cell Fact. 2020, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Abruzzo, A.; Parolin, C.; Ñahui Palomino, R.A.; Dalena, F.; Bigucci, F.; Cerchiara, T.; Luppi, B. Association of Lactobacillus crispatus with fructo-oligosaccharides and ascorbic acid in hydroxypropyl methylcellulose vaginal insert. Carbohydr. Polym. 2016, 136, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Abruzzo, A.; Musazzi, U.M.; Cilurzo, F.; Nicoletta, F.P.; Dalena, F.; Parolin, C.; Vitali, B.; Cerchiara, T.; Luppi, B.; et al. Freeze-Dried Matrices Based on Polyanion Polymers for Chlorhexidine Local Release in the Buccal and Vaginal Cavities. J. Pharm. Sci. 2019, 108. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST), EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 21 May 2021).
- Marangoni, A.; Foschi, C.; Micucci, M.; Nahui Palomino, R.A.; Toschi, T.G.; Vitali, B.; Camarda, L.; Mandrioli, M.; De Giorgio, M.; Aldini, R.; et al. In vitro activity of Spirulina platensis water extract against different Candida species isolated from vulvo-vaginal candidiasis cases. PLoS ONE 2017, 12, e0188567. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
| Candida Isolate | MIC HA (mg/mL) | MIC LCFS (mg/mL) | FICI |
|---|---|---|---|
| C. albicans SO1 | 4 | 62.5 | 1 |
| C. albicans SO2 | 4 | 62.5 | 0.75 |
| C. glabrata SO17 | 4 | 62.5 | 1.5 |
| C. glabrata SO18 | 1 | 62.5 | 1.25 |
| C. tropicalis SO24 | 4 | 62.5 | 1.1 |
| C. krusei SO26 | 4 | 125 | 2.1 |
| Without Matrix | Matrix MCC Alone | Matrix MCC + HA + LCFS | |
|---|---|---|---|
| t = 0 | (1.60 ± 0.18) × 105 | (1.60 ± 0.18) × 105 | (1.60 ± 0.18) × 105 |
| t = 6 h | (1.52 ± 0.25) × 106 | (9.92 ± 1.69) × 105 | (1.66 ± 0.30) × 105 *,# |
| t = 24 h | (3.18 ± 0.85) × 107 | (3.20 ± 0.23) × 107 | (5.92 ± 1.86) × 106 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parolin, C.; Abruzzo, A.; Giordani, B.; Oliver, J.C.; Marangoni, A.; Luppi, B.; Vitali, B. Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy. Antibiotics 2021, 10, 628. https://doi.org/10.3390/antibiotics10060628
Parolin C, Abruzzo A, Giordani B, Oliver JC, Marangoni A, Luppi B, Vitali B. Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy. Antibiotics. 2021; 10(6):628. https://doi.org/10.3390/antibiotics10060628
Chicago/Turabian StyleParolin, Carola, Angela Abruzzo, Barbara Giordani, Josidel C. Oliver, Antonella Marangoni, Barbara Luppi, and Beatrice Vitali. 2021. "Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy" Antibiotics 10, no. 6: 628. https://doi.org/10.3390/antibiotics10060628
APA StyleParolin, C., Abruzzo, A., Giordani, B., Oliver, J. C., Marangoni, A., Luppi, B., & Vitali, B. (2021). Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy. Antibiotics, 10(6), 628. https://doi.org/10.3390/antibiotics10060628