A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Design and Recruitment, Herd Profiles and Management
2.2. Farm and Dam
2.3. Calf Husbandry
2.4. Calf Nutrition
2.5. Collection of Morphometric Data
2.6. Measurement of Passive Transfer and Circulating IGF-1
2.7. Calf Health Monitoring
2.8. Data Analysis and Univariate Statistics
2.9. Multivariable Statistics
3. Results
3.1. Correlated Variables
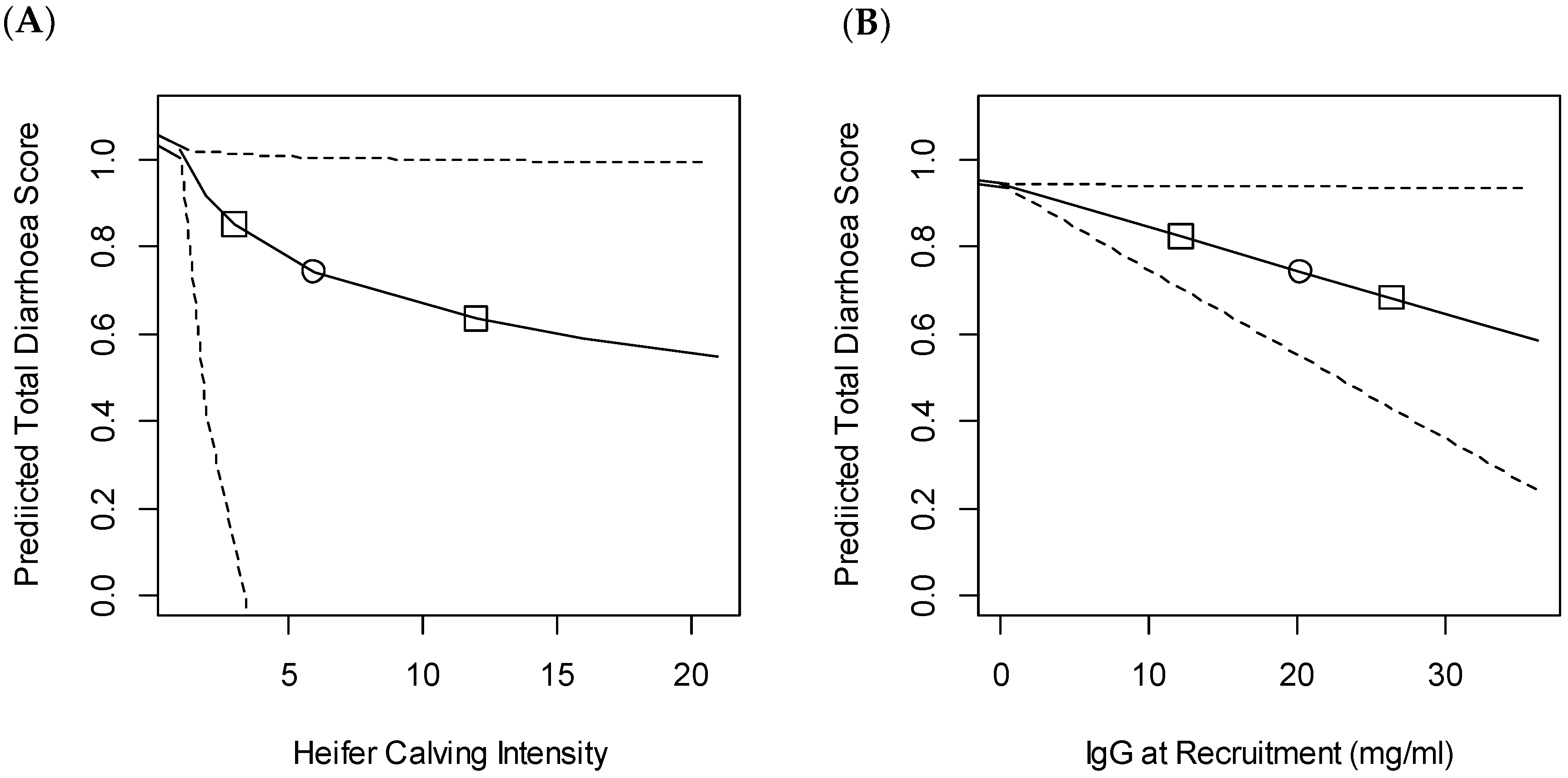
3.2. Diarrhoea
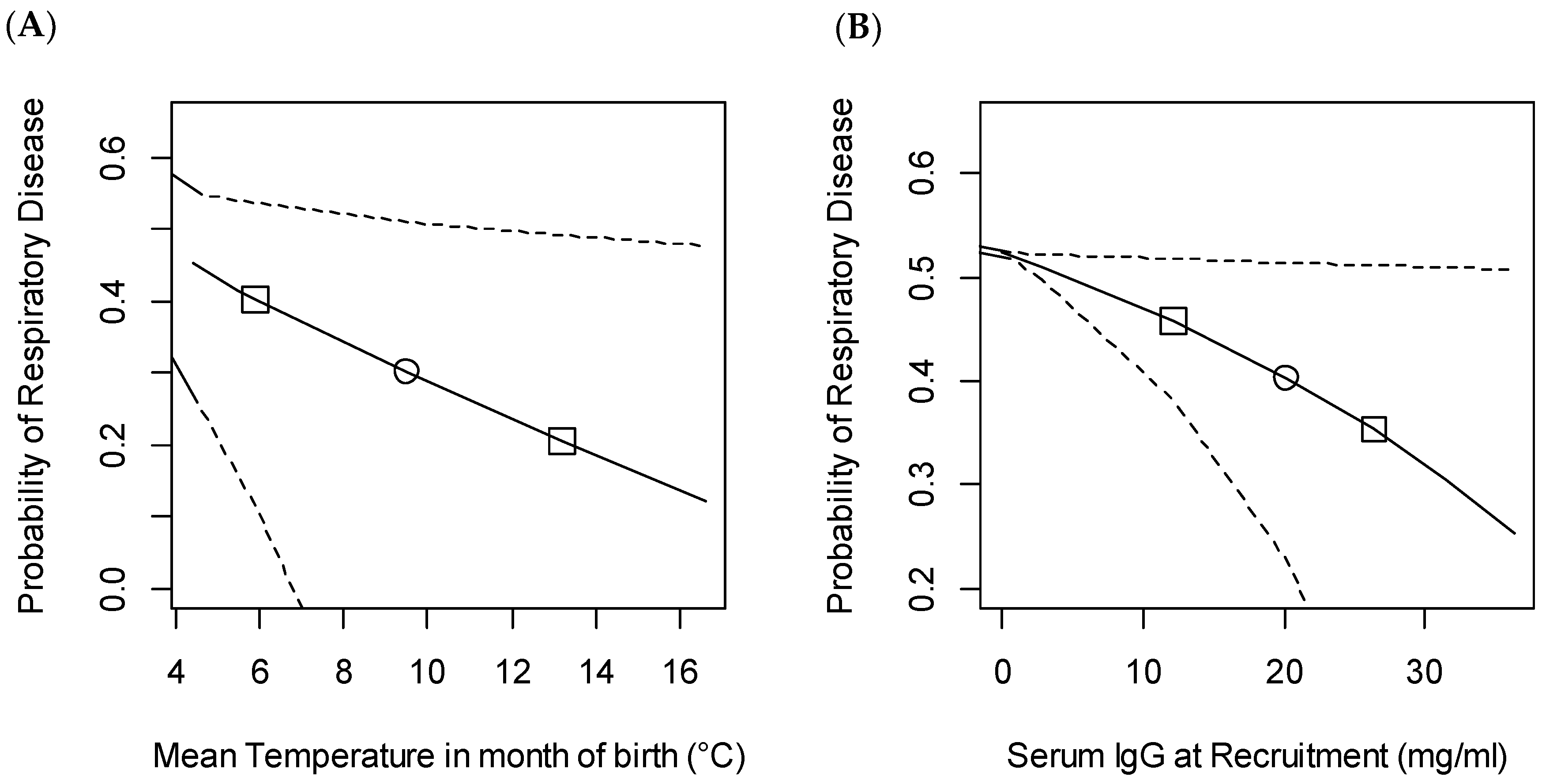
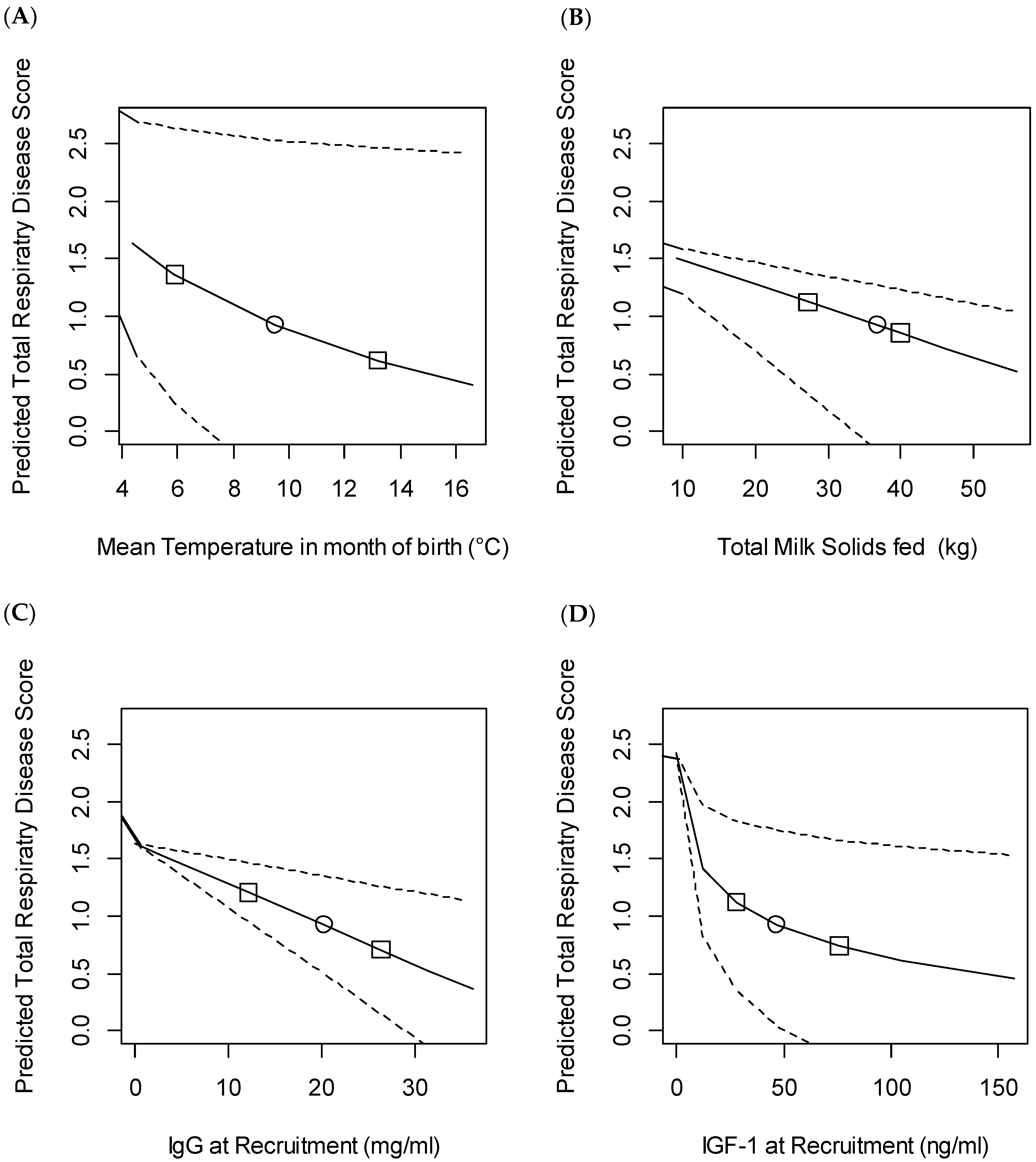
3.3. Bovine Respiratory Disease
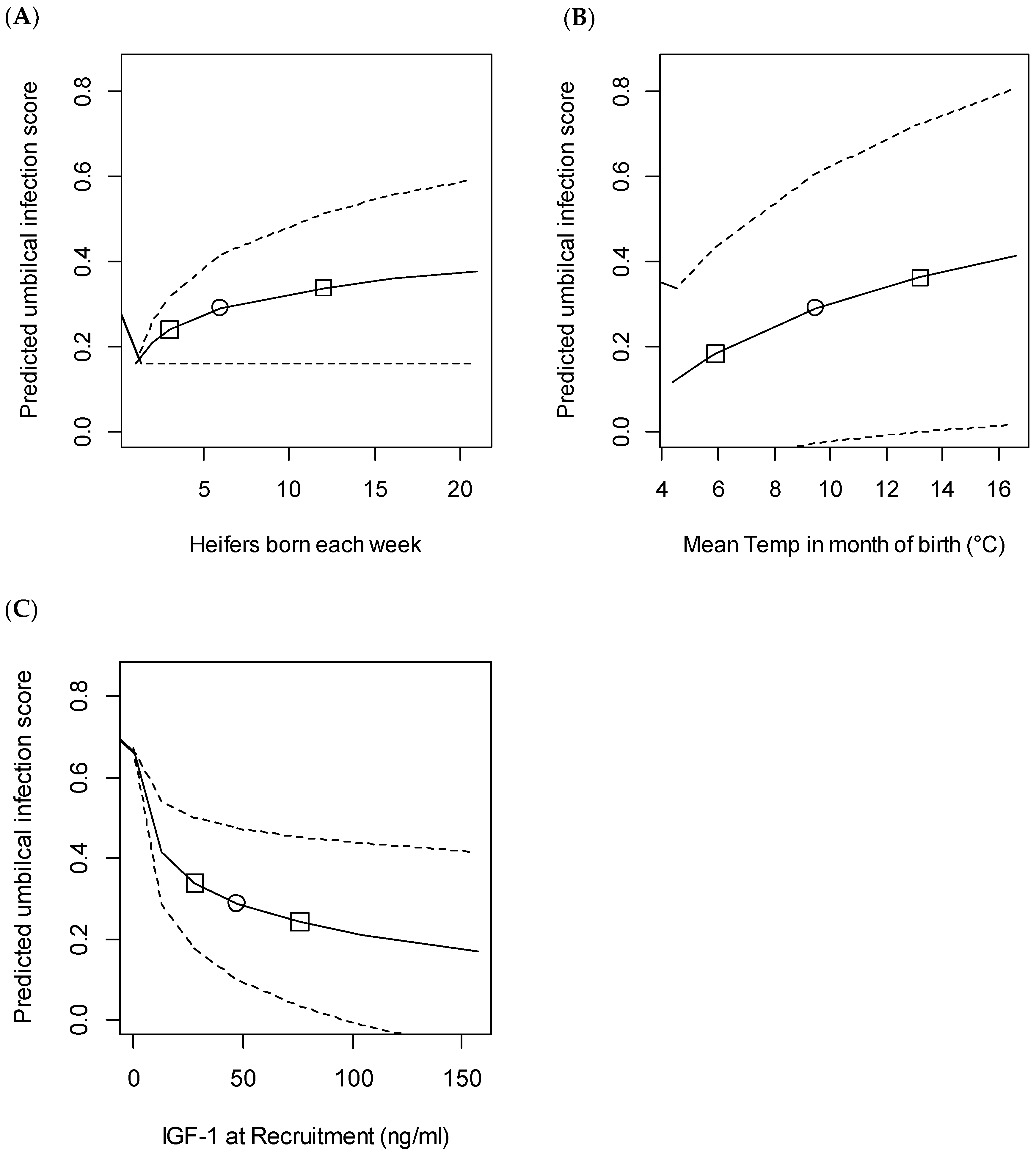
3.4. Umbilical Infection
3.5. Calf Mortality
4. Discussion
4.1. Passive Transfer
4.2. Maternal Factors and IGF-1
4.3. Milk Feeding
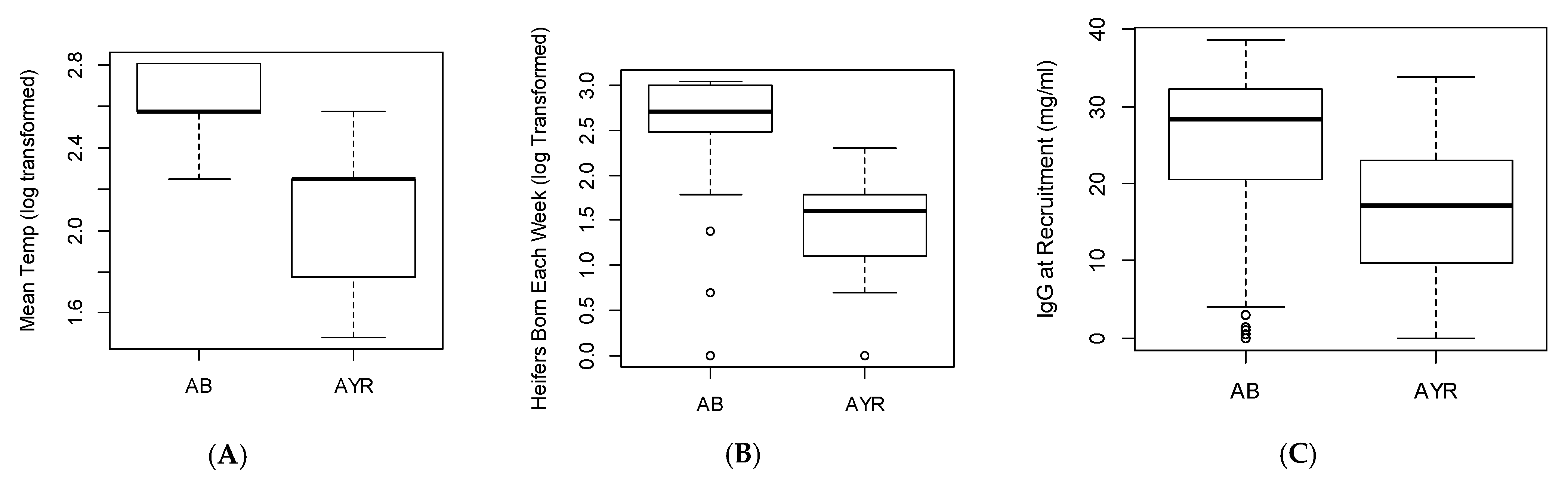
4.4. Breed
4.5. Management and Environmental Factors
4.6. Relevance to Farming Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivula, N.; Ames, T.R.; Marsh, W.E.; Werdin, R.E. Descriptive epidemiology of morbidity and mortality in Minnesota dairy heifer calves. Prev. Vet. Med. 1996, 27, 155–171. [Google Scholar] [CrossRef]
