Middle East Medicinal Plants in the Treatment of Diabetes: A Review
Abstract
1. Introduction
2. Medicinal Plants with Potential Antidiabetic Activity in the Arabian Peninsula
2.1. Oman
2.1.1. Ajuga iva (Lamiaceae)
2.1.2. Moringa pergrina (Moringaceae)
2.1.3. Rhazya stricta (Apocynaceae)
2.2. Qatar
Cynomorium coccineum (Cynomoriaceae)
2.3. Saudi Arabia
2.3.1. Avicennia marina (Avicenniaceae)
2.3.2. Caralluma sinaica (Asclepiadaceae)
2.3.3. Ducrosia anethifolia (Apiaceae)
2.3.4. Jatropha curcas (Euphorbiaceae)
2.3.5. Loranthus acaciae (Loranthaceae)
2.3.6. Lyceum shawii (Solonaceae)
2.3.7. Marrubium vulgare (Lamiaceae)
2.3.8. Moringa oleifera (Moringaceae)
2.3.9. Morus nigra (Moraceae)
2.3.10. Ocimum forskolei (Lamiaceae)
2.3.11. Plicosepalus curviflorus (Loranthaceae)
2.3.12. Retama raetam (Fabaceae)
2.3.13. Rhizophora mucronata (Rhizosphoraceae)
2.3.14. Salvadora persica (Salvadoraceae)
2.4. Yemen
2.4.1. Azadirachta indica (Meliaceae)
2.4.2. Boswellia carterii (Burseraceae)
2.4.3. Cissus rotundifolia (Vitaceae)
2.4.4. Dracaena cinnabari (Dracaenaceae)
2.4.5. Opuntia ficus-indica (Cactaceae)
2.4.6. Pulicaria inuloides (Asteraceae)
2.4.7. Solenostemma argel (Asclepiadaceae)
3. Medicinal Plants with Potential Antidiabetic Activity in Egypt
3.1. Cassia acutifolia (Fabaceae)
3.2. Centaurea alexanderina (Asteraceae)
3.3. Cyperus laevigatus (Cyperaceae)
3.4. Fraxinus ornus (Oleaceae)
3.5. Phoneix dactylifera (Arecaceae)
3.6. Nepeta cataria (Lamiaceae)
3.7. Securigera securidaca (Fabaceae)
3.8. Trigonella stellate (Fabaceae)
3.9. Urtica pilulifera (Urticaceae)
3.10. Zizyphus spina-christi (Rhamnaceae)
4. Medicinal Plants with Potential Antidiabetic Activity in Iran
4.1. Allium ampeloprasum (Liliaceae)
4.2. Allium ascalonicum (Liliaceae)
4.3. Allium sativum (Liliaceae)
4.4. Amygdalus lycioides (Rosaceae)
4.5. Amygdalus scoparia (Rosaceae)
4.6. Arctium lappa (Asteraceae)
4.7. Berberis integerrima (Berberidaceae)
4.8. Brassica napus (Brassicaceae)
4.9. Brassica rapa (Brassicaceae)
4.10. Capparis spinosa (Capparaceae)
4.11. Centaurea bruguierana (Asteraceae)
4.12. Cichorium intybus (Asteraceae)
4.13. Citrullus colocynthis (Cucurbitaceae)
4.14. Cornus mas (Cornaceae)
4.15. Cucumis sativus (Cucurbitaceae)
4.16. Cucurbita pepo (Cucurbitaceae)
4.17. Eryngium caucasicum (Apiaceae)
4.18. Eucalyptus globulus (Myrtaceae)
4.19. Falcaria vulgaris (Apiaceae)
4.20. Ferula assafoetida (Apiaceae)
4.21. Galega officinalis (Fabaceae)
4.22. Gundelia tournefortii (Asteraceae)
4.23. Hordeum vulgare (Poaceae)
4.24. Juglans regia (Juglandaceae)
4.25. Mentha spicata (Lamiaceae)
4.26. Nasturtium officinale (Brassicaceae)
4.27. Otostegia persica (Lamiaceae)
4.28. Pyrus boissieriana (Rosaceae)
4.29. Phoenix dactylifera (Arecaceae)
4.30. Punica granatum (Punicaceae)
4.31. Rhus coriaria (Anacardiaceae)
4.32. Rheum turkestanicum (Polygonaceae)
4.33. Salvia hypoleuca (Lamiaceae)
4.34. Salvia officinalis (Lamiaceae)
4.35. Securigera securidaca (Fabaceae)
4.36. Solanum nigrum (Solanaceae)
4.37. Teucrium polium (Lamiaceae)
4.38. Trigonella foenum-graecum (Fabaceae)
4.39. Vaccinium arctostaphylos (Ericaceae)
4.40. Vitex agnus-castus (Lamiaceae)
4.41. Urtica dioica (Utricaceae)
4.42. Zataria multiflora (Lamiaceae)
4.43. Ziziphus vulgaris (Rhamnaceae)
5. Medicinal Plants with Potential Antidiabetic Activity in Iraq
5.1. Bauhinia variegate (Caesalpiniaceae)
5.2. Momordica charantia (Cucurbitaceae)
5.3. Rheum ribes (Polygonaceae)
6. Medicinal Plants with Potential Antidiabetic Activity in Jordan
6.1. Achillea santolina (Asteraceae)
6.2. Artemisia herba alba (Asteraceae)
6.3. Artemisia sieberi (Asteracea)
6.4. Arum dioscoridis and palaestinum (Araceae)
6.5. Crataegus aronia (Rosaceae)
6.6. Cichorium pumilum (Asteraceae)
6.7. Eryngium creticum (Apiaceae)
6.8. Geranium graveolens (Geraniaceae)
6.9. Phaseolus vulgaris (Fabaceae)
6.10. Pistacia atlantica (Anacardiaceae)
6.11. Tecoma stans (Bignoniaceae)
6.12. Teucrium polium (Lamiaceae)
6.13. Varthemia iphionoides (Asteraceae)
7. Medicinal Plants with Potential Antidiabetic Activity in Lebanon
7.1. Centaurea horrida (Asteraceae)
7.2. Hordeum spontaneum (Poaceae)
7.3. Inula viscosa and Inula vulgaris (Asteraceae)
7.4. Psoralea bituminosa (Fabaceae)
7.5. Salvia libanotica (Lamiaceae)
8. Medicinal Plants with Potential Antidiabetic Activity in Palestine
8.1. Atriplex halimus (Chenopodiaceae)
8.2. Ocimum basilicum (Lamiaceae)
8.3. Sarcopoterium spinosum (Rosaceae)
8.4. Trigonella foenum-graecum (Fabaceae)
8.5. Withania somnifera (Solanaceae)
9. Medicinal Plants with Potential Antidiabetic Activity in Turkey
9.1. Cistus laurifolius (Cistaceae)
9.2. Juniperus oxycedrus (Cupressaceae)
9.3. Heracleum persicum (Apiaceae)
9.4. Juniperus foetidissima and Juniperus sabina (Cupressaceae)
9.5. Origanum minutiflorum (Labmiaceae)
9.6. Salvia triloba (Lamiaceae)
9.7. Thymus praecox (Lamiaceae)
10. Middle East Statistics
11. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mannan, A.; Rupa, B.A.; Azam, N.K.; Ahmed, N.; Hasan, N. A quick review on anti-diabetic plants and action of phytochemicals. Int. J. Adv. Res. 2014, 2, 227. [Google Scholar]
- Sathasivampillai, S.V.; Rajamanoharan, P.R.; Munday, M.; Heinrich, M. Plants used to treat diabetes in Sri Lankan Siddha Medicine–An ethnopharmacological review of historical and modern sources. J. Ethnopharmacol. 2017, 198, 531–599. [Google Scholar] [CrossRef] [PubMed]
- Abuyassin, B.; Laher, I. Diabetes epidemic sweeping the Arab world. World J. Diabetes 2016, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Gupta, M.; Popli, H.; Aggarwal, G. Diabetes mellitus treatment using herbal drugs. Int. J. Phytomedicine 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herb. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Aati, H.; El-Gamal, A.; Shaheen, H.; Kayser, O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomedicine 2019, 15, 2. [Google Scholar] [CrossRef]
- Alwin Robert, A.; Al Dawish, M.A. Microvascular complications among patients with diabetes: An emerging health problem in Saudi Arabia. Diabetes Vasc. Dis. Res. 2019, 16, 227–235. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Bener, A.; Zirie, M.; Janahi, I.M.; Al-Hamaq, A.O.; Musallam, M.; Wareham, N.J. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res. Clin. Pract. 2009, 84, 99–106. [Google Scholar] [CrossRef]
- Yaser, A.J.; Muneer, A.; Abdelhafid, B.; Dauodi, C.; Hammadi, L. Chemical and phytochemical analysis of some antidiabetic plants in Yemen. Int. J. Res. Pharm. 2013, 4, 72–76. [Google Scholar] [CrossRef]
- El Hilaly, J.; Lyoussi, B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and Streptozocin diabetic rats. J. Ethnopharmacol. 2002, 80, 109–113. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Tahraoui, A.; Israili, Z.H.; Lyoussi, B. Acute hypoglycemic, hypocholesterolemic and hypotriglyceridemic effects of continuous intravenous infusion of a lyophilised aqueous extract of Ajuga iva L. Schreber whole plant in Streptozocin-induced diabetic rats. Pak. J. Pharm. Sci. 2007, 20, 261–268. [Google Scholar] [PubMed]
- Boudjelal, A.; Siracusa, L.; Henchiri, C.; Sarri, M.; Abderrahim, B.; Baali, F.; Ruberto, G. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med. 2015, 81, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Jin, H.; Zheng, S.-L.; Xia, P.; Cai, Y.; Ni, X.-J. Phytoecdysteroids from Ajuga iva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed. Pharmacother. 2017, 96, 480–488. [Google Scholar] [CrossRef]
- Hamden, K.; Ayadi, F.; Jamoussi, K.; Masmoudi, H.; Elfeki, A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors 2008, 33, 165–175. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori.—A Review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef]
- El-Alfy, T.S.; Ezzat, S.M.; Hegazy, A.K.; Amer, A.M.; Kamel, G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori.(family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011, 7, 109. [Google Scholar]
- Koheil, M.A.; Hussein, M.A.; Othman, S.M.; El-Haddad, A. In-vivo antioxidant activity of Moringa peregrina against STZ–induced oxidative stress in type 2 diabetic rats. Mol. Clin. Pharm. 2013, 4, 65–75. [Google Scholar]
- Ullah, M.F.; Bhat, S.H.; Abuduhier, F.M. Antidiabetic Potential of Hydro-Alcoholic Extract of M oringa Peregrina Leaves: Implication as Functional Food for Prophylactic Intervention in Prediabetic Stage. J. Food Biochem. 2015, 39, 360–367. [Google Scholar] [CrossRef]
- Reddy, S.H.; Al-Neeri, I.S.; Al-Issaei, H.K.; Al-Jabri, S.M. Effect of selective medicinal plant extract on blood glucose, sperm shape and various physiological parameters. Am. J. Plant Sci. 2015, 6, 1109. [Google Scholar] [CrossRef]
- Al Maharooqi, A.; Al Hilali, M.; Al Hinai, Z.; Unnikrishnan, D. A Review on Medicinal Plant Decne Rhazya Stricta. Adv. Pharm. J. 2016, 1, 119–125. [Google Scholar]
- Baeshen, M.; Khan, R.; Bora, R.; Baeshen, N. Therapeutic potential of the folkloric medicinal plant Rhazya stricta. Biol. Syst. Open Access 2015, 5, 1000151. [Google Scholar] [CrossRef]
- Ahmed, A.; Asad, M.J.; Ahmad, M.S.; Qureshi, R.; Shah, S.I.; Gul, H.; Gulfraz, M. Antidiabetic and hypolipidemic potential of Rhazya stricta Decne extract and its fractions. Int. Curr. Pharm. J. 2015, 4, 353–361. [Google Scholar] [CrossRef]
- Reddy, S.H.; Al-Hinai, A.K.; AL-Yaqoobi, H.H.; Al-Ajmi, F.J. Phytochemical analysis, antimicrobial screening and hypoglycemic effect of some selected medicinal plant extract from Oman. J. Exp. Biol. 2016, 4, 2. [Google Scholar]
- Hao-Cong, M.; Shuo, W.; Ying, L.; KUANG, Y.-Y.; Chao-Mei, M. Chemical constituents and pharmacologic actions of Cynomorium plants. Chin. J. Nat. Med. 2013, 11, 321–329. [Google Scholar]
- Phoboo, S.; Shetty, K.; ElObeid, T. In Vitro assays of anti-diabetic and anti-hypertensive potential of some traditional edible plants of qatar. J. Med. Act. Plants 2015, 4, 22–29. [Google Scholar]
- Das, S.K.; Samantaray, D.; Patra, J.K.; Samanta, L.; Thatoi, H. Antidiabetic potential of mangrove plants: A review. Front. Life Sci. 2016, 9, 75–88. [Google Scholar] [CrossRef]
- Mahera, S.; Saifullah, S.; Ahmad, V.; Mohammad, F. Phytochemical studies on mangrove Avicennia marina. Pak. J. Bot. 2013, 45, 2093–2094. [Google Scholar]
- Okla, M.K.; Alamri, S.A.; Alatar, A.A.; Hegazy, A.K.; Al-Ghamdi, A.A.; Ajarem, J.S.; Faisal, M.; Abdel-Salam, E.M.; Ali, H.M.; Salem, M.Z. Antioxidant, Hypoglycemic, and Neurobehavioral Effects of a Leaf Extract of Avicennia marina on Autoimmune Diabetic Mice. Evid. Based Complementary Altern. Med. 2019, 2019, 1750368. [Google Scholar] [CrossRef]
- Zeid, I.E.M.E.A.; Al-Jaghthmi, O.H.A.; Heba, H.M. Augmentation of Insulin Secretion Induced by Rhizophora Mucronata and Avicennia Marina Extracts in Streptozocin-Induced Diabetic Rats. Int. J. Pharm. Res. Allied Sci. 2019, 8, 14–22. [Google Scholar]
- Hamzevi, A.; Sadoughi, S.D.; Rahbarian, R. The effect of aqueous extract of Avicennia marina (Forsk.) Vierh. leaves on liver enzymes’ activity, oxidative stress parameters and liver histopathology in male diabetic rat. Feyz J. Kashan Univ. Med. Sci. 2017, 21, 305–316. [Google Scholar]
- Habibuddin, M.; Daghriri, H.A.; Humaira, T.; Al Qahtani, M.S.; Hefzi, A.A.H. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on Streptozocin-induced diabetic rabbits. J. Ethnopharmacol. 2008, 117, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, N.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. Biomed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.N.; Patil, R.Y.; Ahirwar, B.; Ahirwar, D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 20–23. [Google Scholar] [CrossRef]
- Aladodo, R. Effects of aqueous root extract of Jatropha curcas on hyperglycaemic and haematological indices in alloxan-induced diabetic rats. Fountain J. Nat. Appl. Sci. 2013, 2, 52–58. [Google Scholar]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.I.; Saad, S.A. Bioactive flavonoid glycosides and antidiabetic activity of Jatropha curcas on Streptozocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 143–156. [Google Scholar]
- Noman, O.M.; Mothana, R.A.; Al-Rehaily, A.J.; Nasr, F.A.; Khaled, J.M.; Alajmi, M.F.; Al-Said, M.S. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. Grown in Saudi Arabia. Saudi Pharm. J. 2019, 24, 724–730. [Google Scholar]
- Sher, H.; Alyemeni, M.N. Evaluation of anti-diabetic activity and toxic potential of Lycium shawii in animal models. J. Med. Plants Res. 2011, 5, 3387–3395. [Google Scholar]
- Elberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel-Sattar, E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in Streptozocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015, 3, 37–44. [Google Scholar] [CrossRef]
- Divi, S.M.; Bellamkonda, R.; Dasireddy, S.K. Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose fed insulin resistant and STZ induced diabetic wistar rats: A comparative study. Asian J. Pharm. Clin. Res. 2012, 5, 67–72. [Google Scholar]
- Al-Malki, A.L.; El Rabey, H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on Streptozocin induced diabetes and diabetic nephropathy in male rats. Biomed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- ABD EL-MAWLA, A.; Mohamed, K.M.; Mostafa, A.M. Induction of biologically active flavonoids in cell cultures of Morus nigra and testing their hypoglycemic efficacy. Sci. Pharm. 2011, 79, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.I.; ElRab, S.M.G.; Khalil, A.F.; Ismael, S.M. Hypoglycemic effect of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits in diabetic rat. Eur. J. Chem. 2014, 5, 65–72. [Google Scholar] [CrossRef]
- Khalil, H.E.; Alharbi, A.G.A.; Ibrahim, I.M. In vitro antidiabetic assessment of Ocimum forskolei L. growing in Saudi Arabia. J. Pharmacogn. Phytochem. 2019, 8, 355–357. [Google Scholar]
- Al-Taweel, A.M.; Perveen, S.; Fawzy, G.A.; Alqasoumi, S.I.; El Tahir, K.E. New flavane gallates isolated from the leaves of Plicosepalus curviflorus and their hypoglycemic activity. Fitoterapia 2012, 83, 1610–1615. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Hanafy, A.; Labib, G.S.; Badr, J.M. Antihyperglycemic activities of extracts of the mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z. Für Nat. C 2014, 69, 391–398. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Alghamdi, H.A.; Ashour, O.M.; Abdel-Naim, A.B.; Ghareib, S.A.; Abdel-Sattar, E.A.; Hajar, A.S. Mechanisms of the antihyperglycemic activity of Retama raetam in Streptozocin-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2448–2453. [Google Scholar] [CrossRef]
- Khan, M.; Ali, M.; Ali, A.; Mir, S. Hypoglycemic and hypolipidemic activities of Arabic and Indian origin Salvadora persica root extract on diabetic rats with histopathology of their pancreas. Int. J. Health Sci. 2014, 8, 45. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Ali, N.A.A.; Setzer, W.N. A survey of chemical compositions and biological activities of Yemeni aromatic medicinal plants. Medicines 2015, 2, 67–92. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Al-Awar, A.A.A.M.; Elias, M.A. Antihyperglycemic and Hypolipidemic Effect of Azadirachta indica Leaves Aqueous Extract in Alloxan-Induced Diabetic Male Rabbits. Int. J. Pharm. Biol. Arch. 2018, 9, 47–51.5. [Google Scholar]
- Patil, P.; Patil, S.; Mane, A.; Verma, S. Antidiabetic activity of alcoholic extract of neem (Azadirachta indica) root bark. Natl. J. Physiol. Pharm. Pharmacol. 2013, 3, 142. [Google Scholar] [CrossRef]
- Nagashayana, G.; Jagadeesh, K.; Shreenivas, P.R. Evaluation of hypoglycemic activity of neem (Azadirachta indica) in albino rats. Iosr J. Dent. Med. Sci. (Iosr-Jdms) 2014, 13, 0411. [Google Scholar]
- Alzohairy, M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018, 32, 1241–1272. [Google Scholar] [CrossRef]
- Satyanarayana, K.; Sravanthi, K.; Shaker, I.A.; Ponnulakshmi, R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J. Ayurveda Integr. Med. 2015, 6, 165. [Google Scholar]
- Mothana, R.A.; Hasson, S.S.; Schultze, W.; Mowitz, A.; Lindequist, U. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia species. Food Chem. 2011, 126, 1149–1154. [Google Scholar] [CrossRef]
- Al-Mehdar, A.A.; Al-Battah, A.M. Evaluation of Hypoglycemic Activity of Boswellia carterii and Cissus rotundifolia in Streptozocin/Nicotinamide-Induced Diabetic Rats. Yemeni J. Med. Sci. 2016, 10, 30–38. [Google Scholar] [CrossRef]
- Farzaei, F.; Morovati, M.R.; Farjadmand, F.; Farzaei, M.H. A mechanistic review on medicinal plants used for diabetes mellitus in traditional Persian medicine. J. Evid.-Based Complement. Altern. Med. 2017, 22, 944–955. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (Boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complementary Med. 2013, 3, 221. [Google Scholar] [CrossRef]
- Onyechi, U.A.; Judd, P.A.; Ellis, P.R. African plant foods rich in non-starch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subjects. Br. J. Nutr. 1998, 80, 419–428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AliMohammed, W.M.; Abbas, A.A.; Mohmmed, H.; Qasem, M.A.; Saleem, H.A.M.; Shikoo, E.Y. Antidiabetic activity of cissus routndifolia leaves. World J. Pharm. Res. 2018, 8, 47–55. [Google Scholar]
- Mohammed, Y.H.E.; Khanum, S.A. Anti-Diabetic Activity of Dracaen cinnabari Balf. f Extracts from Resin in Socotra Island-Yemen. J. Plant Biochem. Physiol. 2016, 4, 100162. [Google Scholar]
- Al-Baoqai, N.; Al-Mahbashi, H.; Al-Adhal, A. antidiabetic and antihyperlipidemic activity of dracaena cinnabari balf. resin ethanolic extract of soqatra island in experimental animals. J. Pharm. Res. 2018, 3, 1–11. [Google Scholar] [CrossRef][Green Version]
- Butterweck, V.; Semlin, L.; Feistel, B.; Pischel, I.; Bauer, K.; Verspohl, E.J. Comparative evaluation of two different Opuntia ficus-indica extracts for blood sugar lowering effects in rats. Phytother. Res. 2011, 25, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeb, G. Effect of prickly pear cactus seeds oil on the blood glucose level of Streptozocin-induced diabetic rats and its molecular mechanisms. Int. J. Herb. Med. 2015, 3, 29–34. [Google Scholar]
- Al-Hajj, N.Q.M.; Sharif, H.R.; Aboshora, W.; Wang, H. In Vitro and in Vivo Evaluation of Antidiabetic Activity of Leaf Essential Oil of Pulicaria inuloides-Asteraceae. J. Food Nutr. Res. 2016, 4, 461–470. [Google Scholar]