- Borderas, T.F.; de Passillé, A.M.; Rushen, J. Behavior of dairy calves after a low dose of bacterial endotoxin. J. Anim. Sci. 2008, 86, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Borderas, T.F.; Rushen, J.; von Keyserlingk, M.A.G.; de Passillé, A.M.B. Automated measurement of changes in feeding behavior of milk-fed calves associated with illness. J. Dairy Sci. 2009, 92, 4549–4554. [Google Scholar] [CrossRef] [PubMed]
- Brickell, J.S.; McGowan, M.M.; Wathes, D.C. Effect of management factors and blood metabolites during the rearing period on growth in dairy heifers on UK farms. Dom. Anim. Endocrinol. 2009, 36, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Soberon, F.; Raffrenato, E.; Everett, R.W.; van Amburgh, M.E. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.F.; Chancellor, N.; Burn, C.C.; Wathes, D.C. Prospective cohort study to assess rates of contagious disease in pre-weaned UK dairy heifers: Management practices, passive transfer of immunity and associated calf health. Vet. Rec. Open 2017, 4, e000226. [Google Scholar] [CrossRef] [Green Version]
- Svensson, C.; Lundborg, K.; Emanuelson, U.; Olsson, S.O. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev. Vet. Med. 2003, 58, 179–197. [Google Scholar] [CrossRef]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J. Dairy Sci. 2018, 101, 9229–9244. [Google Scholar] [CrossRef] [Green Version]
- Virtala, A.M.; Mechor, G.D.; Gröhn, Y.T.; Erb, H.N. Morbidity from nonrespiratory diseases and mortality in dairy heifers during the first three months of life. J. Am. Vet. Med. Assoc. 1996, 208, 2043–2046. [Google Scholar] [PubMed]
- Lombard, J.; Urie, N.; Garry, F.; Godden, S.; Quigley, J.; Earleywine, T.; McGuirk, S.; Moore, D.; Branan, M.; Chamorro, M.; et al. Consensus recommendations on calf- and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef]