- Galala, A.A.; Sallam, A.; Abdel-Halim, O.B.; Gedara, S.R. New ent-kaurane diterpenoid dimer from Pulicaria inuloides. Nat. Prod. Res. 2016, 30, 2468–2475. [Google Scholar] [CrossRef]
- Al-Deen, A.T.; Al-Naqeb, G. Hypoglycemic effect and in vitro antioxidant activity of methanolic extract from Argel (Solenostemma Argel) plant. Int. J. Herb. Med. 2014, 2, 128–131. [Google Scholar]
- Taha, L.E.; Bakhit, S.M.; Al-Sa’aidi, J.A.; Uro, A.B.O. The anti-hyperglycemic effect of Solenostemma argel compared with Glibenclamide. Al-Qadisiyah J. Vet. Med. Sci. 2014, 13, 113–117. [Google Scholar]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Phytochemical Composition, Antioxidant, Antimicrobial and in Vivo Anti-inflammatory Activity of Traditionally Used Romanian Ajuga laxmannii (Murray) Benth.(“Nobleman’s Beard”–Barba Împăratului). Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Al-Maskri, A.Y.; Al-Mahruqi, Z.M.H.; Al-Sabahi, J.N.; Al-Azkawi, A.; Al-Maskari, M.Y. Analytical evaluation of three wild growing Omani medicinal plants. Nat. Prod. Commun. 2011, 6, 1934578X1100601010. [Google Scholar] [CrossRef]
- Ghazanfar, S.A.; Al-Al-Sabahi, A.M. Medicinal plants of northern and central Oman (Arabia). Econ. Bot. 1993, 47, 89–98. [Google Scholar] [CrossRef]
- Norton, J.; Majid, S.A.; Allan, D.; Al Safran, M.; Böer, B.; Richer, R. An Illustrated Checklist of the Flora of Qatar; Browndown Publications: Gosport, UK, 2009. [Google Scholar]
- Tounekti, T.; Mahdhi, M.; Khemira, H. Ethnobotanical Study of Indigenous Medicinal Plants of Jazan Region, Saudi Arabia. Evid. Based Complementary Altern. Med. 2019, 2019, 3190670. [Google Scholar] [CrossRef]
- Albalawi, M.A.D.; Bashir, N.A.O.; Tawfik, A. Anticancer and antifolate activities of extracts of six Saudi Arabian wild plants used in folk medicine. J. Life Sci. 2015, 9, 334–340. [Google Scholar]
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus C aralluma R. Br. J. Pharm. Pharmacol. 2014, 66, 1351–1368. [Google Scholar] [CrossRef]
- Rahman, M.A.; Al-Said, M.S.; Mossa, J.S.; Al-Yahya, M.A.; Al-Hemaid, M.F. A check list of angiosperm flora of Farasan Islands, Kingdom of Saudi Arabia. Pak. J. Biol. Sci. 2002, 5, 1162–1166. [Google Scholar]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupor, D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Staden, J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): A review. S. Afr. J. Bot. 2013, 88, 204–208. [Google Scholar] [CrossRef]
- Kashyap, C.; Ranjeet, K.; Vikrant, A.; Vipin, K. Therapeutic Potency of Ocimum KilimandscharicumGuerke-A Review. Glob. J. Pharmacol. 2011, 5, 191–200. [Google Scholar]
- Zahran, E.M.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. The antiinflammatory activity and LD50 of Ocimum forskolei Benth. family Lamiaceae. J. Adv. Biomed. Pharm. Sci. 2019, 2, 116–120. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Al Naemi, H. Biological activities of Lycium shawii Leaves Extract. Int. J. Pharm. Biol. Arch. 2010, 3, 697–700. [Google Scholar]
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Complementary Med. Res. 2017, 6, 429–452. [Google Scholar] [CrossRef]
- Mossa, J. A study on the crude antidiabetic drugs used in Arabian folk medicine. Int. J. Crude Drug Res. 1985, 23, 137–145. [Google Scholar] [CrossRef]
- Ali, N.; Chhetri, B.; Dosoky, N.; Shari, K.; Al-Fahad, A.; Wessjohann, L.; Setzer, W. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Al Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K.; Al-Khulaidi, A. Ethnopharmacological survey of medicinal plants in Albaha Region, Saudi Arabia. Pharmacogn. Res. 2017, 9, 401. [Google Scholar]
- Batool, N.; Ilyas, N.; Shahzad, A. Asiatic Mangrove (Rhizophora mucronata)–An overview. Eur. Acad. Res. 2014, 2, 3348–3363. [Google Scholar]
- Dholi, S.K.; Raparla, R.; Mankala, S.K.; Nagappan, K. Invivo Antidiabetic evaluation of Neem leaf extract in alloxan induced rats. J. Appl. Pharm. Sci. 2011, 1, 100–105. [Google Scholar]
- Moussaieff, A.; Mechoulam, R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2009, 61, 1281–1293. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Shetty, A.A.; Rana, M.; Preetham, S. Cactus: A medicinal food. J. Food Sci. Technol. 2012, 49, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, N.Q.M.; Wang, H.; Gasmalla, M.A.; Ma, C.; Thabit, R.; Rahman, M.R.T.; Tang, Y. Chemical composition and antioxidant activity of the essential oil of Pulicaria inuloides. J. Food Nutr. Res. 2014, 2, 221–227. [Google Scholar] [CrossRef]
- Al-Hajj, N.Q.M.; Rashid, H.; Al-Hashedi, S.; Thabit, R.; Wang, H.X. Total Phenolic Content and Antioxidant, antimicrobial Activity from Some Yemani Plants. Eur. Acad. Res. II 2014, 8, 10196–10215. [Google Scholar]
- Al-Juhaimi, F.Y.; Shahzad, S.A.; Ahmed, A.S.; Adiamo, O.Q.; Ahmed, I.A.M.; Alsawmahi, O.N.; Ghafoor, K.; Babiker, E.E. Effect of Argel (Solenostemma argel) leaf extract on quality attributes of chicken meatballs during cold storage. J. Food Sci. Technol. 2018, 55, 1797–1805. [Google Scholar] [CrossRef]
- El Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004, 91, 43–50. [Google Scholar] [CrossRef]
- Chenni, A.; Yahia, D.A.; Boukortt, F.; Prost, J.; Lacaille-Dubois, M.; Bouchenak, M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. J. Ethnopharmacol. 2007, 109, 207–213. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Qarawi, A.A.; Bashir, A.K.; Tanira, M.O. Phytochemistry, pharmacology and toxicity of Rhazya stricta Decne: A review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000, 14, 229–234. [Google Scholar]
- Sdiri, M.; Li, X.; Du, W.; El-Bok, S.; Xie, Y.-Z.; Ben-Attia, M.; Yang, B. Anticancer activity of Cynomorium coccineum. Cancers 2018, 10, 354. [Google Scholar] [CrossRef]
- Zucca, P.; Bellot, S.; Rescigno, A. The modern use of an ancient plant: Exploring the antioxidant and nutraceutical potential of the maltese mushroom (Cynomorium coccineum L.). Antioxidants 2019, 8, 289. [Google Scholar] [CrossRef]
- Aljaghthmi, O.; Heba, H.; Zeid, I.A. Antihyperglycemic properties of mangrove plants (Rhizophora mucronata and Avicennia marina): An overview. Adv. Biol. Res. 2017, 11, 161–170. [Google Scholar]
- Elsharkawy, E.R.; Abdallah, E.M.; Shiboob, M.H.; Alghanem, S. Phytochemical, Antioxidant and Antibacterial Potential of Ducrosia anethifolia in Northern Border Region of Saudi Arabia. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.R.; Ho, Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011, 12, 5955–5970. [Google Scholar] [CrossRef] [PubMed]
- Laxane, S.N.; Swarnkar, S.; Zanwar, S.B.; Setty, M.M. Jatropha curcas: A systemic review on pharmacological, phytochemical, toxicological profiles and commercial applications. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 989–1010. [Google Scholar]
- Carels, N. Jatropha curcas: A review. Adv. Bot. Res. 2009, 50, 39–86. [Google Scholar]
- Mariita, R.; Ogol, C.; Oguge, N.; Okemo, P. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop. J. Pharm. Res. 2010, 9, 379–385. [Google Scholar] [CrossRef]
- Ali, S.S.; El-Zawawy, N.A.; Al-Tohamy, R.; El-Sapagh, S.; Mustafa, A.M.; Sun, J. Lycium shawii Roem. & Schult.: A new bioactive antimicrobial and antioxidant agent to combat multi-drug/pan-drug resistant pathogens of wound burn infections. J. Tradit. Complementary Med. 2019, 10, 13–25. [Google Scholar]
- Maizuwo, A.I.; Hassan, A.S.; Momoh, H.; Muhammad, J.A. Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): A review. J. Drug Des. Med. Chem. 2017, 3, 60. [Google Scholar]
- Dalmagro, A.P.; Camargo, A.; da Silva Filho, H.H.; Valcanaia, M.M.; de Jesus, P.C.; Zeni, A.L.B. Seasonal variation in the antioxidant phytocompounds production from the Morus nigra leaves. Ind. Crop. Prod. 2018, 123, 323–330. [Google Scholar] [CrossRef]
- Al-Hajj, N.Q.M.; Rashid, H.; Wang, H.; Thabit, R.; Rashed, M. Antioxident, antimicrobial and pulicaria inuloides and ocimum froskolei: A review. Am. Res. Thoughts 2014, 1, 973–1000. [Google Scholar]
- Fawzy, G.A.; Al-Taweel, A.M.; Perveen, S. Anticancer activity of flavane gallates isolated from Plicosepalus curviflorus. Pharmacogn. Mag. 2014, 10 (Suppl. 3), S519. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Rajesh, P.; Rajaram, R.; Kannan, V.R. A review on Rhizophora genus: Therapeutically important perspective phytochemical constituents. In Bioactive Phytochemicals: Perspectives for Modern Medicine; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2015; Volume 3, pp. 211–234. [Google Scholar]
- Khatak, M.; Khatak, S.; Siddqui, A.; Vasudeva, N.; Aggarwal, A.; Aggarwal, P. Salvadora persica. Pharmacogn. Rev. 2010, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Al-Harrasi, A.; Al-Rawahi, A.; Hussain, J. Chemistry and biology of essential oils of genus boswellia. Evid. Based Complementary Altern. Med. 2013, 2013, 140509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cui, R. Chemical components of Boswellia carterii. Yao Xue Xue Bao= Acta Pharm. Sin. 2002, 37, 633–635. [Google Scholar]
- Basar, S.; Koch, A.; König, W.A. A verticillane-type diterpene from Boswellia carterii essential oil. Flavour Fragr. J. 2001, 16, 315–318. [Google Scholar] [CrossRef]
- Said, A.A.; Aboutabl, E.A.; El Awdan, S.A.; Raslan, M.A. Proximate analysis, phytochemical screening, and bioactivities evaluation of Cissus rotundifolia (Forssk.) Vahl.(Fam. Vitaceae) and Sansevieria cylindrica Bojer ex Hook.(Fam. Dracaenaceae) growing in Egypt. Egypt. Pharm. J. 2015, 14, 180. [Google Scholar]
- Said, A.; Aboutabl, E.A.; Melek, F.R.; Abdel Jaleel Raheem Abdel Jaleel, G.; Raslan, M. Phytoconstituents profiling of Cissus rotundifolia (Forssk.) Vahl. by HPLC-MS/MS, and evaluation of its free radical scavenging activity (DPPH) and cytotoxicity. Trends Phytochem. Res. 2018, 2, 65–74. [Google Scholar]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.; Cioni, P.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Livrea, M.A.; Tesoriere, L. Health benefits and bioactive components of the fruits from Opuntia ficus-indica [L.] Mill. J. Prof. Assoc. Cactus Dev. 2006, 8, 73–90. [Google Scholar]
- Mohamed, S.E.; Azhari, H.E.E.; Rasha, M.; Mona, A.A. Adverse reactions of solenostemma argel leaves, extracts and alkaloids tablets administered to patients. Glob. J. Tradit. Med. Syst. 2013, 2, 14–18. [Google Scholar]
- Teia, F.K.F. A review of Solennostemma argel: Phytochemical, pharmacological activities and agricultural applications. J. Ayurvedic Herb. Med. 2018, 4, 99–101. [Google Scholar]
- Farag, M.; Al-Rehaily, A.; Ahmad, M.S.; Mothana, R.A. Detection of hypoglycemic and antidiabetic fraction in ethanol extract of Jatropha curcas aerial parts. Pharmacol. Pharm. 2014, 5, 663. [Google Scholar] [CrossRef][Green Version]
- El-Beshbishy, H.; Bahashwan, S. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities: An in vitro study. Toxicol. Ind. Health 2012, 28, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, R.; El-Gamal, M.; Abdel-Hady, N.; Hamdy, O. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann. Glob. Health 2015, 81, 814–820. [Google Scholar] [CrossRef]
- EL-Manawaty, M.; Gohar, L. In vitro α-glucosidase inhibitory activity of Egyptian plant extracts as an indication for their antidiabetic activity. Vitro 2018, 11, 360–367. [Google Scholar] [CrossRef]
- Mostafa, N.; Singab, A. Prospective of Herbal Medicine in Egypt. Med. Chem. (Los Angeles) 2018, 8, 116–117. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Ahmed, O.M.; Ahmed, R.R.; Mahmoud, A.; Abdella, E.; Ashour, M.B. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J. Med. Food 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Kubacey, T.M.; Haggag, E.G.; El-Toumy, S.A.; Ahmed, A.A.; El-Ashmawy, I.M.; Youns, M.M. Biological activity and flavonoids from Centaurea alexanderina leaf extract. J. Pharm. Res. 2012, 5, 3352–3361. [Google Scholar]
- Elshamy, A.I.; El-Shazly, M.; Yassine, Y.M.; El-Bana, M.A.; Farrag, A.-R.; Nassar, M.I.; Singab, A.N.; Noji, M.; Umeyama, A. Phenolic Constituents, Anti-Inflammatory and Antidiabetic Activities of Cyperus laevigatus L. Pharmacogn. J. 2017, 9, 828–833. [Google Scholar] [CrossRef]
- Khaliq, T.; Sarfraz, M.; Ashraf, M. Recent progress for the utilization of curcuma longa, Piper nigrum and Phoenix dactylifera seeds against type 2 diabetes. West Indian Med. J. 2015, 64, 527. [Google Scholar]
- Abdelaziz, D.H.; Ali, S.A.; Mostafa, M.M. Phoenix dactylifera seeds ameliorate early diabetic complications in streptozotocin-induced diabetic rats. Pharm. Biol. 2015, 53, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, M.; Khemakhem, B.; Mabrouk, H.B.; El Abed, H.; Makni, M.; Bouaziz, M.; Drira, N.; Marrakchi, N.; Mejdoub, H. Evaluation of anti-diabetic and anti-tumoral activities of bioactive compounds from Phoenix dactylifera L’s leaf: In vitro and in vivo approach. Biomed. Pharmacother. 2016, 84, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.F.; Ebrahim, M.E.; Metawaa, H.M.; Hosni, E.A.-M.A.; Ebrahim, F.M. In vitro and in vivo evaluation of the antidiabetic effect of different extracts of Nepeta cataria in Streptozocin induced diabetic rats. J. Am. Sci. 2010, 6, 364–386. [Google Scholar]
- Ibrahim, R.M.; El-Halawany, A.M.; Saleh, D.O.; El Naggar, E.M.B.; El-Shabrawy, A.E.-R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. De Farmacogn. 2015, 25, 134–141. [Google Scholar]
- Shams Eldin, S.M.; Radwan, M.M.; Wanas, A.S.; Habib, A.-A.M.; Kassem, F.F.; Hammoda, H.M.; Khan, S.I.; Klein, M.L.; Elokely, K.M.; ElSohly, M.A. Bioactivity-Guided Isolation of Potential Antidiabetic and Antihyperlipidemic Compounds from Trigonella stellata. J. Nat. Prod. 2018, 81, 1154–1161. [Google Scholar] [CrossRef]
- Abo-elmatty, D.M.; Essawy, S.S.; Badr, J.M.; Sterner, O. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type2 diabetic rats. J. Ethnopharmacol. 2013, 145, 269–277. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Haghighat, E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr. J. Pharm. Pharmacol. 2012, 6, 2332–2339. [Google Scholar] [CrossRef]
- Michel, C.G.; Nesseem, D.I.; Ismail, M.F. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. J. Ethnopharmacol. 2011, 133, 53–62. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomedicine 2011, 7, 18. [Google Scholar] [CrossRef]
- Soltan, M.M.; Zaki, A.K. Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 2009, 126, 102–107. [Google Scholar] [CrossRef]
- Eissa, T.; Palomino, O.; Carretero, M.; Gómez-Serranillos, M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rabia, A. Ethno-botanic treatments for paralysis (falij) in the Middle East. Chin. Med. 2012, 3, 25949. [Google Scholar] [CrossRef]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Minaiyana, M.; Moattar, F.; Vali, A. Effect of Securigera securidaca seeds on blood glucose level of normal and diabetic rats. Iran. J. Pharm. Sci. 2006, 2, 151–156. [Google Scholar]
- Ali-Shtayeh, M.S.; Yaniv, Z.; Mahajna, J. Ethnobotanical survey in the Palestinian area: A classification of the healing potential of medicinal plants. J. Ethnopharmacol. 2000, 73, 221–232. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.; Jamous, R. Complementary and alternative medicine use amongst Palestinian diabetic patients. Complementary Ther. Clin. Pract. 2012, 18, 12–21. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Yadav, A. A review on Cassia species: Pharmacological, traditional and medicinal aspects in various countries. Am. J. Phytomedicine Clin. Ther. 2013, 1, 291–312. [Google Scholar]
- Selvaraj, Y.; Chander, M.S. Senna-its chemistry, distribution and pharmaceutical value. J. Indian Inst. Sci. 2013, 60, 179. [Google Scholar]
- Al-Dabbas, M.M. Antioxidant activity of different extracts from the aerial part of Moringa peregrina (Forssk.) Fiori, from Jordan. Pak. J. Pharm. Sci. 2017, 30, 2151–2157. [Google Scholar]
- Said-Al Ahl, H.; Naguib, N.Y.; Hussein, M.S. Evaluation growth and essential oil content of catmint and lemon catnip plants as new cultivated medicinal plants in Egypt. Ann. Agric. Sci. 2018, 63, 201–205. [Google Scholar] [CrossRef]
- Tofighi, Z.; Asgharian, P.; Goodarzi, S.; Hadjiakhoondi, A.; Ostad, S.N.; Yassa, N. Potent cytotoxic flavonoids from Iranian Securigera securidaca. Med. Chem. Res. 2014, 23, 1718–1724. [Google Scholar] [CrossRef]
- Irshaid, F.; Mansi, K. The effects of methanol extract derived from Urtica pilulifera leaves on some hematological and biochemical parameters of diabetic rats. Res. J. Biol. Sci. 2009, 4, 675–681. [Google Scholar]
- Mostafa, D.G.; Khaleel, E.F.; Abdel-Aleem, G.A. Inhibition of the hepatic glucose output is responsible for the hypoglycemic effect of Crataegus aronia against type 2 diabetes mellitus in rats. Arch. Biol. Sci. 2018, 70, 277–287. [Google Scholar]
- Abdullah, A.; Bakry, S.; ABD EL-BAKY, A.; Mansour, A. Evaluation of the antioxidative, antidiabetic and antilipidemic effect of bitter mel on seeds (citrullus colocynthis) alcoholic extract on female rats. Al-Azhar Bull. Sci. 2010, 21, 13–26. [Google Scholar]
- Ali, F.T.; Hassan, N.S.; Abdrabou, R.R. Potential activity of Moringa oleifera leaf extract and some active ingredients against diabetes in rats. Int. J. Sci. Eng. Res. 2015, 6, 1490. [Google Scholar]
- Michael, H.N.; Salib, J.Y.; Eskander, E.F. Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytother. Res. 2013, 27, 699–704. [Google Scholar] [CrossRef]
- Niknami, M.; Mirbalouchzehi, A.; Zareban, I.; Kalkalinia, E.; Rikhtgarha, G.; Hosseinzadeh, H. Association of health literacy with type 2 diabetes mellitus self-management and clinical outcomes within the primary care setting of Iran. Aust. J. Prim. Health 2018, 24, 162–170. [Google Scholar] [CrossRef]
- Esteghamati, A.; Larijani, B.; Aghajani, M.H.; Ghaemi, F.; Kermanchi, J.; Shahrami, A.; Saadat, M.; Esfahani, E.N.; Ganji, M.; Noshad, S. Diabetes in Iran: Prospective analysis from first nationwide diabetes report of national program for prevention and control of diabetes (NPPCD-2016). Sci. Rep. 2017, 7, 13461. [Google Scholar] [CrossRef]
- Ghavam-Haghi, F.; Sadeghi Dinani, M. Isolation and identification of Astragalin and 2-methoxy tyrosol from the bulbs of Allium paradoxum. J. Herbmed. Pharmacol. 2017, 6, 114–118. [Google Scholar]
- Nickavar, B.; Yousefian, N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Für Verbrauch. Und Lebensm. 2011, 6, 191–195. [Google Scholar] [CrossRef]