- Hessman, B.E.; Fulton, R.W.; Sjeklocha, D.B.; Murphy, T.A.; Julia, F.; Payton, M.E.; Elisa, A. Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhea virus in a starter feedlot. Am. J. Vet. Res. 2009, 70, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Weary, D.; Von Keyserlingk, M. Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 2011, 94, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Terré, M.; Tejero, C.; Bach, A. Long-term effects on heifer performance of an enhanced-growth feeding programme applied during the preweaning period. J. Dairy Res. 2009, 76, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.T.; Curtis, C.R.; Erb, H.N.; White, M.E. Effect of calfhood morbidity on age at first calving in New York Holstein herds. Prev. Vet. Med. 1988, 6, 253–262. [Google Scholar] [CrossRef]
- Lorenz, I.; Earley, B.; Gilmore, J.; Hogan, I.; Kennedy, E.; More, S.J. Calf health from birth to weaning. III. Housing and management of calf pneumonia. Irish Vet. J. 2011, 64, 14. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, I.; Mee, J.F.; Earley, B.; More, S.J. Calf health from birth to weaning. I. General aspects of disease prevention. Irish Vet. J. 2011, 64, 10. [Google Scholar] [CrossRef]
- Vaarst, M.; Sørensen, J.T. Danish dairy farmers’ perceptions and attitudes related to calf-management in situations of high versus no calf mortality. Prev. Vet. Med. 2009, 89, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.C.; Rushton, J.; Wathes, D.C. A study of dairy heifer rearing practices from birth to weaning and their associated costs on UK dairy farms. Open J. Anim. Sci. 2015, 5, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.F.; Burn, C.C.; Wathes, D.C. Rates and risk factors for contagious disease and mortality in young dairy heifers. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 7th ed.; Brooks/Cole: Boston, MA, USA, 2011. [Google Scholar]
- Met Office UK. Climate Data. 2014. Available online: http://www.metoffice.gov.uk/climate/uk/summaries/2014/annual (accessed on 17 October 2015).
- Johnson, K.F.; Chancellor, N.; Burn, C.C.; Wathes, D.C. Analysis of pre-weaning feeding policies and other risk factors influencing growth rates in calves on 11 commercial dairy farms. Animal 2018, 12, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Brickell, J.S.; McGowan, M.M.; Pfeiffer, D.U.; Wathes, D.C. Mortality in Holstein-Friesian calves and replacement heifers, in relation to body weight and IGF-I concentration, on 19 farms in England. Animal 2009, 3, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuirk, S. Disease management of dairy calves and heifers. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Cortese, V.S.; Kirkpatrick, M.A.; Short, T.H.; Voortman, B. Effect of serum total protein concentration on early-life health and growth of dairy calves. J. Am. Vet. Med. Assoc. 2020, 257, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Garry, F.B.; Tomlinson, S.M.; Garber, L.P. Impacts of dystocia on health and survival of dairy calves. J. Dairy Sci. 2007, 90, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Van Donkersgoed, J.; Ribble, C.S.; Boyer, L.G.; Townsend, H.G. Epidemiological study of enzootic pneumonia in dairy calves in Saskatchewan. Can. J. Vet. Res. 1993, 57, 247–254. [Google Scholar] [PubMed]
- Trotz-Williams, L.A.; Martin, S.W.; Leslie, K.E.; Duffield, T.; Nydam, D.V.; Peregrine, A.S. Association between management practices and within-herd prevalence of Cryptosporidium parvum shedding on dairy farms in southern Ontario. Prev. Vet. Med. 2008, 83, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, J.A.; Grove-White, D.H.; Royal, M.D.; Smith, R.F. Identification and quantification of factors affecting neonatal immunological transfer in dairy calves in the UK. Vet. Rec. 2015, 176, 625. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.L.; Lombard, J.E.; Kopral, C.A.; Garber, L.P.; Winter, A.L.; Hicks, J.A.; Schlater, J.L. Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations. J. Dairy Sci. 2009, 92, 3973–3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, G.C.; Argo, C.M.; Jones, D.; Grove-White, D.H. Impact of feeding and housing systems on disease incidence in dairy calves. Vet. Rec. 2016, 179, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivula, N.; Ames, T.R.; Marsh, W.E. Management practices and risk factors for morbidity and mortality in Minnesota dairy heifer calves. Prev. Vet. Med. 1996, 27, 173–182. [Google Scholar] [CrossRef]
- Windeyer, M.C.; Leslie, K.E.; Godden, S.M.; Hodgins, D.C.; Lissemore, K.D.; Leblanc, S.J. Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age. Prev. Vet. Med. 2014, 113, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.A.; Dohoo, I.R.; Montgomery, D.M.; Bennett, F.L. Associations between passive immunity and morbidity and mortality in dairy heifers in Florida, USA. Prev. Vet. Med. 1998, 34, 31–46. [Google Scholar] [CrossRef]
- Virtala, A.M.; Gröhn, Y.T.; Mechor, G.D.; Erb, H.N. The effect of maternally derived immunoglobulin G on the risk of respiratory disease in heifers during the first 3 months of life. Prev. Vet. Med. 1999, 39, 25–37. [Google Scholar] [CrossRef]
- Gunn, G.J.; Stott, A.W.; Humphry, R.W. Modelling and costing BVD outbreaks in beef herds. Vet. J. 2004, 167, 143–149. [Google Scholar] [CrossRef]
- Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and Mannheimia haemolytica challenge on animal performance, nitrogen balance, and visceral organ mass in beef steers. J. Anim. Sci. 2010, 88, 2179–2188. [Google Scholar] [CrossRef]
- Virtala, A.M.; Mechor, G.D.; Gröhn, Y.T.; Erb, H.N. The effect of calfhood diseases on growth of female dairy calves during the first 3 months of life in New York State. J. Dairy Sci. 1996, 79, 1040–1049. [Google Scholar] [CrossRef]
- Trotz-Williams, L.A.; Wayne Martin, S.; Leslie, K.E.; Duffield, T.; Nydam, D.V.; Peregrine, A.S. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007, 82, 12–28. [Google Scholar] [CrossRef]
- Svensson, C.; Jensen, M.B. Short communication: Identification of diseased calves by use of data from automatic milk feeders. J. Dairy Sci. 2007, 90, 994–997. [Google Scholar] [CrossRef]