- Dehghan, H.; Sarrafi, Y.; Salehi, P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J. Food Drug Anal. 2016, 24, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 2013, 7, 257–266. [Google Scholar]
- Nickavar, B.; Abolhasani, L. Bioactivity-guided separation of an α-amylase inhibitor flavonoid from Salvia virgata. Iran. J. Pharm. Res. Ijpr 2013, 12, 57. [Google Scholar] [PubMed]
- Moradabadi, L.; Kouhsari, S.M.; Sani, M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic insulin and cardiac glut-4 mrnas expression. Iran. J. Pharm. Res. Ijpr 2013, 12, 387. [Google Scholar] [PubMed]
- Ghorbani, A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran:(Part 1): General results. J. Ethnopharmacol. 2005, 102, 58–68. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J. Phytomed. 2013, 3, 254. [Google Scholar]
- Nejad, A.M.; Kamkar, A.; Giri, A.; Pourmahmoudi, A.A. Ethnobotany and folk medicinal uses of major trees and shrubs in Northern Iran. J. Med. Plants Res. 2013, 7, 284–289. [Google Scholar]
- Bonjar, G.S. Screening for antibacterial properties of some Iranian plants against two strains of Escherichia coli. Asian J. Plant Sci. 2004, 3, 310–314. [Google Scholar]
- Baharvand-Ahmadi, B.; Asadi-Samani, M. A mini-review on the most important effective medicinal plants to treat hypertension in ethnobotanical evidence of Iran. J. Nephropharmacology 2017, 6, 3. [Google Scholar]
- Delfan, B.; Bahmani, M.; Kazemeini, H.; Zargaran, A.; Rafieian-Kopaei, M.; Asadi-Samani, M.; Shahsavari, S. Identification of Effective Medicinal Plants for Hyperlipidemia: An Ethnobotanical Study in Lorestan Province, West of Iran. Tradit. Integr. Med. 2016, 1, 28–34. [Google Scholar]
- Sahranavard, S.; Naghibi, F.; Mosaddegh, M.; Esmaeili, S.; Sarkhail, P.; Taghvaei, M.; Ghafari, S. Cytotoxic activities of selected medicinal plants from Iran and phytochemical evaluation of the most potent extract. Res. Pharm. Sci. 2009, 4, 133. [Google Scholar] [PubMed]
- Saki, K.; Bahmani, M.; Rafieian-Kopaei, M.; Hassanzadazar, H.; Dehghan, K.; Bahmani, F.; Asadzadeh, J. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac. J. Trop. Dis. 2014, 4, S895–S901. [Google Scholar] [CrossRef]
- Bakhshi Jouybari, H.; Hosseini, A.S.; Davoodi, A.; Mirzaee, F. Materia medica used in jaundice based on Persian medicine. Res. J. Pharmacogn. 2018, 5, 83–93. [Google Scholar]
- Rajaei, P.; Mohamadi, N. Ethnobotanical study of medicinal plants of Hezar mountain allocated in south east of Iran. Iran. J. Pharm. Res. Ijpr 2012, 11, 1153. [Google Scholar] [PubMed]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Özen, T.; Çöllü, Z.; Korkmaz, H. Antioxidant properties of Urtica pilulifera root, seed, flower, and leaf extract. J. Med. Food 2010, 13, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Mahmoudi, R. Phytochemical properties and hygienic effects of Allium ascalonicum and Pimpinella anisum essential oils in Iranian white brined cheese. J. Essent. Oil Bear. Plants 2012, 15, 1013–1020. [Google Scholar] [CrossRef]
- Karamian, R.; Hosseini, D.A. Screening of total phenol and flavonoid contents, antioxidant and antibacterial activities of the methanolic extract of Allium ampeloprasum L.(Alliaceae) from Iran. Q. J. Sci. Kharazmi Univ. 2014, 14, 225–238. [Google Scholar]
- Kim, S.; Kim, D.-B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.-H. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef]
- Selim, Y.A.; Sakeran, M.I. Effect of Time Distillation on Chemical Constituents and Anti-Diabetic Activity of the Essential Oil from Dark Green Parts of Egyptian Allium ampeloprasum L. J. Essent. Oil Bear. Plants 2014, 17, 838–846. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Heidarian, E.; Kheiri, S.; Rafieian-Kopaei, M. Effect of hydroalcoholic Allium ampeloprasum extract on oxidative stress, diabetes mellitus and dyslipidemia in alloxan-induced diabetic rats. Biomed. Pharmacother. 2017, 86, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Amin, Z.M.; Al-Qattan, K.K.; Shaban, L.H.; Ali, M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in Streptozocin-induced diabetic rats. Int. J. Diabetes Metab. 2007, 15, 108–115. [Google Scholar]
- Liu, C.-T.; Hse, H.; Lii, C.-K.; Chen, P.-S.; Sheen, L.-Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005, 516, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and Streptozocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.; Choudhury, M.; Hossain, M.; Islam, M.; Islam, M.; Sumon, M. Antidiabetic effects of Catharanthus roseus, Azadirachta indica, Allium sativum and glimepride in experimentally diabetic induced rat. Bangladesh J. Vet. Med. 2007, 5, 99–102. [Google Scholar] [CrossRef][Green Version]
- Moezi, L.; Arshadi, S.S.; Motazedian, T.; Seradj, S.H.; Dehghani, F. Anti-diabetic effects of amygdalus lycioides spach in streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr 2018, 17, 353. [Google Scholar] [PubMed]
- Hashemnia, M.; Nikousefat, Z.; Yazdani-Rostam, M. Antidiabetic effect of Pistacia atlantica and Amygdalus scoparia in Streptozocin-induced diabetic mice. Comp. Clin. Pathol. 2015, 24, 1301–1306. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Esfehani, K.N.; Mohammadi, Z.D. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-Streptozocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017, 7, 169. [Google Scholar]
- Ashraf, H.; Heidari, R.; Nejati, V.; Ilkhanipoor, M. Effects of aqueous extract of Berberis integerrima root on some physiological parameters in Streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr 2013, 12, 425. [Google Scholar]
- Ashraf, H.; Heidari, R.; Nejati, V. Antihyperglycemic and antihyperlipidemic effects of fruit aqueous extract of Berberis integerrima Bge. in Streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr 2014, 13, 1313. [Google Scholar]
- Sabahi, Z.; Khoshnood-Mansoorkhani, M.J.; Rahmani Namadi, S.; Moein, M. Antidiabetic and synergistic effects study of anthocyanin fraction from berberis integerrima fruit on Streptozocin-induced diabetic rats model. Trends Pharm. Sci. 2016, 2, 43–50. [Google Scholar]
- Akbari, F.; Khodadadi, S.; Asgari, S.; Shirzad, H.; Mirhoseini, M.; Shahinfard, N.; Rafieian-Kopaei, M. A comparative study on hypoglycemic properties, lipid profile and bioactive components of hydro-alcoholic extracts of cooked and raw Brassica napus. J. Nephropharmacol. 2016, 5, 86. [Google Scholar]
- Fard, M.H.; Naseh, G.; Lotfi, N.; Hosseini, S.M.; Hosseini, M. Effects of aqueous extract of turnip leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J. Phytomed. 2015, 5, 148. [Google Scholar]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharmacother. 2017, 92, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Mahmoodi, M.; Karimi, M.; Hoseini, F.; Heydari, R.; Salehi, M.; Yousefi, A. Effect of hydroalcoholic extract of Capparis spinosa fruit on blood sugar and lipid profile of diabetic and normal rats. Zahedan J. Res. Med. Sci. 2013, 15, 34–38. [Google Scholar]
- Hashemnia, M.; Oryan, A.; Hamidi, A.-R.; Mohammadalipour, A. Blood glucose levels and pathology of organs in alloxan-induced diabetic rats treated with hydro-ethanol extracts of Allium sativum and Capparis spinosa. Afr. J. Pharm. Pharmacol. 2012, 6, 1559–1564. [Google Scholar]
- Mishra, P.R.; Panda, P.K.; Chowdary, K.A.; Panigrahi, S. Antidiabetic and antihyperlipidemic activity of Capparis spinosa extract. Int. J. Pharm. Sci. Rev. Res. 2012, 14, 38–43. [Google Scholar]
- Jalali, M.T.; Mohammadtaghvaei, N.; Larky, D.A. Investigating the effects of Capparis spinosa on hepatic gluconeogenesis and lipid content in Streptozocin-induced diabetic rats. Biomed. Pharmacother. 2016, 84, 1243–1248. [Google Scholar] [CrossRef]
- Khanavi, M.; Taheri, M.; Rajabi, A.; Fallah-Bonekohal, S.; Baeeri, M.; Mohammadirad, A.; Amin, G.; Abdollahi, M. Stimulation of hepatic glycogenolysis and inhibition of gluconeogenesis are the mechanisms of antidiabetic effect of Centaurea bruguierana ssp. belangerana. Asian J. Anim. Vet. Adv. 2012, 7, 1166–1174. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complementary Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef]
- Ghamarian, A.; Abdollahi, M.; Su, X.; Amiri, A.; Ahadi, A.; Nowrouzi, A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru J. Pharm. Sci. 2012, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, M.; Nagib, R.M.; Mamdouh, S.M. Anti-diabetic effect of some herbs and fruit against Streptozocin induced diabetic rats. Glob. Vet. 2014, 12, 541–549. [Google Scholar]
- Shi, C.; Karim, S.; Wang, C.; Zhao, M.; Murtaza, G. A review on antidiabetic activity of Citrullus colocynthis Schrad. Acta Pol. Pharm. 2014, 71, 363–367. [Google Scholar] [PubMed]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.; Chatha, S.A.; Sarker, S.D.; Gilani, A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Hashemnia, M.; Hamidi, A.-R.; Mohammadalipour, A. Effects of hydro-ethanol extract of Citrullus colocynthis on blood glucose levels and pathology of organs in alloxan-induced diabetic rats. Asian Pac. J. Trop. Dis. 2014, 4, 125–130. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Saidu, A.N.; Oibiokpa, F.I.; Olukotun, I.O. Phytochemical screening and hypoglycemic effect of methanolic fruit pulp extract of Cucumis sativus in alloxan induced diabetic rats. J. Med. Plants Res. 2014, 8, 1173–1178. [Google Scholar]
- Karthiyayini, T.; Kumar, R.; Kumar, K.S.; Sahu, R.K.; Roy, A. Evaluation of antidiabetic and hypolipidemic effect of Cucumis sativus fruit in Streptozocin-induced-diabetic rats. Biomed. Pharmacol. J. 2015, 2, 351–355. [Google Scholar]
- Antido, J.W.A.; Gatil, Y.L.B.; Rabajante, N.A.L. Hypoglycemic activity of cucumis sativus extract on alloxan-induced diabetic sprague-dawley rats: A pilot study. Lyceum Philipp. St. Cabrini Coll. Allied Med. Res. 2017, 2, 12–28. [Google Scholar]
- Minaiyan, M.; Zolfaghari, B.; Kamal, A. Effect of hydroalcoholic and buthanolic extract of Cucumis sativus seeds on blood glucose level of normal and Streptozocin-induced diabetic rats. Iran. J. Basic Med. Sci. 2011, 14, 436. [Google Scholar]
- Asgary, S.; Moshtaghian, S.J.; Setorki, M.; Kazemi, S.; Rafieian-Kopaei, M.; Adelnia, A.; Shamsi, F. Hypoglycaemic and hypolipidemic effects of pumpkin (Cucurbita pepo L.) on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2620–2626. [Google Scholar]
- Eslami, S.; Ebrahimzadeh, M.; Moghaddam, A.H.; Nabavi, S.; Jafari, N.; Nabavi, S. Renoprotective effect of Eryngium caucasicum in gentamicin-induced nephrotoxic mice. Arch. Biol. Sci. 2011, 63, 157–160. [Google Scholar] [CrossRef]
- Afshari, M.; Mohammadshahi, M.; Malayeri, A.R.; Zaheri, L. Antidiabetic, Hepato-Protective and Hypolipidemic Effects of Eryngium Caucasicum Extract in Streptozocin-Nicotinamide Induced Type 2 Diabetes in Male Rats. Iraq Med. J. 2019, 3, 11–16. [Google Scholar]
- Mahmoudzadeh-Sagheb, H.; Heidari, Z.; Bokaeian, M.; Moudi, B. Antidiabetic effects of Eucalyptus globulus on pancreatic islets: A stereological study. Folia Morphol. 2010, 69, 112–118. [Google Scholar]
- Dey, B.; Mitra, A. Chemo-profiling of eucalyptus and study of its hypoglycemic potential. World J. Diabetes 2013, 4, 170. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A.; Tahvilian, R.; Moradi, R. Antidiabetic, hematoprotective and nephroprotective effects of the aqueous extract of Falcaria vulgaris in diabetic male mice. Arch. Biol. Sci. 2018, 70, 655–664. [Google Scholar] [CrossRef]
- Rafiey, Z.; Jalili, F.; Sohrabi, M.; Jalili, C. Effects of hydro-alcoholic extract of Falcaria vulgaris on pancreas tissue in Streptozocin-induced diabetic rats. Iran. J. Endocrinol. Metab. 2017, 19, 91–98. [Google Scholar]
- Iranshahi, M.; Alizadeh, M. Antihyperglycemic effect of Asafoetida (Ferula assafoetida Oleo-Gum-Resin) in Streptozocin-induced diabetic rats. World Appl. Sci. J. 2012, 17, 157–162. [Google Scholar]
- Latifi, E.; Mohammadpour, A.A.; Fathi, B.; Nourani, H. Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in Streptozocin-induced diabetic wistar rats. Biomed. Pharmacother. 2019, 110, 197–202. [Google Scholar] [CrossRef]
- Abu-Zaiton, A.S. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak. J. Biol. Sci. 2010, 13, 97. [Google Scholar] [CrossRef]
- Shojaee, S.S.; Vahdati, A.; Assaei, R.; Sepehrimanesh, M. Effect of Galega officinalis leaf powder and Trigonella foenum-graecum seed powder on blood glucose levels and weight gain in a diabetes mellitus rat model. Comp. Clin. Pathol. 2015, 24, 145–148. [Google Scholar] [CrossRef]
- Abtahi-Evari, S.-H.; Shokoohi, M.; Abbasi, A.; Rajabzade, A.; Shoorei, H.; Kalarestaghi, H. Protective Effect of Galega officinalis extract on Streptozocin-induced kidney damage and biochemical factor in diabetic rats. Crescent J. Med. Biol. Sci. 2017, 4, 108–114. [Google Scholar]
- Mohammadi, G.; Zangeneh, M.M.; Rashidi, K.; Zangeneh, A. Evaluation of nephroprotective and antidiabetic effects of Gundelia tournefortii aqueous extract on diabetic nephropathy in male mice. Res. J. Pharmacogn. 2018, 5, 65–73. [Google Scholar]
- Alimoradi, M.; Jalili, C.; Kakeh-Baraei, S.; Tajehmiri, A.; Khodarahmi, R. Effects of Aqueous Extract of Gunnera (Gundelia tournefortii L.) on the Blood Serum Sugar Levels and Changes in the Streptozocin-induced Diabetic Pancreatic Tissue of Rat. Int. J. Sci. Study 2017, 5, 186–191. [Google Scholar]
- Minaiyan, M.; Ghannadi, A.; Movahedian, A.; Hakim-Elahi, I. Effect of Hordeum vulgare L.(Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res. Pharm. Sci. 2014, 9, 173. [Google Scholar]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 2017, 11, 145. [Google Scholar]
- Rahimi, P.; Kabiri, N.; Asgary, S.; Setorki, M. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr J. Pharm. Pharm. 2011, 5, 2655–2661. [Google Scholar]
- Mohammadi, J.; Delaviz, H.; Malekzadeh, J.M.; Roozbehi, A. The effect of hydro alcoholic extract of Juglans regia leaves in Streptozocin-nicotinamide induced diabetic rats. Pak. J. Pharm. Sci. 2012, 25, 407–411. [Google Scholar]
- Mohammadi, J.; Mirzaie, A.; Azizi, A.; Roozbehi, A.; Delaviz, H. The effects of hydroalcoholic extract of Juglans regia leaf on histological changes of Langerhans islet in diabetic rats model. ISMJ 2012, 15, 293–302. [Google Scholar]
- Teymouri, M.; Montaser, K.S.; Ghafarzadegan, R.; Haji, A.R. Study of hypoglycemic effect of Juglans regia leaves and its mechanism. J. Med. Plants 2010, 9, 57–65. [Google Scholar]
- Bayani, M.; Ahmadi-hamedani, M.; Javan, A.J. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian Mentha spicata leaves aqueous extract in diabetic rats. Iran. J. Pharm. Res. Ijpr 2017, 16, 75. [Google Scholar] [PubMed]
- Mousa-Al-Reza Hadjzadeh, Z.R.; Moradi, R.; Ghorbani, A. Effects of hydroalcoholic extract of watercress (Nasturtium officinale) leaves on serum glucose and lipid levels in diabetic rats. Indian J. Physiol. Pharm. 2015, 59, 223–230. [Google Scholar]
- Akbarzadeh, S.; Bazzi, P.; Daneshi, A.; Nabipour, I.; Pourkhalili Kh, M.G.; Sartavi, K.; Abdi, M.; Mirzaei, M.; Bargahi, A. Anti-diabetic effect of Otostegia persica extract on diabetic rats. J. Med. Plant. Res. 2012, 6, 3176–3180. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Pouraboli, I.; Yaghoobi, M.-M.; Mehrabani, M.; Mirtadzadini, S.-M. Antihyperglycemic, antilipid peroxidation, and insulin secretory activities of Otostegia persica shoot extract in Streptozocin-induced diabetic rats and in vitro C187 pancreatic β-cells. Pharm. Biol. 2013, 51, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpoor-Mashhadi, M.R.; Khaksar, Z.; Noorafshan, A.; Mogheisi, B. Stereological study of the effects of orally administrated Otostegia persica extract on pancreatic beta cells in male diabetic rats. Comp. Clin. Pathol. 2014, 23, 761–767. [Google Scholar] [CrossRef]
- Heidari, P.; Rezaei, M.; Sahebi, M.; Khadivi, A. Phenotypic variability of Pyrus boissieriana Buhse: Implications for conservation and breeding. Sci. Hortic. 2019, 247, 1–8. [Google Scholar] [CrossRef]
- Shahaboddin, M.-E.; Pouramir, M.; Moghadamnia, A.-A.; Parsian, H.; Lakzaei, M.; Mir, H. Pyrus biossieriana Buhse leaf extract: An antioxidant, antihyperglycaemic and antihyperlipidemic agent. Food Chem. 2011, 126, 1730–1733. [Google Scholar] [CrossRef]
- Yousefi, F.; Mahjoub, S.; Pouramir, M.; Khadir, F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: Inhibitory effects on α amylase and α glucosidase. Casp. J. Intern. Med. 2013, 4, 763. [Google Scholar]
- Kundakovic, T.; Ciric, A.; Stanojkovic, T.; Sokovic, M.; Kovacevic, N. Cytotoxicity and antimicrobial activity of Pyrus pyraster Burgsd. and Pyrus spinosa Forssk.(Rosaceae). Afr. J. Microbiol. Res 2014, 8, 511–518. [Google Scholar]
- Khan, S.A.; Al Kiyumi, A.R.; Al Sheidi, M.S.; Al Khusaibi, T.S.; Al Shehhi, N.M.; Alam, T. In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L. Asian Pac. J. Trop. Biomed. 2016, 6, 322–329. [Google Scholar] [CrossRef]
- Masmoudi-Allouche, F.; Touati, S.; Mnafgui, K.; Gharsallah, N.; El Feki, A.; Allouche, N. Phytochemical profile, antioxidant, antibacterial, antidiabetic and anti-obesity activities of fruits and pits from date palm (Phoenix dactylifera L.) grown in south of Tunisia. J. Pharmacogn. Phytochem. 2016, 5, 15. [Google Scholar]
- El Abed, H.; Chakroun, M.; Fendri, I.; Makni, M.; Bouaziz, M.; Drira, N.; Mejdoub, H.; Khemakhem, B. Extraction optimization and in vitro and in vivo anti-postprandial hyperglycemia effects of inhibitor from Phoenix dactylifera L. parthenocarpic fruit. Biomed. Pharmacother. 2017, 88, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Mohieldein, A. In vivo evaluation of anti diabetic, hypolipidemic, antioxidative activities of Saudi date seed extract on Streptozocin induced diabetic rats. J. Clin. Diagn. Res. Jcdr 2016, 10, FF06. [Google Scholar] [PubMed]
- Mard, S.A.; Jalalvand, K.; Jafarinejad, M.; Balochi, H.; Naseri, M.K.G. Evaluation of the antidiabetic and antilipaemic activities of the hydroalcoholic extract of Phoenix dactylifera palm leaves and its fractions in alloxan-induced diabetic rats. Malays. J. Med. Sci. Mjms 2010, 17, 4. [Google Scholar] [PubMed]
- Hasona, N.A.S.A.; Qumani, M.A.; Alghassab, T.A.; Alghassab, M.A.; Alghabban, A.A. Ameliorative properties of Iranian Trigonella foenum-graecum L. seeds and Punica granatum L. peel extracts in Streptozocin-induced experimental diabetic guinea pigs. Asian Pac. J. Trop. Biomed. 2017, 7, 234–239. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crop. Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kouhsari, S.M.; Feshani, A.M. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2010, 18, 270. [Google Scholar]
- Ghorbani, A.; Amiri, M.S.; Hosseini, A. Pharmacological properties of Rheum turkestanicum Janisch. Heliyon 2019, 5, e01986. [Google Scholar] [CrossRef]
- Mousa-Al-Reza Hadjzadeh, Z.R.; Khodaei, E.; Malek, M.; Ghanbari, H. Rheum turkestanicum rhizomes possess anti-hypertriglyceridemic, but not hypoglycemic or hepatoprotective effect in experimental diabetes. Avicenna J. Phytomed. 2017, 7, 1. [Google Scholar]
- Estakhr, J.; Javdan, N. Hypoglycemic properties of ethanolic extracts of Salvia hypoleuca in rats. Pharmacologyonline 2011, 3, 354–360. [Google Scholar]
- Zarei, A.; Vaezi, G.; Malekirad, A.A.; Abdollahi, M. Hypoglycemic and hypolipidemic activities of Salvia hydrangea in Streptozocin-induced diabetes in rats. Iran. J. Basic Med. Sci. 2015, 18, 417. [Google Scholar] [PubMed]
- Pouramir, M.; Shahaboddin, M.E.; Moghadamnia, A.-A.; Parastouei, K. To study the effects of Securigera securidaca (L.) seed against alloxan-induced hyperglycemia. J. Med. Plants Res. 2011, 5, 3188–3191. [Google Scholar]
- Ahmadi, A.; Khalili, M.; Margedari, S.; Nahri-Niknafs, B. The effects of solvent polarity on hypoglycemic and hypolipidemic activities of Securigera securidaca (L.) seeds. Drug Res. 2016, 66, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, Z.; Moradi-Afrapoli, F.; Ebrahimi, S.N.; Goodarzi, S.; Hadjiakhoondi, A.; Neuburger, M.; Hamburger, M.; Abdollahi, M.; Yassa, N. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J. Nat. Med. 2017, 71, 272–280. [Google Scholar] [CrossRef]
- Rani, Y.S.; Reddy, V.J.; Basha, S.J.; Koshma, M.; Hanumanthu, G.; Swaroopa, P. A review on Solanum nigrum. World J. Pharm. Pharm. Sci. 2017, 6, 293–303. [Google Scholar]
- Bahramikia, S.; Yazdanparast, R. Phytochemistry and medicinal properties of Teucrium polium L.(Lamiaceae). Phytother. Res. 2012, 26, 1581–1593. [Google Scholar] [CrossRef]
- Sabet, Z.; Roghani, M.; Najafi, M.; Maghsoudi, Z. Antidiabetic effect of Teucrium polium aqueous extract in multiple low-dose Streptozocin-induced model of type 1 diabetes in rat. J. Basic Clin. Pathophysiol. 2013, 1, 34–38. [Google Scholar]
- Afifi, F.U.; Abu-Dahab, R.; Kasabri, V. In vitro modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption by Paronychia argentea, Rheum ribes and Teucrium polium extracts. Jordan J. Pharm. Sci. 2012, 108, 1–34. [Google Scholar]
- Yadav, U.C.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef]
- Hannan, J.M.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.; Abdel-Wahab, Y.H. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef]
- Kannappan, S.; Anuradha, C. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009, 129, 401. [Google Scholar]
- Goodarzi, M.T.; Tootoonchi, A.S.; Karimi, J.; Abbasi Oshaghi, E. Anti-diabetic effects of aqueous extracts of three Iranian medicinal plants in type 2 diabetic rats induced by high fructose diet. Avi. J. Med. Biochem. 2013, 1, 7–13. [Google Scholar]
- Nickavar, B.; Amin, G. Bioassay-guided separation of an α-amylase inhibitor anthocyanin from Vaccinium arctostaphylos berries. Z. Für Nat. C 2010, 65, 567–570. [Google Scholar] [CrossRef]
- Feshani, A.M.; Kouhsari, S.M.; Mohammadi, S. Vaccinium arctostaphylos, a common herbal medicine in Iran: Molecular and biochemical study of its antidiabetic effects on alloxan-diabetic Wistar rats. J. Ethnopharmacol. 2011, 133, 67–74. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Najimi, S.A. Pancreatic protective and hypoglycemic effects of Vitex agnus-castus L. fruit hydroalcoholic extract in D-galactose-induced aging mouse model. Res. Pharm. Sci. 2017, 12, 137. [Google Scholar] [CrossRef]
- Yarizade, A.; Niazi, A.; Kumleh, H.H. Investigation of antiglycation and antioxidant potential of some antidiabetic medicinal plants. J. Pharm. Sci. Res. 2017, 9, 2382–2387. [Google Scholar]
- Gohari, A.; Noorafshan, A.; Akmali, M.; Zamani-Garmsiri, F.; Seghatoleslam, A. Urtica Dioica Distillate Regenerates Pancreatic Beta Cells in Streptozocin-Induced Diabetic Rats. Iran. J. Med. Sci. 2018, 43, 174. [Google Scholar]
- Rahimzadeh, M.; Jahanshahi, S.; Moein, S.; Moein, M.R. Evaluation of α-amylase inhibition by Urtica dioica and Juglans regia extracts. Iran. J. Basic Med. Sci. 2014, 17, 465. [Google Scholar]
- Ranjbari, A.; Azarbayjani, M.A.; Yusof, A.; Mokhtar, A.H.; Akbarzadeh, S.; Ibrahim, M.Y.; Tarverdizadeh, B.; Farzadinia, P.; Hajiaghaee, R.; Dehghan, F. In vivo and in vitro evaluation of the effects of Urtica dioica and swimming activity on diabetic factors and pancreatic beta cells. BMC Complement. Altern. Med. 2016, 16, 101. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluations of cytotoxicity of eight antidiabetic medicinal plants and their effect on GLUT4 translocation. Evid. Based Complement. Altern. Med. 2013, 2013, 549345. [Google Scholar]
- Kavoosi, G. Zataria multiflora essential oil reduces diabetic damages in Streptozocin-induced diabetic rats. Afr. J. Biotechnol. 2011, 10, 17632–17639. [Google Scholar]
- Moein, S.; Pimoradloo, E.; Moein, M.; Vessal, M. Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of Labiatae plants extracts: As a model of antidiabetic compounds properties. Biomed. Res. Int. 2017, 2017, 7319504. [Google Scholar] [CrossRef]
- Solati, J.; Soleimani, N. Antihyperglycemic and antihyperlipidemic effects of Ziziphus vulgaris L. onreptozocin-induced diabetic adult male Wistar rats. Acta Diabetol. 2010, 47, 219–223. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Salim, S.Y.; Assaf, M.H.; Abdel-Hady, R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol. 2005, 101, 129–138. [Google Scholar] [CrossRef]
- Sadeghi, M.; Zolfaghari, B.; Senatore, M.; Lanzotti, V. Antifungal cinnamic acid derivatives from Persian leek (Allium ampeloprasum Subsp. Persicum). Phytochem. Lett. 2013, 6, 360–363. [Google Scholar] [CrossRef]
- Ghasemi, P.A.; Momeni, M.; Bahmani, M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 368–385. [Google Scholar] [CrossRef]
- Niroumand, M.C.; Farzaei, M.H.; Razkenari, E.K.; Amin, G.; Khanavi, M.; Akbarzadeh, T.; Shams-Ardekani, M.R. An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red Crescent Med. J. 2016, 18, e22361. [Google Scholar]
- Shams-Ghahfarokhi, M.; Shokoohamiri, M.-R.; Amirrajab, N.; Moghadasi, B.; Ghajari, A.; Zeini, F.; Sadeghi, G.; Razzaghi-Abyaneh, M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia 2006, 77, 321–323. [Google Scholar]
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Naghdi, N.; Rafieian-Kopaei, M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 2016, 5, 44. [Google Scholar] [CrossRef]
- Nasab, F.K.; Khosravi, A.R. Ethnobotanical study of medicinal plants of Sirjan in Kerman Province, Iran. J. Ethnopharmacol. 2014, 154, 190–197. [Google Scholar] [CrossRef]
- Delfan, B.; Saki, K.; Bahmani, M.; Rangsaz, N.; Delfan, M.; Mohseni, N.; Babaeian, Z. A study on anti-diabetic and anti-hypertension herbs used in Lorestan province, Iran. J. Herbmed. Pharmacol. 2014, 3, 71–76. [Google Scholar]
- Saeidnia, S.; Gohari, A.R. Importance of Brassica napus as a medicinal food plant. J. Med. Plants Res. 2012, 6, 2700–2703. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Moradi, M.-T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017, 6, 118. [Google Scholar] [CrossRef]
- Taghavi, M.; Nazari, M.; Rahmani, R.; Sayadi, A.; Hajizadeh, M.; Mirzaei, M.; Ziaaddini, H.; Hosseini-Zijoud, S.; Mahmoodi, M. Outcome of Capparis spinosa fruit extracts treatment on liver, kidney, pancreas and stomach tissues in normal and diabetic rats. Med. Chem. 2014, 4, 717–721. [Google Scholar] [CrossRef]
- Rahimi, R.; Amin, G.; Ardekani, M.R.S. A review on Citrullus colocynthis Schrad.: From traditional Iranian medicine to modern phytotherapy. J. Altern. Complementary Med. 2012, 18, 551–554. [Google Scholar] [CrossRef]
- Abdollahi, B.; Abbasi, M.M.; Milani, P.Z.; Nourdadgar, A.S.; Khojasteh, S.M.B.; Nejati, V. Hydro-methanolic extract of Cornus mas L. and blood glucose, lipid profile and hematological parameters of male rats. Iran. Red Crescent Med. J. 2014, 16, e17784. [Google Scholar] [CrossRef][Green Version]
- Khoshbakht, K.; Hammer, K.; Pistrick, K. Eryngium caucasicum Trautv. cultivated as a vegetable in the Elburz Mountains (Northern Iran). Genet. Resour. Crop Evol. 2007, 54, 445–448. [Google Scholar] [CrossRef]
- Nowbandegani, A.S.; Kiumarcy, S.; Rahmani, F.; Dokouhaki, M.; Khademian, S.; Zarshenas, M.M.; Faridi, P. Ethnopharmacological knowledge of Shiraz and Fasa in Fars region of Iran for diabetes mellitus. J. Ethnopharmacol. 2015, 172, 281–287. [Google Scholar] [CrossRef]
- Choobkar, N.; Kakoolaki, S.; Mohammadi, F. The biological effects of herbal medicine, Falcaria vulgaris: An article review. Iran. J. Aquat. Anim. Health 2017, 3, 74–81. [Google Scholar] [CrossRef][Green Version]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Dadashzadeh Mehrabani, G.; Ahmadi, M.; Ranjbar, R.; Safi-AldinArdebily, M. Chemistry, pharmacology and medicinal properties of Heracleum persicum Desf. Ex Fischer: A review. J. Med. Plants Res. 2012, 6, 1813–1820. [Google Scholar]
- Sadeghi, Z.; Akaberi, M.; Valizadeh, J. Otostegia persica (Lamiaceae): A review on its ethnopharmacology, phytochemistry, and pharmacology. Avicenna J. Phytomed. 2014, 4, 79. [Google Scholar]
- Farzaei, M.H.; Shams-Ardekani, M.R.; Abbasabadi, Z.; Rahimi, R. Scientific evaluation of edible fruits and spices used for the treatment of peptic ulcer in traditional Iranian medicine. Isrn Gastroenterol. 2013, 2013, 136932. [Google Scholar] [CrossRef]
- Shakiba, M.; Kariminik, A.; Parsia, P. Antimicrobial activity of different parts of Phoenix dactylifera. Int. J. Mol. Clin. Microbiol. 2011, 1, 107–111. [Google Scholar]
- Abedi, A.; Parviz, M.; Karimian, S.M.; Rodsari, H.R.S. Aphrodisiac activity of aqueous extract of Phoenix dactylifera pollen in male rats. Adv. Sex. Med. 2013, 3, 28. [Google Scholar] [CrossRef]
- Amirghofran, Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J. Immunol. 2010, 7, 65–73. [Google Scholar]
- Jazayeri, S.B.; Amanlou, A.; Ghanadian, N.; Pasalar, P.; Amanlou, M. A preliminary investigation of anticholinesterase activity of some Iranian medicinal plants commonly used in traditional medicine. Daru J. Pharm. Sci. 2014, 22, 17. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R.; TaghavizadehYazdi, M.E. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad, Iran. Iran. J. Pharm. Res. IJPR 2014, 13, 157. [Google Scholar]
- Javdan, N.; Estakhr, J. Evaluation of the effects of Salvia hypoleuca on the expression of cytokines: IL-6, IL-10 and TNF-α in high fat diet-fed mice towards a cure for diabetes mellitus. Pharmacologyonline 2011, 2, 842–852. [Google Scholar]
- Naghibi, F.; Mosadegh, M.; Mohammadi, M.S.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 4, 63–79. [Google Scholar]
- Bahmani, M.; Shirzad, H.; Mirhosseini, M.; Mesripour, A.; Rafieian-Kopaei, M. A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L). J. Evid. Based Complement. Altern. Med. 2016, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Avizeh, R.; Najafzadeh, H.; Pourmahdi, M.; Mirzaee, M. Effect of glibenclamide and fruit extract of Zizyphus spina-christi on alloxan-induced diabetic dogs. J. Appl. Res. Vet. Med. 2010, 8, 109. [Google Scholar]
- Abd, F.A.E.-R.A.; Ali, R.F.M. Proximate compositions, phytochemical constituents, antioxidant activities and phenolic contents of seed and leaves extracts of Egyptian leek (Allium ampeloprasum var. kurrat). Eur. J. Chem. 2013, 4, 185–190. [Google Scholar]
- Fattorusso, E.; Iorizzi, M.; Lanzotti, V.; Taglialatela-Scafati, O. Chemical composition of shallot (Allium ascalonicum Hort.). J. Agric. Food Chem. 2002, 50, 5686–5690. [Google Scholar] [CrossRef]
- Mohammadi-Motlagh, H.-R.; Mostafaie, A.; Mansouri, K. Anticancer and anti-inflammatory activities of shallot (Allium ascalonicum) extract. Arch. Med. Sci. Ams 2011, 7, 38. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Foods 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Babaei, H.; Sadeghpour, O.; Nahar, L.; Delazar, A.; Nazemiyeh, H.; Mansouri, M.R.; Poursaeid, N.; Asnaashari, S.; Moghadam, S.B.; Sarker, S.D. Antioxidant and vasorelaxant activities of flavonoids from Amygdalus lycioides var. horrida. Turk. J. Biol. 2008, 32, 203–208. [Google Scholar]
- Takallozadeh, M.; Bashtani, M.; Farhangfar, H. Evaluation of the nutritive value of wild almond seed (Amygdalus scoparia) and its effect on performance, milk fatty acid composition and antioxidant activity in lactating goats. J. Livest. Sci. Technol. 2019, 7, 21–31. [Google Scholar]
- Alemardan, A.; Asadi, W.; Rezaei, M.; Tabrizi, L.; Mohammadi, S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crop. Prod. 2013, 50, 276–287. [Google Scholar] [CrossRef]
- Paul, S.; Geng, C.A.; Yang, T.H.; Yang, Y.P.; Chen, J.J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L.(cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Sahu, J. Cucumis sativus (cucumber): A review on its pharmacological activity. J. Appl. Pharm. Res. 2015, 3, 4–9. [Google Scholar]
- Zengin, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J. Phytochemical characterization and bioactivities of five Apiaceae species: Natural sources for novel ingredients. Ind. Crop. Prod. 2019, 135, 107–121. [Google Scholar] [CrossRef]
- Goorani, S.; Zangeneh, M.M.; Koohi, M.K.; Seydi, N.; Zangeneh, A.; Souri, N.; Hosseini, M.-S. Assessment of antioxidant and cutaneous wound healing effects of Falcaria vulgaris aqueous extract in Wistar male rats. Comp. Clin. Pathol. 2019, 28, 435–445. [Google Scholar] [CrossRef]
- Lupak, M.; Khokhla, M.; Hachkova, G.Y.; Kanyuka, O.; Klymyshyn, N.; Chajka, Y.P.; Skybitska, M.; Sybirna, N. The alkaloid-free fraction from Galega officinalis extract prevents oxidative stress under experimental diabetes mellitus. Ukr. Biochem. J. 2015, 87, 78–86. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Azimi, N. Gundelia: A systematic review of medicinal and molecular perspective. Pak. J. Biol. Sci. 2013, 16, 1238–1247. [Google Scholar] [CrossRef]
- Alkan, E.E.; Celik, I. The therapeutics effects and toxic risk of Heracleum persicum Desf. extract on Streptozocin-induced diabetic rats. Toxicol. Rep. 2018, 5, 919–926. [Google Scholar] [CrossRef]
- Changxing, L.; Dongfang, D.; Lixue, Z.; Saeed, M.; Alagawany, M.; Farag, M.; Chenling, M.; Jianhua, L. Heracleum persicum: Chemical composition, biological activities and potential uses in poultry nutrition. World’s Poult. Sci. J. 2019, 75, 207–218. [Google Scholar] [CrossRef]
- Sinha, A.; Meena, A.; Panda, P.; Srivastava, B.; Gupta, M.; Padhi, M. Phytochemical, pharmacological and therapeutic potential of Hordeum vulgare Linn.-a review. Asian J. Res. Chem. 2012, 5, 1303–1308. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid. Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Arun, N.; Singh, D. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012, 3, 1240. [Google Scholar]
- Saeidnia, S.; Ghamarinia, M.; Gohari, A.R.; Shakeri, A. Terpenes from the root of Salvia hypoleuca Benth. Daru J. Pharm. Sci. 2012, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran. J. Pharm. Res. Ijpr 2013, 12, 801. [Google Scholar] [PubMed]
- Zameer, S.; Najmi, A.K.; Vohora, D.; Akhtar, M. A review on therapeutic potentials of Trigonella foenum graecum (fenugreek) and its chemical constituents in neurological disorders: Complementary roles to its hypolipidemic, hypoglycemic, and antioxidant potential. Nutr. Neurosci. 2018, 21, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Kazempour, N.; Taghizadeh, M. In vitro antimicrobial and antioxidant activity of Vaccinium arctostaphylos L. extracts. J. Biol. Act. Prod. Nat. 2013, 3, 241–247. [Google Scholar]
- Saedi Dezaki, E.; Mahmoudvand, H.; Sharififar, F.; Fallahi, S.; Monzote, L.; Ezatkhah, F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm. Biol. 2016, 54, 752–758. [Google Scholar] [CrossRef]
- FathiMoghaddam, H.; Mokhtari, M.; Kamaei, L.; Ahangarpour, A. Effects of Avicennia Marina leaves aqueous and hydro alcoholic extract on Streptozocin-induced diabetic male rats. J. Rafsanjan Univ. Med. Sci. 2011, 10, 245–254. [Google Scholar]
- Kazemian, M.; Abad, M.; reza Haeri, M.; Ebrahimi, M.; Heidari, R. Anti-diabetic effect of Capparis spinosa L. root extract in diabetic rats. Avicenna J. Phytomed. 2015, 5, 325. [Google Scholar]
- Shahraki, A.; Shahraki, M. The comparison of eucalyptus aqueous extract and insulin on blood sugar and liver enzymes in diabetic male rats. Zahedan J. Res. Med. Sci. 2013, 15, 25–28. [Google Scholar]
- Pashazadeh, M.; Mirzazadeh, J.; Alizadeh, A. Effect of Fabaceae (Galega Officinalis L.) consumption on levels of blood glucose, lipids and lipoproteins in Streptozocin-induced diabetic rats. Bull. Env. Pharm. Life Sci. 2015, 4, 127–132. [Google Scholar]
- Rajaei, Z.; Mousa-Al-Reza Hadjzadeh, R.M.; Ghorbani, A.; Saghebi, A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in Streptozocin-induced diabetic rats. Adv. Biomed. Res. 2015, 4, 33. [Google Scholar] [PubMed]
- Ahangarpour, A.; Mohammadian, M.; Dianat, M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran. J. Med. Sci. 2012, 37, 181. [Google Scholar] [PubMed]
- Abusaib, M.; Ahmed, M.; Nwayyir, H.A.; Alidrisi, H.A.; Al-Abbood, M.; Al-Bayati, A.; Al-Ibrahimi, S.; Al-Kharasani, A.; Al-Rubaye, H.; Mahwi, T. Iraqi Experts Consensus on the Management of Type 2 Diabetes/Prediabetes in Adults. Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1179551420942232. [Google Scholar] [CrossRef]
- Ali, N.S.M.; Allela, O.Q.; Salih, H.M.; Ahmed, I.H. Prevalence of Type 2 Diabetes Associated Complications in Kurdistan Region Iraq. J. Basic Clin. Pharm. 2019, 10, 1–6. [Google Scholar]
- Aljobouri, A.M.; Rashid, K.I.; Ibrahim, S.A.; Zyer, A.; Abbas, Z.N. Study the effect of Bauhinia variegate Linn. ethanolic extract on reducing glucose and lipid levels of white albino mice. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 652–658. [Google Scholar]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Al-Abassi, N.N.; Ibrahem, A.M. Study Antidiabetic Effect of Momordica Charantia (bitter gourd) seeds on Alloxan Induced Diabetic Rats. Iraqi J. Vet. Med. 2010, 34, 165–170. [Google Scholar]