- Langel, S.N.; Wark, W.A.; Garst, S.N.; James, R.E.; McGilliard, M.L.; Petersson-Wolfe, C.S.; Kanevsky-Mullarky, I. Effect of feeding whole compared with cell-free colostrum on calf immune status: The neonatal period. J. Dairy Sci. 2015, 98, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.; McGuirk, S.M.; Bennett, T.B.; Cook, N.B.; Nordlund, K.V. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. J. Dairy Sci. 2006, 89, 4014–4025. [Google Scholar] [CrossRef] [Green Version]
- Mee, J.F. Newborn dairy calf management. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Bauer, M.K.; Breier, B.H.; Harding, J.E.; Veldhuis, J.D.; Gluckman, P.D. The fetal somatotropic axis during long term maternal undernutrition in sheep: Evidence for nutritional regulation in utero. Endocrinology 1995, 136, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Osgerby, J.C.; Wathes, D.C.; Howard, D.; Gadd, T.S. The effect of maternal undernutrition on ovine fetal growth. J. Endocrinol. 2002, 173, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowden, A.L. Endocrine regulation of fetal growth. Reprod. Fert. Dev. 1995, 7, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Fowden, A.L. Growth and metabolism. In Fetal Growth and Development; Harding, R., Bocking, A.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 44–69. [Google Scholar]
- Smith, J.M.; Van Amburgh, M.E.; Díaz, M.C.; Lucy, M.C.; Bauman, D.E. Effect of nutrient intake on the development of the somatotropic axis and its responsiveness to GH in Holstein bull calves. J. Anim. Sci. 2002, 80, 1528–1537. [Google Scholar] [CrossRef]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Ramenghi, L.A.; Löfqvist, C.; Smith, L.E.; Hård, A.L. Role of insulin like growth factor 1 in fetal development and in the early postnatal life of premature infants. Am. J. Perinatol. 2016, 33, 1067–1071. [Google Scholar] [CrossRef] [Green Version]
- Oherle, K.; Acker, E.; Bonfield, M.; Wang, T.; Gray, J.; Lang, I.; Bridges, J.; Lewkowich, I.; Xu, Y.; Ahlfeld, S.; et al. Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs. Immunity 2020, 52, 275–294.e9. [Google Scholar] [CrossRef]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Kim, S.B.; Ki, K.S.; Choi, Y.J. Pre- and postweaning performance of Holstein female calves fed milk through step-down and conventional methods. J. Dairy Sci. 2007, 90, 876–885. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7 (Suppl. 1), 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular metabolism turns immune regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walls, J.; Sinclair, L.; Finlay, D. Nutrient sensing, signal transduction and immune responses. Semin. Immunol. 2016, 28, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Ollivett, T.L.; Nydam, D.V.; Linden, T.C.; Bowman, D.D.; Van Amburgh, M.E. Effect of nutritional plane on health and performance in dairy calves after experimental infection with Cryptosporidium parvum. J. Am. Vet. Med. Assoc. 2012, 241, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, L.E.; Cobb, C.J.; Carroll, J.A.; Ballou, M.A. The effects of early weaning on innate immune responses of Holstein calves. J. Dairy Sci. 2011, 94, 2545–2556. [Google Scholar] [CrossRef]
- Johnson, R.W. The concept of sickness behavior: A brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 2002, 87, 443–450. [Google Scholar] [CrossRef]
- Kelley, K.W.; Bluthé, R.-M.; Dantzer, R.; Zhou, J.-H.; Shen, W.-H.; Johnson, R.W.; Broussard, S.R. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003, 17 (Suppl. 1), S112–S118. [Google Scholar] [CrossRef]
- Lundborg, G.K.; Svensson, E.C.; Oltenacu, P.A. Herd-level risk factors for infectious diseases in Swedish dairy calves aged 0-90 days. Prev. Vet. Med. 2005, 68, 123–143. [Google Scholar] [CrossRef]
- Gulliksen, S.M.; Jor, E.; Lie, K.I.; Hamnes, I.S.; Løken, T.; Akerstedt, J.; Osterås, O. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J. Dairy Sci. 2009, 92, 5057–5066. [Google Scholar] [CrossRef]
- de Graaf, D.C.; Vanopdenbosch, E.; Ortega-Mora, L.M.; Abbassi, H.; Peeters, J.E. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 1999, 29, 1269–1287. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Foote, M.R.; Miller, B.L.; Fowler, M.; Johnson, T.E.; Horst, R.L. Effects of chronic environmental cold on growth, health, and select metabolic and immunologic responses of preruminant calves. J. Dairy Sci. 2009, 92, 6134–6143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRC. Nutrient Requirements of Dairy Cattle, 3rd ed.; National Academy of Science: Washington, DC, USA, 2001. [Google Scholar]