- Naqishbandi, A.M.; Josefsen, K.; Pedersen, M.E.; Jäger, A.K. Hypoglycemic activity of Iraqi Rheum ribes root extract. Pharm. Biol. 2009, 47, 380–383. [Google Scholar] [CrossRef]
- Raafat, K.; Aboul-Ela, M.; El-Lakany, A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch. Pharmacal Res. 2014, 1–10. [Google Scholar] [CrossRef]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. J. Ethnopharmacol. 2011, 133, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Dizaye, K.F.; Sultan, A.H.; Banna, H.B. Immunohistochemical and biochemical study of the effect of Rheum ribes on the pancreas of diabetic rats. Zanco J. Med. Sci. 2019, 23, 394–402. [Google Scholar] [CrossRef]
- Al-douri, N.A. A survey of medicinal plants and their traditional uses in Iraq. Pharm. Biol. 2000, 38, 74–79. [Google Scholar] [CrossRef]
- Öztürk, M.; Aydoğmuş-Öztürk, F.; Duru, M.E.; Topçu, G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007, 103, 623–630. [Google Scholar] [CrossRef]
- Al-Sanafi, A. The Pharmacological Importance of Bauhinia variegata. A Review. Int. J. Pharma Sci. Res. (Ijsr) 2013, 12, 160–164. [Google Scholar]
- Tartik, M.; Darendelioglu, E.; Aykutoglu, G.; Baydas, G. The various biological activities of Rheum ribes extract on different types of cell. Türk Doğa Ve Fen Dergİsİ 2015, 1, 7–12. [Google Scholar]
- Al-Awwadi, N.A.J. Anti diabetics effect of Achillea santolina aqueous leaves extract. Int. J. Med. Plants Res. 2013, 4, 151–156. [Google Scholar]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L. Modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption with Achillea santolina, Eryngium creticum and Pistacia atlantica extracts: In vitro evaluation. J. Exp. Integr. Med. 2012, 2, 245–254. [Google Scholar] [CrossRef]
- Al-Aboudi, A.; Afifi, F.U. Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm. Biol. 2011, 49, 221–239. [Google Scholar] [CrossRef]
- Dabe, N.E.; Kefale, A.T. Antidiabetic effects of Artemisia species: A systematic review. Anc. Sci. Life 2017, 36, 175. [Google Scholar]
- Irshaid, F.; Mansi, K.; Aburjai, T. Antidiabetic effect of essential oil from Artemisia sieberi growing in Jordan in normal and alloxan induced diabetic rats. Pak. J. Biol. Sci. 2010, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Khayoon, H.A.; Ali, A.H.; Kadhim, T.A.; Abdulhadi, H.A. Antidiabetic effect of Artemisia sieberi in rabbits that induced diabetic by alloxan. Health Perspect. 2001, 109, 69. [Google Scholar]
- Afifi, F.U.; Kasabri, V.; Litescu, S.C.; Abaza, I.M. In vitro and in vivo comparison of the biological activities of two traditionally and widely used Arum species from Jordan: Arum dioscoridis Sibth & Sm. and Arum palaestinum Boiss. Nat. Prod. Res. 2016, 30, 1777–1786. [Google Scholar] [PubMed]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. De Farmacogn. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Al-Hallaq, E.K.; Kasabri, V.; Abdalla, S.S.; Bustanji, Y.K.; Afifi, F.U. Anti-obesity and antihyperglycemic effects of Crataegus aronia extracts: In vitro and in vivo evaluations. Food Nutr. Sci. 2013, 4, 972–983. [Google Scholar]
- Alkofahi, A.S.; Abdul-Razzak, K.K.; Alzoubi, K.H.; Khabour, O.F. Screening of the Anti-hyperglycemic activity of some medicinal plants of Jordan. Pak. J. Pharm. Sci. 2017, 30, 907–912. [Google Scholar]
- Kikowska, M.; Dworacka, M.; Kędziora, I.; Thiem, B. Eryngium creticum–ethnopharmacology, phytochemistry and pharmacological activity. A review. Rev. Bras. De Farmacogn. 2016, 26, 392–399. [Google Scholar] [CrossRef]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. Evaluation of the acute antihyperglycemic effects of four selected indigenous plants from Jordan used in traditional medicine. Pharm. Biol. 2011, 49, 687–695. [Google Scholar] [CrossRef]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L.; Shawash, H. In vitro effects of Geranium graveolens, Sarcopoterium spinosum and Varthemia iphionoides extracts on pancreatic MIN6 proliferation and insulin secretion and on extrapancreatic glucose diffusion. Int. J. Diabetes Dev. Ctries. 2013, 33, 170–177. [Google Scholar] [CrossRef]
- Boukhris, M.; Bouaziz, M.; Feki, I.; Jemai, H.; El Feki, A.; Sayadi, S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis. 2012, 11, 81. [Google Scholar] [CrossRef]
- Atchibri, A.; Brou, K.; Kouakou, T.; Kouadio, Y.; Gnakri, D. Screening for antidiabetic activity and phytochemical constituents of common bean (Phaseolus vulgaris L.) seeds. J. Med. Plants Res. 2010, 4, 1757–1761. [Google Scholar]
- Yazdan, P.R.; Esmaeili, M.A.; Ashrafi, H.J. Teucrium polium extract effects pancreatic function of Streptozocin diabetic rats: A histopathological examination. Iran. Biomed. J. 2005, 9, 81–85. [Google Scholar]
- Afifi, F.; Saket, M.; Jaghabir, M.; Al-Eisawi, D. Effect of Varthemia iphionoides on blood glucose level of normal rats and rats with streptozocin-induced diabetes mellitus. Curr. Ther. Res. 1997, 58, 888–892. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef]
- Nawash, O.; Shudiefat, M.; Al-Tabini, R.; Al-Khalidi, K. Ethnobotanical study of medicinal plants commonly used by local bedouins in the badia region of Jordan. J. Ethnopharmacol. 2013, 148, 921–925. [Google Scholar] [CrossRef]
- Hudaib, M.; Mohammad, M.; Bustanji, Y.; Tayyem, R.; Yousef, M.; Abuirjeie, M.; Aburjai, T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 2008, 120, 63–71. [Google Scholar] [CrossRef]
- Azab, A. Arum: A plant genus with great medicinal potential. Eur. Chem. Bull. 2017, 6, 59–68. [Google Scholar] [CrossRef]
- Al-Hallaq, E.K.; Afifi, F.U.; Abdalla, S.S. Evaluation of the hypocholesterolemic effect and phytochemical screening of the hydroethanolic extract of Crataegus aronia from Jordan. Nat. Prod. Commun. 2012, 7, 1934578. [Google Scholar] [CrossRef]
- Aburjai, T.; Hudaib, M.; Tayyem, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294–304. [Google Scholar] [CrossRef]
- Oran, S.A.; Al-Eisawi, D. Ethnobotanical survey of the medicinal plants in the central mountains (North-South) in Jordan. J. Biodivers. Environ. Sci. 2015, 6, 381–400. [Google Scholar]
- Awwad, O.; Abu-Dahab, R.; Abaza, I.F.; Alabbassi, R.; Majdalawi, L.; Afifi, F.U. Effect of the galling aphid of Baizongia pistaciae L. on composition and biological activities of essential oils of Pistacia atlantica Desf. growing wild in Jordan. J. Essent. Oil Bear. Plants 2017, 20, 791–800. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [PubMed]
- Afifi, F.U.; Kasabri, V. Pharmacological and phytochemical appraisal of selected medicinal plants from Jordan with claimed antidiabetic activities. Sci. Pharm. 2013, 81, 889–932. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.E.-H.H.; El-Sayed, M.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Afifi-Yazar, F.U.; Kasabri, V.; Abu-Dahab, R. Medicinal plants from Jordan in the treatment of cancer: Traditional uses vs. in vitro and in vivo evaluations–Part 1. Planta Med. 2011, 77, 1203–1209. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Alu’datt, M.; Al-Shara, B.; Abu-Zaiton, A. In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ Cult. (Pctoc) 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Aguilar-Santamaría, L.; Ramírez, G.; Nicasio, P.; Alegría-Reyes, C.; Herrera-Arellano, A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2009, 124, 284–288. [Google Scholar] [CrossRef]
- Twaij, H.A.; Al-Dujaili, E.A. Evaluation of the anti-diabetic and anti-ulcer properties of some Jordanian and Iraqi medicinal plants; a screening study. JMED Res. 2014, 2014, 539605. [Google Scholar] [CrossRef][Green Version]
- Raafat, K.; Boukhary, R.; Aboul-Ela, M.; El-Lakany, A. Endogenous Lebanese plants treating diabetes and related complications. Nat. Prod. Chem. Res. 2013, 1, 112–120. [Google Scholar]
- Rashid, K.; Kumar, C.S.; Haleel, P. Healthcare Benefits of Hordeumvulgare L (Barley): A Phyto-Pharmacological Review. Res. J. Pharmacol. Pharmacodyn. 2017, 9, 207–210. [Google Scholar] [CrossRef]
- Seca, A.M.; Grigore, A.; Pinto, D.C.; Silva, A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.; Ela, M.; Raafat, K.; El-Lakany, A. Role of antioxidants in the antidiabetic potential of two indigenous Lebanese Inula species. Med. Aromat. Plants S 2015, 2, 2167-0412. [Google Scholar]
- Lemouchi, R.; Selles, C.; Medjdoub, H.; Tabti, B. Assessment of possible efficacy of aqueous leaves extract of Psoralea bituminosa L. for anti-hyperglycaemic activity. Asian Pac. J. Trop. Dis. 2015, 5, 575–578. [Google Scholar] [CrossRef]
- Al Ayoubi, S.; Raafat, K.; El-Lakany, A.; Aboul-Ela, M. Phytochemical Investigation of Psoralea bituminosa L. and its Anti-Diabetic Potentials. Pharmacogn. J. 2018, 10, 841–853. [Google Scholar] [CrossRef]
- Boukhary, R.; Aboul-ElA, M.; Al-Hanbali, O.; El-Lakany, A. Phenolic compounds from Centaurea horrida L growing in Lebanon. Phyto 2017, 9, 1–4. [Google Scholar] [CrossRef]
- Nelly, A.; Annick, D.-D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar]
- Arnold, N.; Baydoun, S.; Chalak, L.; Raus, T. A contribution to the flora and ethnobotanical knowledge of Mount Hermon, Lebanon. Fl Medit 2015, 25, 13–55. [Google Scholar]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The pharmacological properties of Salvia essential oils. J. Appl. Pharm. Sci. 2013, 3, 122. [Google Scholar]
- Mohammedi, Z. Resistance, pharmacology properties and nutritional value of a shrub from arid environments Atriplex halimus. Res. J. Med. Plant. 2016, 10, 10–18. [Google Scholar] [CrossRef][Green Version]
- Ezeani, C.; Ezenyi, I.; Okoye, T.; Okoli, C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J. Intercult. Ethnopharmacol. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Smirin, P.; Taler, D.; Abitbol, G.; Brutman-Barazani, T.; Kerem, Z.; Sampson, S.R.; Rosenzweig, T. Sarcopoterium spinosum extract as an antidiabetic agent: In vitro and in vivo study. J. Ethnopharmacol. 2010, 129, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, K.; Smirin, P.; Sampson, S.R.; Rosenzweig, T. Insulin-sensitizing and insulin-mimetic activities of Sarcopoterium spinosum extract. J. Ethnopharmacol. 2014, 155, 362–372. [Google Scholar] [CrossRef]
- Elyasiyan, U.; Nudel, A.; Skalka, N.; Rozenberg, K.; Drori, E.; Oppenheimer, R.; Kerem, Z.; Rosenzweig, T. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. Bmc Complement. Altern. Med. 2017, 17, 356. [Google Scholar] [CrossRef]
- Rozenberg, K.; Rosenzweig, T. Sarcopoterium spinosum extract improved insulin sensitivity in mice models of glucose intolerance and diabetes. PLoS ONE 2018, 13, e0196736. [Google Scholar] [CrossRef]
- Olaiya, C.O.; Soetan, K.O. A review of the health benefits of fenugreek (Trigonella foenum-graecum L.): Nutritional, Biochemical and pharmaceutical perspectives. Am. J. Soc. Issues Hum. 2014, 1, 3–12. [Google Scholar]
- Goyal, S.; Gupta, N.; Chatterjee, S. Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J. Toxicol. 2016, 2016, 1250387. [Google Scholar] [CrossRef]
- Puri, D.; Prabhu, K.; Dev, G.; Agarwal, S.; Murthy, P. Mechanism of antidiabetic action of compound GII purified from fenugreek (Trigonella foenum graecum) seeds. Indian J. Clin. Biochem. 2011, 26, 335–346. [Google Scholar] [CrossRef][Green Version]
- Anwer, T.; Sharma, M.; Pillai, K.K.; Iqbal, M. Effect of Withania somnifera on Insulin Sensitivity in Non-Insulin-Dependent Diabetes Mellitus Rats. Basic Clin. Pharmacol. Toxicol. 2008, 102, 498–503. [Google Scholar] [CrossRef]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, R.; Kasthurirengan, S.; Mariashibu, T.S.; Rajesh, M.; Anbazhagan, V.R.; Kim, S.C.; Ganapathi, A.; Choi, C.W. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009, 10, 2367–2382. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Sharma, N.; Dhaliwal, H.S.; Sharma, V. A systematic and comprehensive review on Withania somnifera (L.) dunal-an indian ginseng. J. Pharm. Res. Int. 2015, 7, 63–75. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef]
- Jaradat, N. Ethnopharmacological survey of natural products in palestine. An-Najah Univ. J. Res. 2005, 19, 1880. [Google Scholar]
- Ali-Shtayeh, M.; Yaghmour, R.M.-R.; Faidi, Y.; Salem, K.; Al-Nuri, M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M. Herbal medicines in cancer care in the Palestinian authority. Eur. J. Integr. Med. 2011, 3, 125–131. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Shawahna, R.; Eid, A.M.; Al-Ramahi, R.; Asma, M.K.; Zaid, A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016, 178, 1–8. [Google Scholar] [CrossRef]
- Kaileh, M.; Berghe, W.V.; Boone, E.; Essawi, T.; Haegeman, G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J. Ethnopharmacol. 2007, 113, 510–516. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Zaid, A.N.; Al-Ramahi, R.; Alqub, M.A.; Hussein, F.; Hamdan, Z.; Mustafa, M.; Qneibi, M.; Ali, I. Ethnopharmacological survey of medicinal plants practiced by traditional healers and herbalists for treatment of some urological diseases in the West Bank/Palestine. BMC Complement. Altern. Med. 2017, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.U.; Sreenivasulu, M.; Chengaiah, B.; Reddy, K.J.; Chetty, C.M. Herbal medicines for diabetes mellitus: A review. Int. J. Pharmtech. Res. 2010, 2, 1883–1892. [Google Scholar]
- Nathiya, S.; Durga, M.; Devasena, T. Therapeutic role of Trigonella foenum-graecum [Fenugreek]–a review. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 74–80. [Google Scholar]
- Kulkarni, S.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Kadan, S.; Sasson, Y.; Saad, B.; Zaid, H. Gundelia tournefortii antidiabetic efficacy: Chemical composition and GLUT4 translocation. Evid. Based Complement. Altern. Med. 2018, 2018, 8294320. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crop. Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Zengin, G.; Guler, G.O.; Aktumsek, A.; Ceylan, R.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medicinal plants from Turkey. Adv. Pharmacol. Sci. 2015, 2015, 410675. [Google Scholar] [CrossRef]
- Orhan, N.; Hoçbaç, S.; Orhan, D.D.; Asian, M.; Ergun, F. Enzyme inhibitory and radical scavenging effects of some antidiabetic plants of Turkey. Iran. J. Basic Med. Sci. 2014, 17, 426. [Google Scholar]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crop. Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Topal, M.; Bingol, Z.; Cetin Çakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22, 1511–1526. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Zengin, G.; Aladag, M.O.; Ozparlak, H.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Aumeeruddy, M.Z. HPLC-MS/MS chemical characterization and biological properties of Origanum onites extracts: A recent insight. Int. J. Environ. Health Res. 2019, 29, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop. Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. Ijpr 2013, 12, 367. [Google Scholar]
- Kültür, Ş. An ethnobotanical study of Kırklareli (Turkey). Phytol. Balc. 2008, 14, 279–289. [Google Scholar]
- Yücel, E.; Özel, A.N. The plants consumed as food in Kemaliye (Erzincan/Turkey) district and other typical foods in this region. 2013. Biol. Divers. Conserv. 2013. [Google Scholar]
- Özbel, Y.; Özbilgin, A. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol. 2007, 30, 439–445. [Google Scholar]
- Bagci, E.; Elkiran, O.; Evren, H. Constituents of the essential oils of Helichrysum graveolens (Bieb.) Sweet from Turkey. Asian J. Chem. 2013, 25, 7254. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yeşilada, E. A study of antidiabetic and antioxidant effects of Helichrysum graveolens capitulums in Streptozocin-induced diabetic rats. J. Med. Food 2007, 10, 396–400. [Google Scholar] [CrossRef]
- Ugulu, I. Traditional ethnobotanical knowledge about medicinal plants used for external therapies in Alasehir, Turkey. Int. J. Med. Arom. Plants 2011, 1, 101–106. [Google Scholar]
- Kupeli, E.; Orhan, I.; Yesilada, E. Evaluation of some plants used in Turkish folk medicine for their anti-inflammatory and antinociceptive activities. Pharm. Biol. 2007, 45, 547–555. [Google Scholar] [CrossRef]
- Baser, K.; Kürkçüoglu, M.; Tarimcilar, G.; Kaynak, G. Essential oils of Mentha species from Northern Turkey. J. Essent. Oil Res. 1999, 11, 579–588. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Eryigit, F.; Cengiz, M.; Tepe, B.; Cakir, A.; Mete, E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectrosc. Lett. 2012, 45, 352–358. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Şüküroğlu, M.; Orhan, D.D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC–TOF-MS analysis. J. Ethnopharmacol. 2013, 146, 859–865. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Pekcan, M.; Orhan, D.D.; Bedir, E.; Ergun, F. Identification of hypoglycaemic compounds from berries of Juniperus oxycedrus subsp. oxycedrus through bioactivity guided isolation technique. J. Ethnopharmacol. 2012, 139, 110–118. [Google Scholar] [CrossRef]
- Orhan, N.; Orhan, D.D.; Gökbulut, A.; Aslan, M.; Ergun, F. Comparative Analysis of Chemical Profile, Antioxidant, In-vitro and In-vivo Antidiabetic Activities of Juniperus foetidissima Willd. and Juniperus sabina L. Iran. J. Pharm. Res. Ijpr 2017, 16, 64. [Google Scholar]
- Orhan, N.; Aslan, M.; Demirci, B.; Ergun, F. A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves. J. Ethnopharmacol. 2012, 140, 409–415. [Google Scholar] [CrossRef]
- Orhan, N.; Berkkan, A.; Orhan, D.D.; Aslan, M.; Ergun, F. Effects of Juniperus oxycedrus ssp. oxycedrus on tissue lipid peroxidation, trace elements (Cu, Zn, Fe) and blood glucose levels in experimental diabetes. J. Ethnopharmacol. 2011, 133, 759–764. [Google Scholar]
- Dehghan, H.; Sarrafi, Y.; Salehi, P.; Ebrahimi, S.N. α-Glucosidase inhibitory and antioxidant activity of furanocoumarins from Heracleum persicum. Med. Chem. Res. 2017, 26, 849–855. [Google Scholar] [CrossRef]
- Afrisham, R.; Aberomand, M.; Ghaffari, M.A.; Siahpoosh, A.; Jamalan, M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of α-amylase. J. Bot. 2015, 2015, 824683. [Google Scholar]
- Kiliçgün, H.; Korkmaz, M.; Kılıçgün, H. Hepatoprotective and antidiabetic activity of origanum minutiflorum grown wild in Turkey. Bothalia J. 2014, 44, 3. [Google Scholar]
- Cam, M.E.; YILDIZ, S.; ERTAŞ, B.; ACAR, A.E.; TAŞKIN, T.; Kabasakal, L. Antidiabetic effects of Salvia triloba and Thymus praecox subsp. skorpilii var. skorpilii in a rat model of Streptozocin/nicotinamide-induced diabetes. Marmara Pharm. J. 2017, 21, 818–827. [Google Scholar] [CrossRef]
- Durmuşkahya, C.; Öztürk, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malays. 2013, 42, 1431–1438. [Google Scholar] [CrossRef]
- Tumen, I.; Süntar, I.; Keleş, H.; Küpeli Akkol, E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid. Based Complement. Altern. Med. 2012, 2012, 728281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cetin, H.; Yanikoglu, A. A study of the larvicidal activity of Origanum (Labiatae) species from southwest Turkey. J. Vector Ecol. 2006, 31, 118–122. [Google Scholar] [CrossRef]
- Doğan, A.; Çelik, İ. Healing effects of sumac (Rhus coriaria) in Streptozocin-induced diabetic rats. Pharm. Biol. 2016, 54, 2092–2102. [Google Scholar]
- Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Goto, K. Traditional medicine in Turkey. I: Folk medicine in Northeast anatolia. J. Ethnopharmacol. 1991, 35, 191–196. [Google Scholar] [CrossRef]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Azaz, A.D.; Irtem, H.A.; Kurkcuoǧlu, M.; Baser, K.H.C. Composition and the in vitro antimicrobial activities of the essential oils of some Thymus species. Z. Für Nat. C 2004, 59, 75–80. [Google Scholar] [CrossRef]
- Chaouche, T.M.; Haddouchi, F.; Atik-Bekara, F.; Ksouri, R.; Azzi, R.; Boucherit, Z.; Tefiani, C.; Larbat, R. Antioxidant, haemolytic activities and HPLC–DAD–ESI–MSn characterization of phenolic compounds from root bark of Juniperus oxycedrus subsp. oxycedrus. Ind. Crop. Prod. 2015, 64, 182–187. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; El-Eraky, W.; Ibrahim, M.; Mabry, T. Antiinflammatory and ulcerogenic activities of Salvia triloba extracts. Fitoterapia 2006, 77, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, S.; McDaniel, T. Floras of the Middle East: A quantitative analysis and biogeography of the flora of Iraq. Edinb. J. Bot. 2016, 73, 1–24. [Google Scholar] [CrossRef]
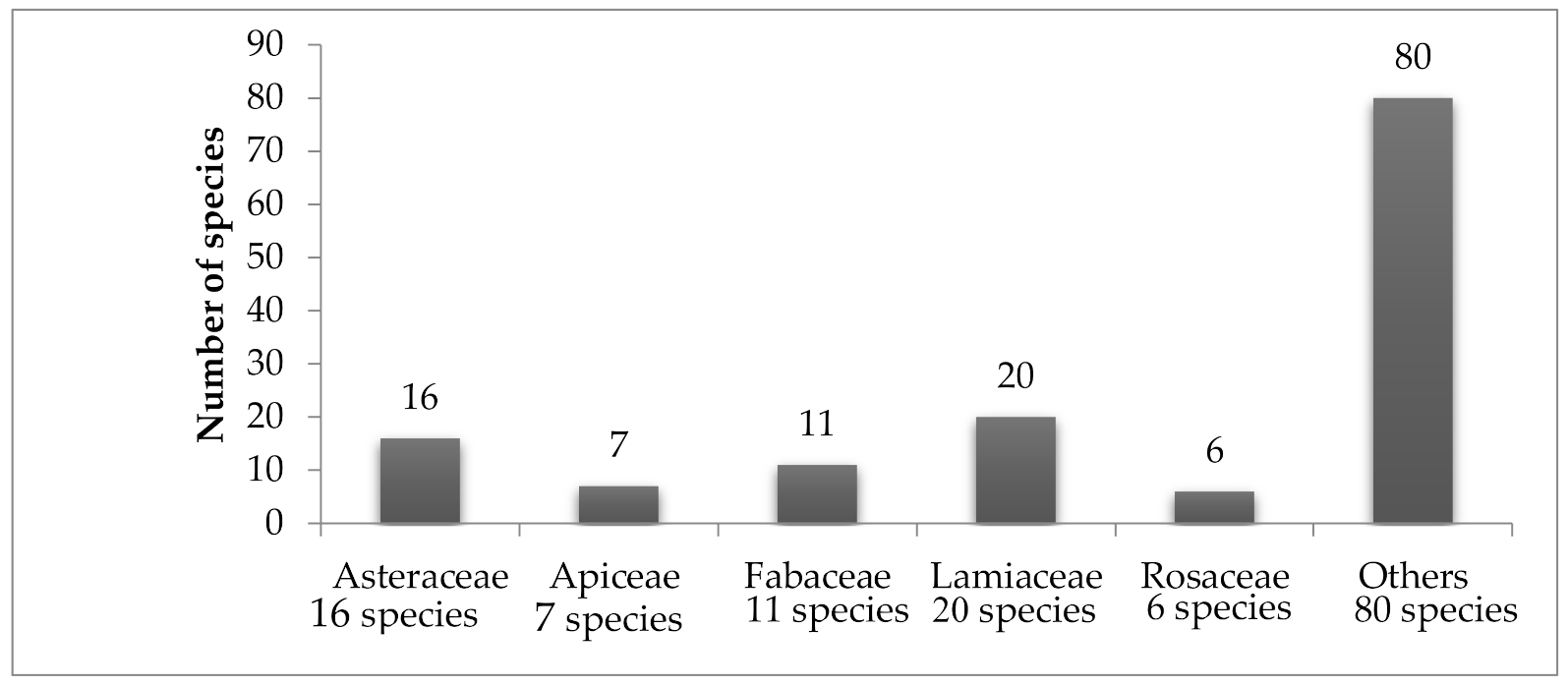

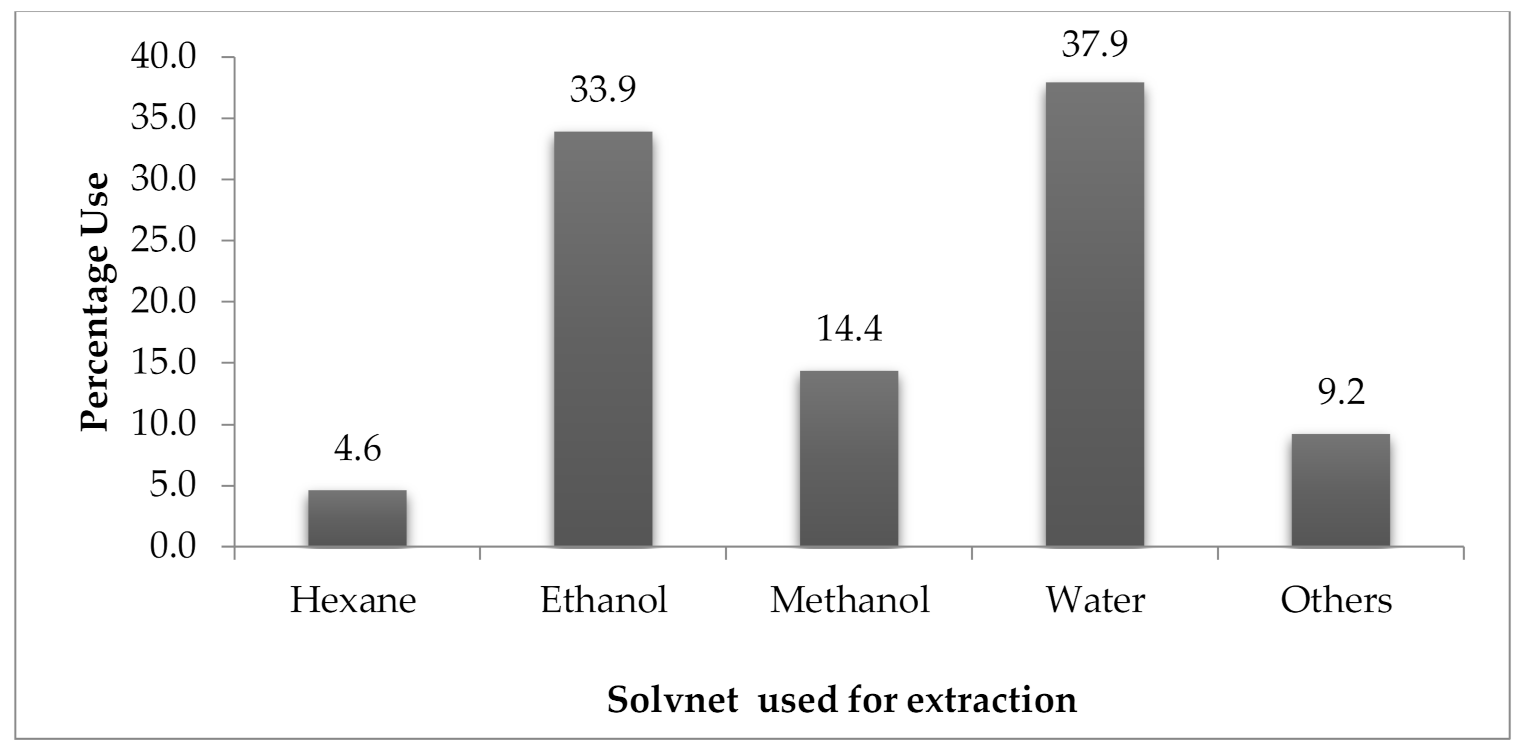
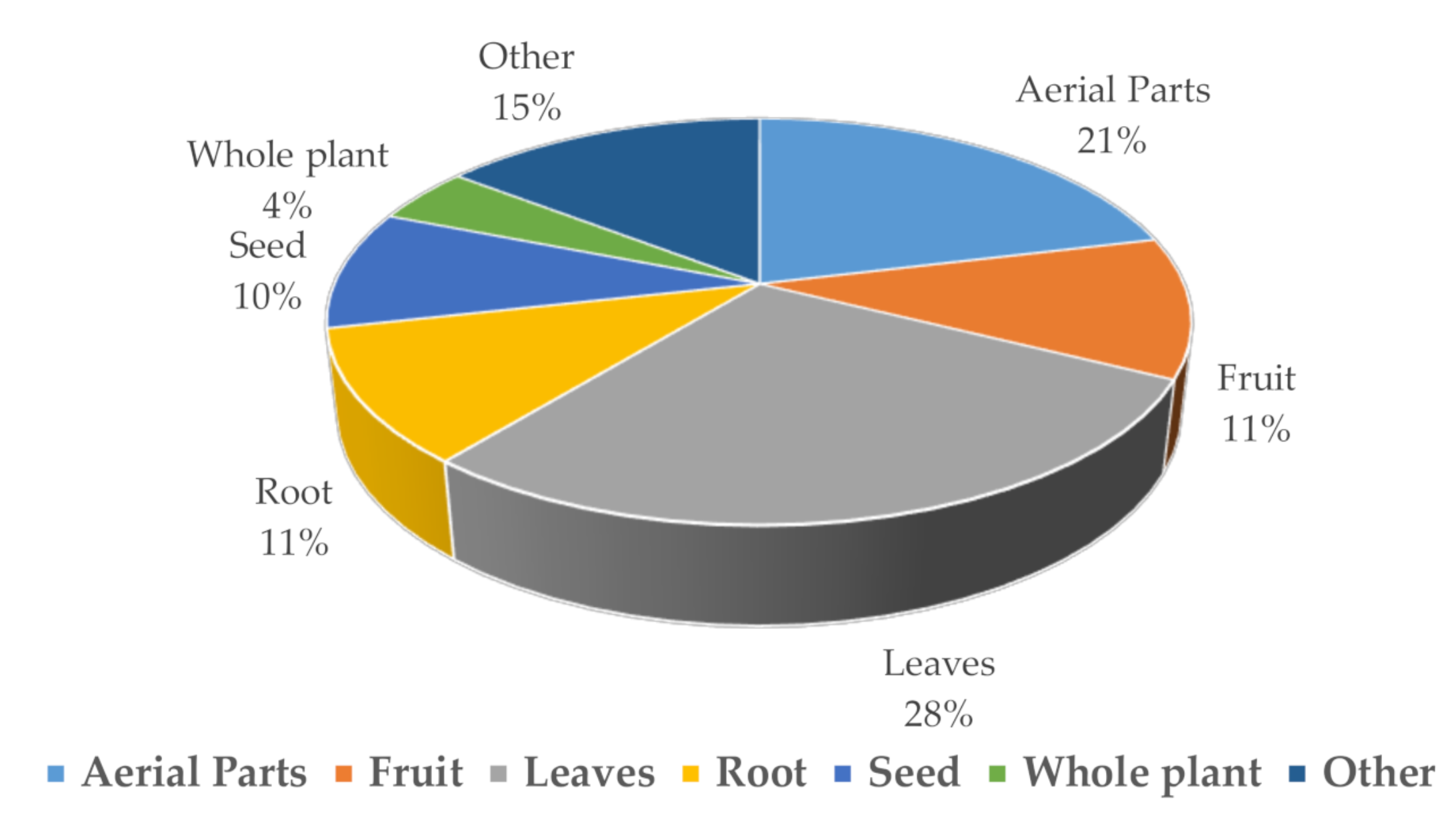
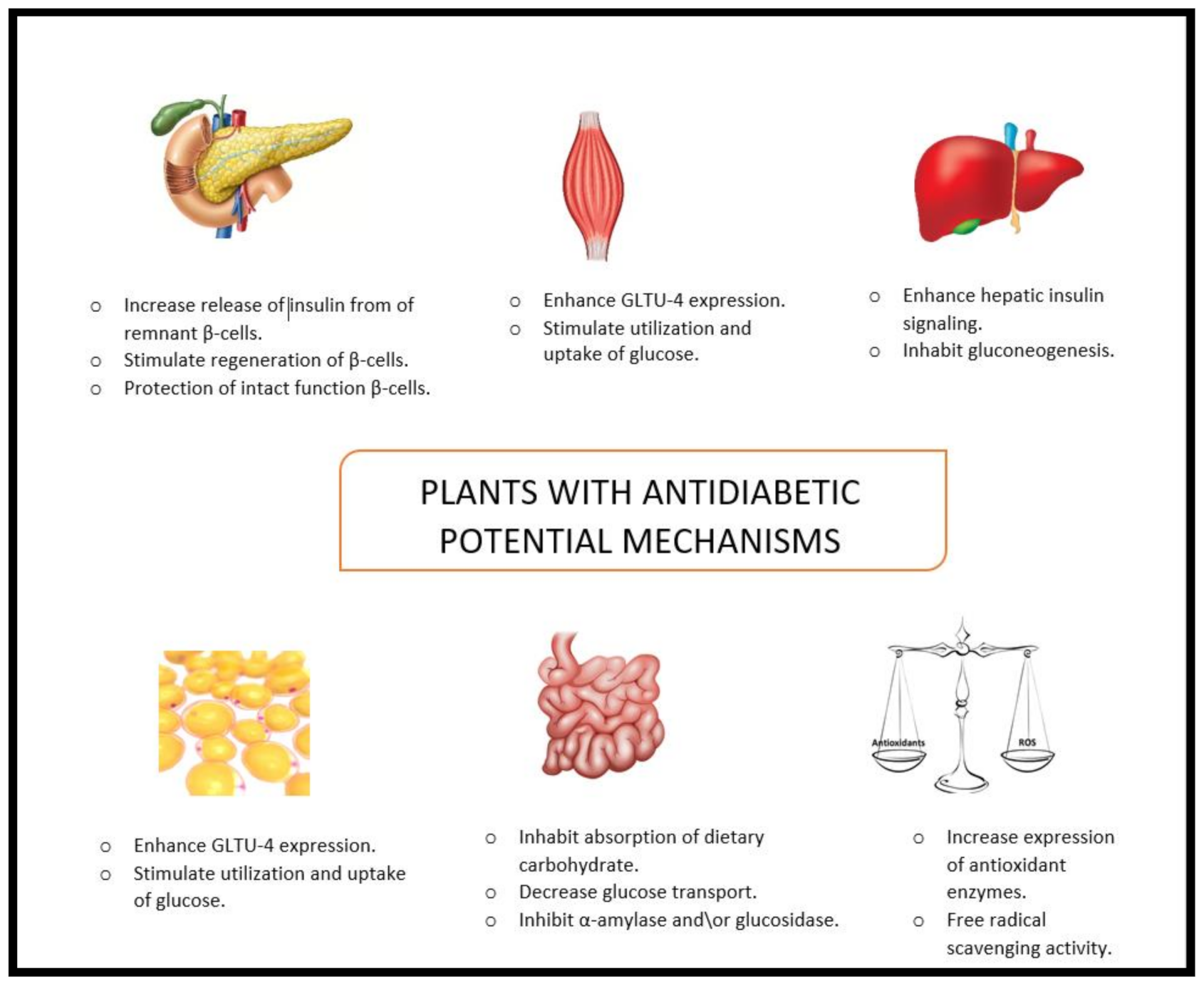
| County | Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|---|
| Oman | Ajuga iva | Chendgoura | Lamiaceae | Anthelmintic, analgesia, diuretic agent, diabetes | Leaf, stem | [71,72] |
| Moringa peregrina | Shua | Moringaceae | Convulsions or infantile paralysis, diabetes | Pod oil, seed | [17,73] | |
| Rhayza stricta | Harmal | Apocynaceae | Bad breath, chest pain, conjunctivitis, constipation, diabetes, fever, skin rash, anthelmintic, increase lactation | Seed, whole plant | [74] | |
| Qatar | Cynomorium coccineum | Tarthuth | Cynomoriaceae | The roots are edible and were sold in earlier times as a vegetable (Qatar) used as an aphrodisiac in Bahrain | Flower, root | [75] |
| Saudi Arabia | Avicennia marina | Shoura | Avicenniaceae | Smallpox, sores, pruritic, induce women infertility, diabetes | Branches | [76] |
| Caralluma sinaica | DedElkalba | Asclepiadaceae | Anticancer, diabetes, leprosy, obesity, rheumatism | Leaf | [77,78,79] | |
| Ducrosia anethifolia | Not mentioned | Apiaceae | Analgesic, anxiety, backache, carminative, colic, cold, galactogogue, insomnia, pain reliever for headache, useful for irregularities of menstruation (Iranian folk medicine) | Aerial parts, seed, whole herb | [80] | |
| Jatropha curcas | Kharat | Euphorbiaceae | Ailments related to skin, cancer, digestive disease, diabetes, infectious disease, respiratory disease | Barkk, fruit, latex, leaf, root, seed | [79,81] | |
| Loranthus acaciae | Not mentioned | Loranthaceae | Antipyretic, cancer, colds, cosmetic, ear aches, headache, hypertension, mosquito Repellent, obesity, rheumatoid diseases, skin infections | Leaf | [82,83] | |
| Lycium shawii | Awsaj | Solonaceae | Diabetes, hypotensive agent | Flower, shoot | [39,84] | |
| Marrubiumvulgare | Not mentioned | Lamiaceae | Return to reference | Leaves, flowers, stem | [85] | |
| Moringa oleifera | Getha | Moringaceae | Diabetes, ascites, splenic enlargement, inflammatory swellings, abdominal tumors, colic, dyspepsia, fever, ulcers, paralysis, lumbago, skin diseases | Fruit | [86] | |
| Ocimum forskolei | Basil | Lamiaceae | Cosmetic, digestive agent, spasm, mosquito’s repellent factor, relieves fever, skin infections | Leaf | [45,87] | |
| Saudi Arabia | Plicosepalus curviflorus | EnamElTalh | Loranthaceae | Cancer, diabetes | Stem | [46] |
| Retama raetam | Al-retem | Fabaceae | Diabetes | Fruit | [88] | |
| Rhizophora mucronata | Kindale | Rhizosphoraceae | Elephantiasis, hematoma, hepatitis, ulcers, diarrhea or gastric motility disorder, diabetes | Bark, branches, flower, fruit, leaf | [76,89] | |
| Salvadora persica | Miswak | Salvadoraceae | Oral and dental cleaning tool | Bark | [49] | |
| Yemen | Azadirachta indica | Neem | Meliaceae | Diabetes, GIT disorder, general health promoter, leprosy, respiratory disorders | Leaf, bark | [90] |
| Boswellia carterii | Olibanum | Burseraceae | Anti-inflammatory, bruises, infected sores psychoactive, reduce the loss of blood in the urine from schistosomiasis infestation, tranquilizer, wound healing, various non-pharmaceutical applications | Resin | [58,91] | |
| Cissus rotundifolia | Arabian wax | Vitaceae | Appetizer, antipyretic carbuncles, dengue fever, malaria, rheumatic pain, snake bites | Leaf, root, stem, | [58] | |
| Dracaena cinnabari | Damm Al-Khwain | Agavaceae | Burn, control of bleeding, diarrhea, fractures, fevers, healing of fractures, hemorrhage, pain, stimulation of circulation, sprains, ulcers, wounds | Resin | [63,92] | |
| Opuntia ficus-indica | Cactus | Cactaceae | Allergy, analgesic, anti-inflammatory, dyspnoea, fatigue, glaucoma, gastric ulcer, health supporting nutrient, healing wounds, hypoglycemic agent, liver diseases, prostate cancer, urological problems | Flower, fruit | [93] | |
| Yemen | Pulicaria inuloides | False fleabane | Asteraceae | Anthelmintic, carminative, cold, diuretic, fever, inflammation, insect repellent, pain, intestinal disorder, pyritic conditions in urogenetic organs | Flowers | [94,95] |
| Solenostemma argel | Argel | Asclepiadaceae | Diabetes, cardiovascular disorders, gastrointestinal problems, kidney and liver diseases, pain, respiratory tract infections, urinary tract infections | Bark, leaf, stem | [69,96] |
| Country | Name | Chemical Constituents | Scientific Reports | Reference |
|---|---|---|---|---|
| Oman | Ajuga iva | Anthocyanins, ecdysteroids, flavones, glycosides, terpenes; diterpenes, triterpenes, tannins, withanolides | Antibacterial, antifungal, antihypertensive, antimycobacterial, antiplasmodial, hypoglycaemic, larvae and insect antifeedant activity | [12,16,97,98] |
| Moringa | Flavonoid, isothiocyanate, glycosides, phytosterol, polyphenol, triterpene, volatile oil | Anticancer, antioxidant, antimicrobial, antidiabetic, anti-inflammatory, anti-spasmodic, hypertension, hepatotoxicity, lipid lowering activity, memory disorders | [17] | |
| Rhazya stricta | Indole alkaloid, flavonoids, carbolines, alkaloids with β-carboline nucleus (akuammidine, rhazinilam tetrahydrosecamine), triterpenes, tannins, volatile bases | Antioxidant, blood pressure, diabetes mellitus, immunomodulation effect | [22,99] | |
| Qatar | C. coccineum | Flavonoids, organic acids, scharrides, lipids | Anticancer, antidiabetic, antioxidant, anti-tyrosinase antimicrobial, cardioprotective, immunity-improving, neuroprotective, increase and folliculogenesis, testicular development, spermatogenesis | [100,101] |
| Saudi Arabia | Avicennia marina | Alkaloids, glycosides, flavonoids, phenols, saponins, tannins, terpenoids | Antidiabetic, anti-inflammatory, antimicrobial, antioxidant, antiviral | [102] |
| Caralluma sinaica | Coumarin, flavonoids, glycosides, phenolic alkaloids, steroids, tannins | Antidiabetic agent, antimicrobial | [78] | |
| Ducrosia anethifolia | Monoterpenes hydrocarbons(essential oil), coumarins | Anticancer, antidiabetic, anti-inflammatory, antimicrobial, anxiolytic, radical scavenging | [80,103] | |
| Saudi Arabia | Jatropha curcas | C yclic peptide alkaloids, flavonoid, lignans, saponins, terpenes, diterpenoids | Antioxidant, anticancer, anti-inflammatory, gastroprotective, anthelmintic, antidiarrhea activity, antiulcer | [103,104,105,106] |
| Loranthus acaciae | Alkaloid, cardiac glycosides, flavonoids saponins tannins, terpenoids, catechin | Analgesic, antidiabetic, anti-inflammatory, antimicrobial, antitumor, antioxidant, antihepatotoxic, antiviral | [38,107] | |
| Lycium shawii | Alkaloids, flavonoids, phenolics, tannins, saponins, glycosides, terpenoids, steroids, coumarins | Antioxidant, diuretic, laxative, anticancer, antimicrobial anti-inflammatory, tonic agent | [108] | |
| Marrubiumvulgare | Flavonoid, phenylpropanoids, terpenes, sesqui and diterpenes | Antihypertensive, analgesic, anti-inflammatory, antioxidant | [85] | |
| Moringa oleifera | Carotenoids, glucosinolates, flavonoids, phenolic acids, polyunsaturated fatty acids, tocopherols | Antidyslipidemic, anthelmintic, antihyperglycemic, anti-inflammatory, antimicrobial, antioxidant, apoptotic properties, antiproliferative, anti-ulcer, antiurolithiatic, hepatoprotective | [109] | |
| Morus nigra | Alkaloids, flavonoids, phenols | Antihyperlipidemic, antidepressant, antioxidant, neuroprotective | [110] | |
| Saudi Arabia | Ocimum forskolei | Alkaloids, essential oils, flavonoids, glycosides, phenylpropanoids, saponins, steroids, tannins | Activities against bacteria and dermatophytes, antihyperglycemic, nematicidal activity, weak antioxidant | [87,111] |
| Plicosepalus curviflorus | Flavonol rhamnosides, sesquiterpene lactones | Antihepatotoxic, antidiabetic, cytotoxic activities | [112] | |
| Retama raetam | Flavonoid, quinolizidine alkaloids | Antibacterial, antihyperlipidemic, antihypertensive, antioxidant, diuretic, hypoglycemic. | [48] | |
| Rhizophora mucronata | Alkaloids, anthocyanidins, anthraquinone, carotenoids, catechin, flavonoids, phenolics, saponin, steroids, triterpene | Antimicrobial, antioxidant, hepatoprotective | [89,102,113] | |
| Salvadora persica | Essential oil, organosulphur compounds, saponin, β-sitosterol | Anticonvulsant, antifertility, antimicrobial, analgesic, antiplaque, aphrodisiac, antipyretic, antiulcer, astringent, diuretic, hypolipidemic, stomachic activities. | [49,114] | |
| Yemen | Azadirachta indica | Alkaloid, aromatic compound, flavonoid, flavone, isoprenoidssesquiterpenes, terpenes;triterpenoids tetraanortriterpenoid, saponins, limonoids | Antibacterial, antidiabetic, antifertility, antihypercholesteremic. antimalarial, antioxidant, antiulcer, anti-tumor | [9,58] |
| Boswellia carterii | Pentacyclic triterpenoid, volatile oil | Anti-inflammatory, neuroprotective. | [115,116,117] | |
| Yemen | Cissus rotundifolia | Steroids, flavonoids, β-sitosterol | Analgesic, antidiabetic, anti-inflammatory, antiparasitic, antiulcerative, antioxidant, hepatoprotective activity | [62,118,119] |
| Dracaena cinnabari | Flavone, chalcone | Antidiarrheal, anti-hemorrhagic, anti-inflammatory antimicrobial, antiviral activity, antitumor activity, antiulcer, immunomodulatory, antioxidant | [92] | |
| Opuntia ficus-indica | Flavonoid glycoside, oxygenated monoterpenes, sesquiterpene hydrocarbons, aldehydes with non-terpenic structure | Antioxidant, antiulcer, cardioprotective, hepatoprotective | [120,121] | |
| Pulicaria inuloides | Essential oil, monoterpenes, diterpenes, sesquiterpene lactones and caryophyllane derivatives | Antimicrobial, antifungal, antimalaria, insecticides properties | [94,95] | |
| Solenostemma argel | Phenolic acids, flavones, glycosylated flavonoids, polyphenols, β-carotene, β-sitosterol, terpenes, mono and triterpenes | Antibacterial, antifungal, antioxidant | [69,96,122,123] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Ajuga iva | Root, stem, leaf | Ethanol, maceration | Streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Moringa pergrina | Leaf | Chloroform, soxhlet | Mice | 1 mL of 0.5%, 1%, 1.5%, or 2%, 21 days | Weight reduction, influenced the reproductive system | [21] |
| Pteropyrum scoparium | Leaf, root, stem | Ethanol, maceration | streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Rhazya stricta | Root, stem, leaf | Ethanol, maceration | Streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Cynomorium coccineum | Inner flesh of stem | Water, reflux | In vitro | Antioxidant activity associated with angiotensin converting enzyme | [27] | |
| Avicennia marina | Leaf | Ethanol, maceration | Streptozocin diabetic rats | 2 mg/gm, 4 weeks | Protected liver, improved the neurobehavioral changes, decreased inflammatory cells aggregation, vacuolation, and hemorrhage. | [30] |
| Leaf | Water, decoction | Streptozocin diabetic rats | 400 mg/kg, 6 weeks | Significant increase in the muscle levels of CAT, SOD and glutathione | [31] | |
| Caralluma sinaica | Aerial parts, root | Ethanol (80%), maceration | Streptozocin diabetic rats | 100 mg/kg, 30 days | Reversed streptozocin effect on glycogen content | [33] |
| Ducrosia anethifolia | Aerial parts | Ethanol (80%),maceration | Streptozocin diabetic rats | 500 mg/kg, 45 days | Normalized liver enzymes, total protein, lipid, cholesterol levels, antioxidant markers, glycolytic, and elevated level of kidney biomarkers | [34] |
| Jatropha curcas | Aerial parts | Ethanol (96%), chloroform, hexane, maceration | Alloxan diabetic mice | 400 mg/kg, 1 day | Safe up to dose of 5 g/kg | [124] |
| Loranthus acaciae | Leaf, stem | Ethanol, soxhlet | Alloxan diabetic rats, normal rats | 500 mg/kg, 1 week | LD50 of the extract and its fractions was more than 5 g/kg, a potent anti-inflammatory and antioxidant effect was detected for the chloroform fraction | [38] |
| Lycium shawii | Aerial parts | Ethanol, decoction | Alloxan diabetic rats, normal rats | 500 mg/kg, o.p or i.p, 90 days | None of the mice died up to 3 g/kg dose. Prolonged L. shawii treatment is toxic. | [39] |
| Marrubium vulgare | Aerial parts | Methanol, maceration | Streptozocin diabetic rats | 500 mg/kg, 28 days | Significant reduction in plasma TC, TG, LDL, increased HDL | [40] |
| Moringa oleifera | Seed | Powder | Streptozocin diabetic rats | 50, 100 mg/kg, 4 weeks | Restoration of the normal histology of both kidney and pancreas | [42] |
| Morus nigra | Leaf | Ethylalcohol, maceration | Streptozocin diabetic rats | 500 mg/kg, 10 days | Significant increase in insulin level | [43] |
| Ocimum basilicum | Leaf | Water, infusion | In vitro | 20, 18.2, 16.3, 14.5 mg/mL | Significant dose-dependent inhibition of sucrase, maltase and pancreatic α-amylase | [125] |
| Ocimum forskolei | Leaf, stem | Methanol (70%), maceration | In vitro | 10, 20, 30 mg/mL | The inhibitory activity (IC50) of both leaf and stems methanol extracts are almost the same | [45] |
| Plicosepalus curviflorus | Leaf | Serial exhaustive extraction, maceration | Mice | Extract: 500 mg/kg, i.p Isolated compounds 50, 100 mg/kg, i.p | The mixture of the isolated compounds synergized with each other. | [45] |
| Whole plant | Methanol, maceration | High-fat diet followed by injection of streptozocin diabetic rats | Extract or solid lipid nanoparticles: 250 mg/kg, 4 weeks | The SLN preparation with the highest lipid content gave the best result of reduction of hyperglycemia and insulin resistance | [45] | |
| Retama raetam | Fruit | Methanol, maceration | Streptozocin diabetic rats | 100, 250, 500 mg/kg, 4 weeks | The extract significantly inhibits glucose absorption by rat isolated intestine | [48] |
| Rhizophora mucronata | Leaf | Water | Streptozocin diabetic rats | 400 mg/kg, 6 weeks | Significant increase in the muscle levels of CAT, GSH and SOD | [31] |
| Salvadora persica | Root | Water, soxhlet | Streptozocin diabetic rats | 250, 500 mg/kg, 21 days | Significant decrease in TC, TG, LDL, VLDL, increase in HDL | [49] |
| Azadirachta indica | Leaf | Water, percolation | Alloxan diabetic rabbits | 200, 400 mg/kg, 25 days | Decrease in serum TG, cholesterol, LDL, increase in HDL | [51] |
| Boswellia carterii | Resin | Water, decoction | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Decrease in the serum cholesterol and TG | [58] |
| Cissus rotundifolia | Aerial parts | Water, maceration | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Decrease in serum cholesterol and TG | [58] |
| Leaf | Water, maceration | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Significant decrease in urea, ALT, AST | [62] | |
| Dracaena cinnabari | Resin | Ethanol 99%, maceration | Alloxan diabetic rats | 100, 300 mg/kg, 14 days | Antihyperlipidemic effect | [64] |
| Dracaena cinnabari | Resin | Serial exhaustive extraction, soxhlet | In vitro MCF-7 cell line | 100 μg/mL | Glucose uptake inducing activity of ethylacetate extract was found to be higher than Metformin | [63] |
| Opuntia ficus-indica | Seed | Hexane, p-ether, chloroform, maceration | Streptozocin-diabetic rats | 0.2, 0.4, 0.6 g/kg, twice daily, 21 days | Reducing the expression of the PCK1 gene while increasing the expression of Slc2a2 gene in the liver tissue. | [66] |
| Pulicaria inuloides | Leaf | Oil extract, stem distillation | Streptozocin diabetic rats | 400 mg/kg, 21 days | Inhibitory effect on α-glycosidase and α-amylase | [67] |
| Solenostemma argel | Leaf | Methanol, maceration | Methylprednisol-one diabetic rats | 1 gm/kg, 14 days | Antioxidant activity | [69] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cassia acutifolia | Not mentioned | Fabaceae | Constipation | Not mentioned | [141] |
| Centaurea alexanderina | Not mentioned | Asteraceae | Antimalarial, bitter tonic, diuretic, mild astringent, stomachic | Not mentioned | [130] |
| Fraxinus ornus | HabElderdar | Oleaceae | Diabetes | Not mentioned | [129] |
| Moringa peregrina | Hadendowa | Moringaceae | Edible, fever, headache, earache, burns, disinfectant, adenopathy, skin disorders, itching, soothe rash, purify water, wound, cancer, laxative, cathartic, malnutrients, ascites, leprosy, swellings | Flower, leaf, root, seed | [142,143] |
| Nepeta Cataria | Qatram, hashishat al-her (Arabic name) | Lamiaceae | Asthma, bronchitis, cough, diarrhea, gastrointestinal, respiratory hyperactivedisorders | Leaf | [135,144] |
| Phoneix dactylifera | Not mentioned | Arecaceae | Aphrodiasiac, tonic | Pollen, male flower | [145] |