- Hepola, H. Milk feeding systems for dairy calves in groups: Effects on feed intake, growth and health. Appl. Anim. Behav. Sci. 2003, 80, 233–243. [Google Scholar] [CrossRef]
- Perez, E.; Noordhuizen, J.; Vanwuijkhuise, L.; Stassen, E. Management factors related to calf morbidity and mortality rates. Livest. Prod. Sci. 1990, 25, 79–93. [Google Scholar] [CrossRef]
- Bartels, C.J.M.; Holzhauer, M.; Jorritsma, R.; Swart, W.A.J.M.; Lam, T.J.G.M. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 2010, 93, 162–169. [Google Scholar] [CrossRef]
- Bach, A. Ruminant Nutrition Symposium: Optimizing performance of the offspring: Nourishing and managing the dam and postnatal calf for optimal lactation, reproduction, and immunity. J. Anim. Sci. 2012, 90, 1835–1845. [Google Scholar] [CrossRef] [Green Version]
- Stanton, A.L.; Kelton, D.F.; LeBlanc, S.J.; Wormuth, J.; Leslie, K.E. The effect of respiratory disease and a preventative antibiotic treatment on growth, survival, age at first calving, and milk production of dairy heifers. J. Dairy Sci. 2012, 95, 4950–4960. [Google Scholar] [CrossRef] [Green Version]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 2014, 8 (Suppl. 1), 91–104. [Google Scholar] [CrossRef] [Green Version]
| Variable | Estimate | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 0.68 | 0.42 to 1.1 | 0.143 |
| Breed—Holstein | (ref) | ||
| Breed—Ayrshire | 0.45 | 0.11 to1.91 | 0.317 |
| Breed—Friesian | 0.60 | 0.26 to 1.38 | 0.317 |
| Breed—Friesian × Jersey | 0.93 | 0.26 to 3.29 | 0.913 |
| Breed—Friesian × Other | 0.50 | 0.05 to 5.14 | 0.558 |
| Breed—Jersey | 0.21 | 0.06 to 0.81 | 0.026 |
| Breed—Jersey × Other | 0.85 | 0.22 to 3.32 | 0.819 |
| Breed—Scandinavian Red× | 1.63 | 0.82 to 3.26 | 0.215 |
| Calving Pattern—Autumn Block | (ref) | ||
| Calving Pattern—All Year Round | 1.73 | 1.01 to 2.96 | 0.049 |
| Variable | Estimate | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 1.22 | 0.88 to 1.56 | <0.01 |
| Heifer calving intensity (log transformed) ‡ | −0.16 | −0.30 to 0.009 | 0.039 |
| IgG in the first week of life (mg/mL) | −0.01 | −0.019 to 0 | 0.064 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 10.79 | 2.25 to 51.77 | 0.003 |
| Breed—Holstein | (ref) | ||
| Breed—Other | 0.63 | 0.33 to 1.1 | 0.097 |
| Group management—continuous flow | (ref) | ||
| Group management—fixed group | 0.42 | 0.17 to 0.96 | 0.040 |
| Mean temperature in month of birth (°C, log transformed) | 0.54 | 0.29 to 0.97 | 0.042 |
| IgG at recruitment (mg/mL) | 0.98 | 0.95 to 1 | 0.030 |
| Variable | Estimate | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 6.02 | 4.17 to 7.75 | <0.01 |
| Group management—continuous flow | (ref) | ||
| Group management—fixed group | −1.14 | −2.07 to −0.21 | <0.01 |
| Mean temperature in month of birth (°C, log transformed) | −0.93 | −1.56 to −0.22 | <0.01 |
| Total milk solids fed preweaning (kg) | −0.031 | −0.051 to −0.012 | 0.002 |
| IgG at recruitment (mg/mL) | −0.025 | −0.056 to −0.014 | 0.001 |
| IGF-1 at recruitment (ng/mL, log transformed) | −0.389 | −0.617 to −0.176 | 0.001 |
| Variable | Estimate | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 0.044 | 0.03 to 0.38 | 0.008 |
| Heifer calving intensity (log transformed) | 0.071 | 0 to 0.14 | 0.049 |
| Mean temperature in month of birth (°C, log transformed) | 0.222 | 0.08 to 0.36 | 0.002 |
| IGF-1 at recruitment (ng/mL, log transformed) | −0.099 | −0.15 to −0.05 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.F.; Chancellor, N.; Wathes, D.C. A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves. Animals 2021, 11, 378. https://doi.org/10.3390/ani11020378
Johnson KF, Chancellor N, Wathes DC. A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves. Animals. 2021; 11(2):378. https://doi.org/10.3390/ani11020378
Chicago/Turabian StyleJohnson, Kate F., Natalie Chancellor, and D. Claire Wathes. 2021. "A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves" Animals 11, no. 2: 378. https://doi.org/10.3390/ani11020378
APA StyleJohnson, K. F., Chancellor, N., & Wathes, D. C. (2021). A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves. Animals, 11(2), 378. https://doi.org/10.3390/ani11020378