| Securigera securidaca | Not mentioned | Fabaceae | Antidiabetic, antihyperlipidemic | Seed | [146] |
| Trigonella stellate | Not mentioned | Fabaceae | Dantidiabetic, antihyperlipidemic | Not mentioned | [137] |
| Urtica pilulifera | Nettle (Palestine) | Urticaceae | A diuretic, antiasthmatic, anti-inflammatory, hypoglycemic, hemostatic, antidandruff and astringent | Whole plant | [138,147] |
| Zizyphus spina-christi | Nabka, Seder (Palestine) | Rhamnaceae | Swellings, pain, and heat, eye inflammation, constipation, heartburn, diarrhea, wound, diuretic, liver problems, anus problems | Leaf, root, seed | [148] |
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Cassia acutifolia | Anthraquinone, phenolic glycoside | Laxative | [149,150] |
| Centaurea alexanderina | Sesquiterpene lactones, flavonoids | Hypoglycemia, cytotoxicity | [130] |
| Cyperus laevigatus | Quinones, flavonoids, sesquiterpenes, Steroids, essential oils | Anti-inflammatory, hepatoprotective, gastroprotective, antimalarial, antidiabetic activities | [131] |
| Fraxinus ornus | Alkaloid, coumarins, flavonoid, phenylethanoids, secoiridoid, tannin | Antimicrobial, antioxidant, anti-inflammatory | [129] |
| Moringa peregrina | Flavonoid | Antioxidant | [151] |
| Nepeta cataria | Essential oil, urosolic acid, β-sisoterol, campesterol, α-amyrin, β-amyrin, neptalactones, alkaloids, carenolides, tannins, saponins, coumarins | Antibacterial, antifungal, analgesic | [135,152] |
| Phoneix dactylifera | Phenolic acid, sterol, carotenoid, flavonoid, procyanidins, anthocyanins | Immune system, nephroprotective, hepatoprotective, gastrointestinal protective, antioxidant, antihyperlipidemic activity, anticancer | [145] |
| Securigera securidaca | Alkaloid, cardiac glycosides, coumarins flavonoid, saponins, tannins | Antihyperlipidemic, chronotropic, diabetes, diuretic, gastroprotective | [153] |
| Trigonella stellate | Flavonoids; isoflavonoid, phenolic compounds | Antihyperlipidemic, antioxidant | [137] |
| Urtica pilulifera | Lectins, b-sitosterol, phenolic acids triterpene | Antitumor, astringent, asthma, antidandruff, diuretic, diabetes, deputative, galactogogue | [154] |
| Zizyphus spina-christi | Triterpenoid saponin glycosides, polyphenol, tannin, flavonoid, cyclopeptides, cardiac glycoside, essential oil, alkaloid | Antinociceptive, antioxidant, antifungal, antibacterial, antidiabetic | [139,140] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Cassia acutifolia | Leaf | Ethanol 80%, maceration | Nicotinamide-Streptozocin diabetic mice | 10, 50 mg/kg, 1 week | No effect on serum insulin level | [129] |
| Crataegus aronia | Whole plant | Water, maceration | High-fat diet with small dose of streptozocin diabetic rats | 500 mg/kg, 60 days | Lowered serum lipid levels, hepatic glycogen, hepatic lipid peroxidation, tumor necrosis factor α, interleukin, enhanced the level of reduced glutathione, superoxide dismutase, hepatic mRNA expression of the insulin receptor A isoform, glucose 6-phosphatase | [155] |
| Centaurea alexanderina | Leaf | Methanol 80%, reflux | Streptozocin diabetic rats | 600 mg/kg, 60 days | Anti-inflammatory activity was seen for the extract | [130] |
| Citrullus colocynthis | Seed | Ethanol | Alloxan diabetic rats | 50 mg/kg, 8 weeks | Decrease in lipid peroxidation, total cholesterol, triglyceride, total, direct bilirubin, increase on glutathione, lactate dehydrogenase, ALT, AST, ALP, total lipid were significantly increased in both normoglycemic and hyperglycemic rats | [156] |
| Cyperus laevigatus | Aerial parts | Methanol 70%, maceration | Streptozocin diabetic rats | 50mg/kg, 14 days | Decreasing levels of NO, glucagon, promoted paraoxonase activity | [131] |
| Fraxinus ornus | Fruit | Ethanol 80%, maceration | Nicotinamide-Streptozocin diabetic mice | 10, 50 mg/kg, 1 week | No effect on insulin serum level | [129] |
| Moringa oleifera | Leaf | Ethanol, maceration | Alloxan diabetic rats | 150 mg/kg, 21 days | Quercetin has the greatest potential antidiabetic activity in the extract, followed by chlorogenic acid and moringinine | [157] |
| Moringa peregrina | Aerial parts | Ethanol 95%, percolation | Streptozocin diabetic rats | Extracts: 25 mg/kg, fractions: 50 mg/kg, 1 day | The n-hexane fraction was the only fraction that showed a highly significant antihyperglycemic activity | [18] |
| Seed | ethanol 70%, soxhlet | Streptozocin diabetic rats | 150 mg/kg, 30 days | The extracts exerted protective effects against lipid peroxidation ethanolic extract > aqueous extract | [19] | |
| Nepeta cataria | Flowering aerial parts, seed | P-ether, ethyl acetate, ethanol 70%, soxhlet | Streptozocin diabetic rats | 50 g/kg, 30 days | All extracts had significant scavenging of free radicals abilities, normalized liver function and inhibited lipid synthesis | [135] |
| Phoenix dactylifera | Seed | Aqueous suspension | Streptozocin diabetic rats | 1 gm/kg, 4 weeks | Protective effects for kidney, liver | [133] |
| Epicarp | Isolated compounds | Alloxan diabetic rats | 20 mg/kg, 30 days | Significant decrease in ALT, AST, cholesterol, TG, increase in glutathione peroxidase and superoxide dismutase in liver | [158] | |
| Securigera securidaca | Flower | Ethanol 90%, maceration | Alloxan diabetic rats | 100, 200, 400 mg/kg | The extract was safe up to a dose of 2000 mg/kg (body weight), reduction in serum TG and total cholesterol levels | [136] |
| Trigonella stellate | Aerial parts, root | Ethanol 90%, maceration | Human hepatoma (HepG2) cell line | 12.5, 25, 50 μg/mL | Activation of PPARα and PPARγ (fractions and isolated comp) | [137] |
| Urtica pilulifera | Aerial parts | Methanol, maceration | High-fat, low-dose streptozocin diabetic rats | 250, 500 mg/kg | A significant antioxidant, anti-inflammatory effect | [138] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Allium paradoxum | Alezi | Liliaceae | Acne, food flavoring | Raw vegetable | [161,163] |
| Allium ascalonicum | Not mentioned | Alliaceae | Diabetes | Not mentioned | [166] |
| Allium sativum | Sarimsaq, sir | Alliaceae | Diabetes, digestive system, blood fat, rabies | Bulb | [166,167,168] |
| Arctium lappa | Palvarg | Asteraceae | Antiscorbutic, antioxidant, blood purifiers, constipation, diuretic, disinfectant, gout | Berries, leaf, root | [169] |
| Buxus hyrcana | Shemshad | Buxaceae | Broken bone, toothache | Leaf | [167] |
| Calendula officinalis | Marigold, HamisheBahar | Asteraceae | Blood cleanser, eczema, dermal disorders, sudorific | Flower | [168,170] |
| Camellia sinensis | Chai Sabz | Theaceae | Anticancer, blood pressure, antihyperlipidemia, hepatitis, obesity | Leaf | [168,170] |
| Convolvulus persicus | Not mentioned | Convolvulaceae | Not mentioned | Not mentioned | [72] |
| Cinnamomum zeylanicum | Darchin | Lauraceae | Antibacterial, antioxidant, hypertension, diabetes | Bark | [164] |
| Coriandrum sativum | Geshniz | Apiaceae | Acne, antiseptic, appetizer, aphrodisiac, aromatic, calmative, flatulence, jaundice | Fruit | [168,170] |
| Crataegus oxyacantha | Sorkhevalik | Rosaceae | Diabetes, hypertension | Leaf | [164] |
| Hibiscus sabdariffa | Chay-e-makki | Malvaceae | Antioxidant, diabetes, hypertension | Flower | [164] |
| Juglans regia | Gerdu | Juglandaceae | Antidiarrheal, eczema, hair color | Fruit, leaf | [168] |
| Morus alba | Toot | Moraceae | Constipation, diabetes blood lipid reduction | Leaf | [164] |
| Olea europaea | Zeytoun | Oleaceae | Anti-hemorrhoids, blood lipid, diabetes, dermal allergy, hypertension, kidney stone, laxative, renal problems | Fruit, leaf, seed | [167,168,171,172] |
| Parrotia persica | Not mentioned | Hamamelidaceae | Antifever, broken bone, food coloring and flavoring | Bark | [163,173] |
| Pimpinella affinis | Taretizakebaghi | Apiaceae | Asthma, antimicrobial, antispasmodic, carminative, cholera, diuretic, migraine, narcotic | Flowering shoot, Seed | [163,174] |
| Primula heterochroma | Not mentioned | Primulaceae | Food flavoring | Not mentioned | [163] |
| Portulaca oleracea | Khorfeh | Portulaceae | Antibacterial, antiviral, diabetes, enhancing immunity | Seed | [164] |
| Rubus fruticosus | Tameshk | Rosaceae | Anti-infection, anticramp, hypertension, food flavoring, narcotic | Leaf | [164] |
| Ruscus hyrcanus | Butcher’s broom | Asparagaceae | Appetizer, antibleeding, antilaxative, anti-nephritis, anti-infection, antivaricose, aperient, diuretic, jaundice, laxative, vasoconstrictor | Leaves, fruit | [163,175] |
| Smilax excelsa | Not mentioned | Smilacaceae | Antieczema, diuretic, food flavoring | Not mentioned | [163] |
| Syzygium aromaticum | Alezi | Liliaceae | Acne, digestive disorder food flavoring | Raw vegetable | [164] |
| Teucrium polium | Kalpoore | Buxaceae | Analgesic, aperient, antiepileptic, antihairloss, antiheadache, anti-infection, antimalaria, antipneumonia, antirheumatism, carminative, constipation, febrifuge, stomachache | Aerial part | [164,176] |
| Trigonella foenum-graecum | Sic lefuit fenugreek | Fabaceae | Not mentioned | Not mentioned | [164,170] |
| Urtica dioica | Nettle | Urticaceae | Diabetes | Aerial parts, seed | [177] |
| Urtica pilulifera | Kara Isırgan (Turkey) | Urticaceae | Antidandruff, anti-inflammatory, asthma, astringent, blood purifier, diuretic, diabetes, enhancement of hemoglobin concentration, hemostatic, lower urinary tract infection, stimulating tonic (Egypt) | Whole plant, leaf | [138,178] |
| Vaccinium arctostaphylos | Darchin | Lauraceae | Antibacterial, antioxidant, blood pressure, diabetes | Bark | [164] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Allium ampeloprasum | Tarreh (Iran) Kurrat (Eygept) | Liliaceae | Asthma, antiseptic, diuretic, goat, vasodilatory, expectorant, constipation, hemoptysis, obesity, hemorrhoids, headache and as a diuretic, emmenagoug | Leaf, bulb | [182,183,277,278] |
| Allium sativum | Som, sir | Liliaceae | Antifungal, antiprotozoal, anthelminthic, antiviral, disinfectant, antitumor, gastric hepatic disorders, diabetes, hypertension, hypercholesterolemia, immunodeficiency syndromes | Bulb | [279,280,281] |
| Amygdalus lycioides | BadamTalkhkuhi | Rosacese | Antimicrobial, anti-inflammatory, diabetes | Aerial parts, root | [188] |
| Amygdalus scoparia | Badam-Koohi-Arzhan | Rosaceae | Appetizer, cardiovascular and respiratory diseases, headache, rheumatism, wound healing | Leaf | [281] |
| Arctium lappa | Baba adam | Asteraceae | Diabetes | Root, leaf | [190] |
| Berberis integerrima | Zarch | Berberidaceae | Abdominal ache, depurative, diabetes, hypertension | Fruit, root, leaf, stem | [176,282,283] |
| Brassica napus | Colza | Brassicaceae | anti-goat, anti-inflammatory, anti-scurvy, diuretic, bladder and hepatic and kidney colic | Root, seed | [284] |
| Brassica rapa | Kolza | Brassicaceae | Diabetes | Root, seed, leaf | [285] |
| Capparis spinosa | Shomisheytoni | Capparaceae | Diabetes, diuretic, gout arthritis, liver disorders, neurological conditions, rheumatoid, paralysis | Fruit, leaf | [283,286] |
| Centaurea bruguierana | Baad-Avard | Asteraceae | Hypoglycemic | Aerial fruiting parts | [201] |
| Cichorium intybus | Chicory or Kasni | Asteraceae | Antipyretic, depurative, diabetes, choleretic, eupeptic, hypotension, laxative, stomachic, tonic | Whole plant | [202,203] |
| Citrullus colocynthis | Hanzal | Cucurbitaceae | Abortifacient, antiepileptic, analgesic, anti-inflammatory, diabetes, hair growth-promoting | Seeds, root, pulp, fruits | [279,287] |
| Cornus mas | Not mentioned | Cornaceae | Anemia, chickenpox, diarrhea, diabetes, digestion problems, hepatitis A, heal wounds inflammatory bowel disease, fever, kidney stones, malaria, measles, pyelonephritis, rickets, sore throat, sunstroke, urinary tract infections, ulcer | Leaf, flowers, fruits | [208,288] |
| Cucumis sativus | Tokhm-e-khiyar | Cucurbitaceae | Anti-fever, demulcent, diabetes | Seed | [212] |
| Cucurbita pepo | Kadohalvai | Cucurbitaceae | Diabetes | Seed, fruit | [285] |
| Eryngium caucasicum | Aweiyeh | Apiaceae | Flavoring | Leaf | [289] |
| Eucalyptus globules | Okaliptus | Myrtaceae | Diabetes | Leaf | [290] |
| Falcaria vulgaris | Paghazou | Apiaceae | Diabetes, gastric and duodenal ulcers, wound healing | Stem, leaf | [291] |
| Ferula assafoetida | “Anghouzeh”, “Khorakoma” “Anguzakoma” | Apiaceae | Asthma, epilepsy, flatulence, influenza, intestinal parasites, stomach ache, weak digestion | Oleo-gum resin | [221] |
| Galega officinalis | Galega | Fabaceae | Diabetes | Flower | [290] |
| Gundelia tournefortii | Kangar | Asteraceae | Antiparasite, diabetes | Leaf, root | [281,292] |
| Heracleum persicum | Glopar | Apiaceae | Carminative | Fruit | [293] |
| Hordeum vulgare | Jo dosar | Poaceae | Diabetes | Seed, bran | [285] |
| Juglan regia | Gerdoo | Juglandaceae | Antidiarrhea, antiparasitic, blood purifier, diabetes, hemorroides, venous insufficiency | Leaf | [228,281] |
| Mentha spicata | Nana, pooneh | Lamiaceae | Antispasmodic, carminative, diabetes, digestive, stomach pain-relieving agent, | Aerial parts, leaves, essence | [233] |
| Nasturtium officinale | Bakalu | Brassicaceae | Jaundice in children, abortion | Aerial parts, leaf | [292] |
| Otostegia persica | ShekarShafa | Lamiaceae | Antipyretic, antihyperlipidemia, analgesic, arthritis, cardiac distress, carminative, cough, diabetes, headache, laxative, parasite repellent, reducing palpitation, regulating blood pressure, rheumatoid toothache, stomachache, morphine withdrawal | Aerial Parts | [285,294] |
| Phoenix dactylifera | Khorma, Tarooneh | Arecaceae | Aphthous, antidepressant, bladder and nerve tonic, diabetes, pertussis, rheumatoid arthritis sedative, tranquilizer, wound healer | Spathe, pollen, leaf | [145,295,296,297] |
| Pistacia atlantica | Zhevi | Anacardiaceae | Diabetic ulcers, hyperglycemia | Juice | [283] |
| Punica granatum | Anar | Punicaceae | Astringent, burns, diarrhea, hematopoiesis, gastrointestinal disorders inflammation, pain, oral aphthous, sore throat | Fruit | [298,299] |
| Pyrus boissieriana | Not mentioned | Rosaceae | Anticramp, antihypertensive, food flavoring, anti-infection, narcotic, | Not mentioned | [163] |
| Rheum turkestanicum | Eshghan | Polygonaceae | Depurative, diabetes, hypertension jaundice | Root | [300] |
| Rhus coriaria | Sumac | Anacardiaceae | Adjustment blood lipid, diabetes, diarrhea, dysentery, hemorrhoids, gout, reduction of uric acid, wound healing table spice | Not mentioned | [248,249] |
| Salvia hydrangea | Gol-e Arooneh | Anti-inflammatory, antispasmodic, analgesic, cold | Leaf | [253] | |
| Salvia hypoleuca | Lamiaceae | diabetes, hemorrhoids, insecticide, purgative | [301] | ||
| Salvia officinalis | Maryam | Lamiaceae | Antiseptic diabetes, diuretic, dyspepsia, emmenagogue, fever | Leaf, flower | [302] |
| Securigera securidaca | GandehTalkheh | Fabaceae | Diabetes, hypertension, hyperlipidemia | Seed | [256] |
| Solanum nigrum | Hava | Solonaceae | Antibacterial, anticonvulsant, antipyretic, antioxidant, anti-inflammatory, antitumorigenic, cytotoxicity, diabetes, diuretic, enteric diseases, fever, hepatoprotective, inflammation, mycotic infection, pain, ulcer, sexually transmitted diseases | Aerial parts, fruit | [257,285] |
| Teucrium polium | Kalpooreh | Lamiaceae | Abdominal pain, cold, diabetes, indigestion, urogenital diseases | Aerial parts | [259] |
| Trigonella foenum-graecum | Shanbalileh | Fabaceae | Backache, bladder cooling reflex, cold, cough, diabetes, emollient, hepatitis, gastrointestinal disorders, loss of appetite, mouth odor, splenomegaly, undesired odor of body and sweat, skin disease, red spot of eye, tonic | Leaf, seed, aerial part | [285,303] |
| Urtica dioica | Gazane, chitchitiodghin, Gazgazuk, Aragh | Urticaceae | Allergic rhinitis, diabetes, digestive, diuretic, hypertension, galactogogue, inflammation, prostatic hyperplasia, rheumatoid arthritis, tonic | Root, aerial parts, leaf | [268,284] |
| Vaccinium arctostaphylos | Qaraqat, Cyah-gileh | Ericaceae | Diabetes, hypertension | Berries | [265] |
| Zataria multiflora | Avishan | Lamiaceae | Anesthetic, antinociceptive, antispasmodic, flavoring-preserving foods/drinks, irritable bowel syndrome, respiratory tract infections | Not mentioned | [304] |
| Zizyphus spina-christi | Sedr | Rhamnaceae | Antiseptic, antifungal, anti-inflammatory, bronchitis, cough, dysentery, nausea, sedative, skin diseases, wound healing, tuberculosis, vomiting, abdominal pains associated with pregnancy | Leaf, fruit, seed | [305] |
| Name | Chemical Constituents | Biological and Pharmacological Activities | Reference |
|---|---|---|---|
| Allium ampeloprasum | Cinnamic acid derivatives, nitrates, flavonoids, polysaccharides, glucosinolates, organosulphur compounds | Antibacterial, antioxidant, antifungal, protect skin against damage due to pathogenic agents, alleviate gastrointestinal diseases, inflammatory, hepatotoxicity | [183,277,306] |
| Allium ascalonicum | Volatile sulfur compounds, saponins, flavonoids, furostanol saponin | Antibacterial, anti-fungal, antihelicobacter pylori, beneficial hematological influences, antioxidant, peroxynitrite-scavenging capacity | [307,308] |
| Allium sativum | Organo-sulphur compounds, volatile sulphur compounds (diallyl sulphide), polyphenols | Hypoglycemic, cardiac diseases, antiseptic, toothache, antihyperlipidemia, anthelmintic, antihypertensive | [166,168,309] |
| Amygdalus lycioides | Alkaloids, amygdalin, flavonoid, phenolic compound, terpenoids, | Antioxidant, anticancer, anti-inflammatory, antiaging, antimicrobial, decrease the risk of colonic cancer, increase HDL cholesterol, reduce LDL cholesterol, cardioprotective | [189,310] |
| Amygdalus scoparia | Amygdalin, flavonoids, amino acids, linoleic acid | Antioxidant | [311] |
| Arctium lappa | Flavonoids, lignans, phenols, saponins, tannin | Anti-inflammatory, hepatoprotective, free radical scavenging activities | [190] |
| Berberis integerrima | Anthocyanins, alkaloids, berberine | Anticonvulsant, bleeding, diarrhea, fever, gastrointestinal disease, hepatitis, malaria, swollen gums teeth, sore throat, bile inflammation, reducing blood cholesterol | [191,312] |
| Brassica napus | Anthocyanin, cinnamic acid hydroxyl derivatives, flavonoids, erucic acid | Antioxidant | [194,284] |
| Brassica rapa | Flavonoids, phenylpropanoid derivatives, indole alkaloids, sterol glucosides, glucosinolates and isothiocyanate | Analgesic, anticancer, anti-inflammatory, antimicrobial, antioxidant, cardiovascular and hypolipidemic effect, diabetes, hepatoprotective, metabolic syndrome neuroprotective, obesity | [195,313] |
| Capparis spinosa | alkaloids, lipids, polyphenols, flavonoids, Tocopherols, carotenoids, glycosides, capparic acids, tannins | Antioxidant, antimicrobial, anticancer, antiallergic, hepatoprotective effects | [199,286] |
| Centaurea bruguierana | Sesquiterpene lactone, flavonoid (kaempferol, rutin, quercetin) | Antiplasmodial, antipeptic ulcer | [201] |
| Cichorium intybus | Aliphatic compounds, terpenoids, saccharides, methoxycoumarin cichorine, flavonoids, essential oils and anthocyanins | Antimicrobial, anthelmintic, antimalarial, diabetes, hepatoprotective, gastroprotective, anti-inflammatory, analgesic, antioxidant, tumor inhibitory, antiallergic | [202] |
| Citrullus colocynthis | a bitter compound (Cucurbitacins), colocynthein, colocynthetin, pectin, gum, flavonoids, alkaloid (choline), volatile terpenoids, fixed oil albuminoids, tocopherols and carotenes | Antidiabetic, hypolipidemic, antimicrobial, anti-inflammatory, antioxidant, cytotoxic, insecticidal, antiallergic | [206,287] |
| Cornus mas | Sugar, organic acids, tannins, anthocyanins, phenolic acid, tannin, vitamin C, flavonoid, iridoids, terpene (mono, tri), Carotenoids | Antimicrobial, antidiabetic, antiobesity, hypolipidemic and anti-atherosclerotic, cytotoxicity, hepatoprotective, renalprotective, neuroprotective, anti-inflammatory, antioxidant, antiplatelet, cardioprotective, antiglaucomic, reproductive organ -protective, radioprotective, aldose redustase inhibitory | [231,288,314] |
| Cucurbita pepo | Tetra cyclic triterpens, saponins, proteins, fibers, polysaccharides, miberal, carotenoid, tocopherol | Antioxidant, lipid-lowering, hepatoprotective, anticarcinogenic, antimicrobial, antidiabetic | [213] |
| Cucumis sativus | Flavonoids, saponin, tannin, steroid | Antimicrobial, antitumor, antacid, carminative, wound healing, against ulcerative colitis, skin irritation, hypoglycemic and hypolipidemic | [315] |
| Eucalyptus globulus | Euglobals, essential oils, hydrocarbons | Antidiabetic, anti-bacterial, antiplaque, antitumor, antiviral, antifungal, antihistaminic, anti-inflammatory, antioxidant, antimalarial | [217] |
| Falcaria vulgaris | Volatile oil, phenolics, flavonoid, | Anti-inflammatory, antioxidant, antibacterial, antifungal, antiviral, and bleeding inhibitor activities | [316,317] |
| Ferula assafoetida | Coumarine, flavonoids, gum, phenolic acids terpene, volatile oil | Antioxidant, antiviral, antifungal, cancer chemopreventive, antidiabetic, antispasmodic, hypotensive, molluscicide | [220,221] |
| Galega officinalis | Alkaloid, flavonoid | Antioxidant | [318] |
| Gundelia tournefortii | Coumarin, terpene, sterol, essential oil, phenolic compounds, | Antibacterial, anti-inflammatory, hypolipemic activity, hepatoprotective, antiplatelet, antioxidant | [319] |
| Heracleum persicum | Alkaloids, flavonoids, furanocoumarin, terpenoids; triterpenes, volatile substances | Anti-inflammatory, immunomodulatory, growth enhancer, antioxidant, anticonvulsant, analgesic, hypocholesterolaemic agent | [320,321] |
| Hordeum vulgare | Soluable fiber components especially β-glucans | Anti-inflammatory, antilactagogue, antimutagenic, antiviral, astringent, antioxidant, antiprotozoal, aphrodisiac, demulcent, digestive, diuretic, expectorant, febrifuge, hypocholesterolemic, refrigerant, sedative, stomachic, tonic, poultice for burns and wounds | [322] |
| Juglans regia | Flavonoids, phenolics, tannins, flavonoids, phytosterols, tocopherol | Antioxidant, antidiabetic, antihypertensive, antimicrobial, anticancer, liver and kidney protection, lipid-lowering effect | [228] |
| Mentha spicata | Flavonoids, terpenoids, monoterpenes phenols | Antimicrobial, antispasmodic, antiplatelet, insecticidal, neutraceutical and cosmetic industries | [233] |
| Nasturtium officinale | carotenoids, polyphenols, vitamin C, vitamin A and α-tocopherol | Antimicrobial, antioxidant, antiestrogenic, anticarcinogenic, for the prevention of cancer | [234] |
| Otostegia persica | Alkaloids, essential oil, flavonoids, tannin, terpenes, mono, di and sesquiterpene, steroids | Antimicrobial, antioxidant, antidiabetic, antiglycation, anti-aphids, hepatoprotective | [235,294] |
| Olea europaea | Lignan like compounds, lignan glycoside, coumarin, flavonoid, oleuropein, phenolics, hydroxytyrosol derivatives, secoiridoid, secoiridoid glycosides, triterpene | Antidiabetic, anticancer, antimicrobial, antioxidant, antihypertensive, enzyme inhibitory activities, anti-inflammatory, antinociceptive activities, gastroprotective, neuroprotective | [323] |
| Phoenix dactylifera | Alkaloids, anthocyanins, carotenoids, flavonoids, phenolics, procyanidins, sterols, vitamins, tannins. | Anticancer, antioxidant, antimutagenic, antihemolytic, antiviral, antifungal, anti-inflammatory, hepatoprotective, gonadotropic activity immunostimulant, nephroprotective, | [145] |
| Punica granatum | Alkaloid, anthocyanin, catechin, ellagitannins, flavonoid, sterol, tannins | anticancer, antidiabetic, anti-inflammatory, antimicrobial, healing activity | [324] |
| Pyrus boissieriana | Arbutin, glycosides, flavonoids, phenols | Antioxidant, antihyperlipidemic, bactericidal and antifungal effects, diabetes | [163,240] |
| Rheum turkestanicum | Anthraquinone, flavonoid, phytosterol, phenolic glycosides | Anticancer, cardioprotective, diabetes, nephroprotective, hepatoprotective, neuroprotective | [250] |
| Rhus coriaria | tannins, anthocyanins, various organic acids such as malic and citric acids, fatty acids, vitamins, flavonoids and terpenoids, essential oil | Antimicrobial, antifungal, antiviral, antioxidant, anti-inflammatory, hepatoprotective, xanthine oxidase inhibition, hypoglycemic, cardiovascular protective activities | [248] |
| Salvia hydrangea | Flavonoids, phenolic acid, terpenoids | Antioxidant | [253] |
| Salvia hypoleuca | Essential oil, lactones, isomeric epoxides, monolactone and hypoleuenoic acid, sterols, terpenes, sesqui, di and triterpenes | Antioxidant | [325,326] |
| Salvia officinalis | Flavonoids, carnosic acid, rosmarinic acid | Antioxidant | [302] |
| Securigera securidaca | Cardenolides, coumarins, dihydrobenzofuran derivatives, flavonoids, steroidal and pentacyclic triterpenoid-type saponins | Antiulcerogenic, antiepileptic, chronotropic, diuretic, hypokalemic | [136,256] |
| Solanum nigrum | Catechin, caffeic acid, epicatechin, flavonoids, glycoalkaloids, glycoproteins, polysaccharides, polyphenolic compounds, protocatechuic acid | Antimicrobial, anti-HCV, anti-ulcer, analgesic, anti-inflammatory, antidiarrheal, anticancer, antiseizure, cardioprotective, cytotoxic activity diabetes, immunostimulant, hepatoprotective, larvicidal activity | [257] |
| Teucrium polium | Terpenoids, mono, di and sesquiterpene, flavonoids, neoclerodane, polyphenols | Antioxidant, antimutagenic, anticancer, anti-inflammatory, antinociceptive, antispasmodic, antiulcer, antimicrobial, diabetes, hepatoprotective, hypolipidemic | [258,259] |
| Trigonella foenum-graecum | Flavonoids, alkaloids, coumarins, vitamins, steroidal saponins | Anti-inflammatory, antilipidemic, antioxidant, antimicrobial, antiulcer, anticarcinogenic, carminative, diabetes, hypocholesterolemic, hepatoprotective, galactogogue | [261,285,327] |
| Vaccinium arctostaphylos | Anthocyanins, flavonoids, phenolic acids | Antioxidant, antimicrobial | [265,328] |
| Urtica dioica | Alkaloids, agglutinin, lecithin, flavonoid, phenolic acids, stigmasterol, terpenes, coproporphyrin, lignan, and violaxanthin, coxamarins | Antiproliferative, antioxidant, antidandruff, anti-inflammatory, antimicrobial, cancer, coronary heart disease, diabetes, joint pain reduction, urinary tract infection, psychotic disorders, viral and parasitic diseases | [271] |
| Zataria multiflora | Volatile oil | Antioxidant, antinociceptive, anti-inflammatory, antibacterial, antiviral, antifungal, immunostimulant, pain-relieving | [273,329] |
| Zizyphus spina-christi | Flavonoid, isoquinoline alkaloid, cyclopeptide, saponin, triterpenes | Analgesic effect, antioxidant, antidiabetic, antifungal, antibacterial, antinociceptive | [275,305] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Allium ampeloprasum | Bulb | Ethanol 70%, maceration | Alloxan diabetic rats | 400, 800 mg/kg, 8 weeks | Prevent diabetes associated complications; decreased the levels of serum lipids and MDA, significantly increased the activity of antioxidant enzymes | [183] |
| Allium ascalonicum | Bulb | Methanol 80%, soxhlet | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks | Elevated expression of both insulin and glucose transporters transcripts | [166] |
| Allium sativum | Bulb | Methanol 80%, soxhlet | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks | Elevated expression of both insulin and glucose transporters transcripts | [166] |
| Arctium lappa | Root | Methanol 40%, maceration | nicotinamide-Streptozocin diabetic rats | 200, 300 mg/kg, 28 days | Decreased level of triglyceride, vLDL, and alkaline phosphatase | [190] |
| Amygdalus lycioides | Spach branches | Methanol 50%, maceration | Streptozocin diabetic rat | 125, 250, 500, 1000 mg/kg, 2 weeks | Decreased total cholesterol, LDL, TG, Cr, and alkaline phosphatase levels, increased ALT, ASTT, total number and numerical density of β-cells increased | [188] |
| Amygdalus scoparia | Fruit | Hexane, maceration | Streptozocin diabetic rat | 1 mL/kg 15 days | Improve dregeneration of B cells | [189] |
| Leaf | Aqueous, hydroalcoholic solvent | Streptozocin diabetic rats | 30, 60, 120 mg/kg, 3 days | Increase in the serum insulin level | [330] | |
| Avicennia marina | Leaf | Water, soxhlet | Streptozocin diabetic rats | 100 and 200 mg/kg, i.p., one month, alternate day | Decreased the serum levels of the liver enzymes and tissue level of MDA, and the activity of the liver tissue’s antioxidant enzymes was increased | [32] |
| Berberis integerrima | Root | Water, maceration | Streptozocin diabetic rat | 250 and 500 mg/kg, 6 weeks | Significant decrease in TG, cholesterol, LDL, ALT, AST, ALP, total bilirubin, Cr and urea, increase in HDL-cholesterol and total protein | [191] |
| Fruit | Water, maceration | Streptozocin diabetic rats | 250 and 500 mg/kg, 6 weeks | Extract did not possess the hypoglycemic and hypolipidemic activity. | [192] | |
| Fruit | Anthocyanin fraction | Streptozocin diabetic rats | 200, 400 and 1000 mg/kg | Significantly increased liver glycogen and body weight | [193] | |
| Brassica napus | Juice | Water, decoction | Alloxan diabetic rats | 4 weeks | Decreased TG, cholesterol and LDL | [194] |
| Brassica rapa | Leaf | Water, maceration | Alloxan diabetic rats | 200, 400 mg/kg | Decreased ALT, cholesterol, LDL, HDL, increased TG, AST | [195] |
| Capparis spinosa | Fruit | Ethanol 80%, soxhlet | Alloxan diabetic rats | 300 mg/kg, 12 days | There was no evidence of regeneration in the liver of diabetic rats, brings the blood glucose significantly toward normal values from day 2 onwards | [198] |
| Fruit | Ethanol 70%, maceration, soxhlet | Streptozocin diabetic rats | 5 mg/kg | Dose-dependent decrease in blood sugar, decrease in triglycerides | [197] | |
| Root | Ethanol 70%, maceration | Streptozocin diabetic rats | 0.2, 0.4 g/kg | Decreased LDL, AL, ALP, insulin levels did not increase, increased HDL | [331] | |
| Fruit | Water, decoction | Streptozocin diabetic rats | 20 mg/kg, 28 days | No significant influence on the insulin level, decreased blood TG, cholesterol content, reduced the mRNA expression, enzyme activities of glucose-6- phosphatase and phosphoenolpyruvate carboxykinase in liver | [200] | |
| Centaurea bruguierana | Aerial fruiting parts | Water, dichloromethane, ethylacetate, methanol, percolation | Streptozocin, alloxan diabetic rats | 200, 400 mg/kg | Aqueous extract showed best effect | [201] |
| Cichorium intybus | Seed | Water, maceration | Streptozocin, Streptozocin and niacinamide diabetic rats | ------- | Normalization of blood ALT, TG, total cholesterol, HB1C | [203] |
| Citrullus colocynthis | Seed | Ethanol 80%, maceration | Alloxan diabetic rat | 300 mg/kg, 12 days | Enhanced regeneration of B-cells, increased size of pancreatic islet, improvement of hepatic tissue | [207] |
| Cornus mas | Fruit | Ethanol 70%, maceration | Alloxan diabetic rat | 2 gm/kg, 1 month | Less severe hepatic portal inflammation, antidyslipidemia effects | [208] |
| Cucurbita pepo | Fruit | Powder | Alloxan diabetic rat | 1, 2 gm/kg 4 weeks | Reduced cholesterol, TG, LDL and CRP levels | [213] |
| Cucumis sativus | Seed | Ethanol 75%, percolation buthanol, maceration | Normal and streptozocin diabetic rat | 0.2, 0.4, 0.8 g/kg, 9 days | The extracts were not effective in reducing blood glucose levels in normal and diabetic rats | [212] |
| Eucalyptus globulus | Leaf | Water, decoction | Streptozocin diabetic mice | 20, 62.5 g/kg eucalyptus in the diet, and 2.5 g/L extract in drinking water, 4 weeks | Dose-dependent amelioration of diabetic states by partial restoration of pancreatic β-cells | [216] |
| Leaf | Water, hot maceration | Streptozocin diabetic rats | 2.5 mg/mL, 4 weeks | Increase ALT, AST and ALP activity | [332] | |
| Falcaria vulgaris | Aerial parts | Water, maceration | Streptozocin diabetic rat | 200, 600, 1800 μg/kg | Hematoprotective and nephroprotective activity | [218] |
| Ferula assa-foetida | Oleo-gum resin | Ethanol 70%, maceration | Streptozocin diabetic rat | 150, 250 mg/kg, 6 weeks | Protective effect of liver and kidney damage | [221] |
| Oleo-gum resin | Water, maceration | Streptozocin diabetic rat | 50, 100, 300 mg/kg, 4weeks | 50 mg/kg significantly lowered the serum glucose concentration | [219] | |
| Galega officinalis | Leaf | Powder | Streptozocin diabetic rats | 1.5, 3 g/kg | Body weight-reducing properties | [223] |
| Root | Ethanol, maceration | Streptozocin diabetic rats | 150 mg/kg, o.p, i.p, 6 weeks | Decreased LDL, leptin, increased TG, VLDL, AST | [333] | |
| Not mentioned | Hydroalcoholic solvent | Streptozocin diabetic rats | 50 mg/kg, i.p, 20 days | Decreased urea and creatinine | [224] | |
| Gundelia tournefortii | Aerial parts | Water, maceration | Alloxan diabetic rats | 5, 10, 20, 40 mg/kg, 20 days | Alleviation of diabetic complications such as nephropathy | [225] |
| Shoots | Water | Streptozocin diabetic rats | 400 mg/kg, 21 days | Recovery of pancreas tissue | [226] | |
| Horddeum vulgare | Seed | Ethanol 75%, percolation and alkaline extract | Streptozocin diabetic rats | 0.1, 0.25, 0.5 g/kg, 11 days | Restored body weight, long term benefits | [227] |
| Not mentioned | Hexane oil extraction | Alloxan diabetic rats | i.p administration of oil, 6 weeks | Reduced insulin, HbA1C, total cholesterol, LDL, VLDL, HDL and TG | [229] | |
| Juglans regia | Leaf | Ethanol 90%, maceration | Streptozocin-nicotinamide diabetic rats | 200 mg/kg, 1 month | Reduced HbA1C, total cholesterol, LDL and TG | [230] |
| Leaf | Methanol 70%, maceration | Streptozocin diabetic rats | 200, 400 mg/kg, 4 weeks | Significant increase in diameter and number of β-cells compared to diabetic control group | [231] | |
| Leaf | Methanol 70%, maceration | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks | Significant inhibition of α-glucosidase, maltaseand sucrase enzymes | [232] | |
| Mentha spicata | Leaf | Water, soxhlet | Alloxan diabetic rats | 300 mg/kg, 21 days | LD50 ˃ 1500 mg/kg (body weight), decreasedcholesterol | [233] |
| Nasturtium officinale | Aerial parts | Water, maceration | Streptozocin diabetic rats | 100, 200 mg/kg, 4 weeks | Decrease in total cholesterol and LDL | [234] |
| Otostegia persica | Aerial parts | Water, decoction | Streptozocin diabetic rats | 100, 200, 400 mg/kg, 1 month | Reduced TG | [235] |
| Aerial parts | Methanol, soxhlet | Streptozocin diabetic rats, C187 β-cell line | 200, 300, 400 mg/kg | Decreased MDA and increased GSH levels in the liver | [236] | |
| Otostegia persica | Aerial parts | Ethanol 50%, percolation | Streptozocin diabetic rat | 500 mg/kg | Helped prevent the entering of the remaining β-cells into some pathologic changes, such as hypertrophy | [237] |
| Phoenix dactylifera | Leaf | Ethanol extract, maceration | Alloxan diabetic rat | Extract: 100, 200, 400 mg/kg, 14 days Fractions: 50, 100, 200 mg/kg, 14 days | Decrease in water intake, serum TG and cholesterol, increase in plasma insulin level | [246] |
| Pistacia atlantica | Fruit | Hexane extract, maceration | Streptozocin diabetic rat | 1 mL/kg, 15 days | Improvedregeneration of β cells | [189] |
| Punica granatum | Fruit | Methanol extract, maceration | Streptozocin diabetic guinea pigs | 500 mg/kg, 4 weeks | Enhanced glutathione content as well as the activity of catalase, decrease in total cholesterol and TG | [247] |
| Pyrus boissieriana | Leaf | Methanol, maceration | Alloxan diabetic rat | 500, 1000 mg/kg, 4 days | Decreased serum TG cholesterol, increased antioxidant status | [239,240] |
| Rheum turkestanicum | Rhizome | Water, decoction | Streptozocin diabetic rats | 200, 400, 600 mg/kg, 3 weeks | Decreased triglycerides, no hypoglycemic or hepatoprotective effect in diabetic rats | [251] |
| Rhus coriaria | Fruit | Ethanol, maceration | Alloxan diabetic rats | 200, 400 mg/kg, 21 days | Increased serum HDL, superoxide dismutase and catalase activities, reduced LDL, inhibited maltase and sucrase activities | [249] |
| Not mentioned | Water | Alloxan diabetic rats | 50, 100, 250 mg/kg, 28 days | Increase catalase activities in liver and kidney | [248] | |
| Salvia hydrangea | Aerial parts | Ethanol 90%, maceration | Streptozocin diabetic rats | 100, 200 mg/kg, 21 days | Reduce blood fat | [253] |
| Salvia hypoleuca | Whole plant | Ethanol, maceration | Alloxan and normal diabetic rat | 250, 450 mg/kg, 14 days | Non-significant reductions for the non-diabetic groups that received extracts. | [252] |
| Salvia officinalis | Leaves | Methanol, soxhlet | Alloxan diabetic rat | 250, 500 mg/kg, 21 days | Elevated expression of both Ins and Glut-4 transcripts | [166] |
| Securigera securidaca | Seed | Aqueous suspension | Alloxan diabetic rat | 2, 4 g/kg, 3 days, intra-gastric gavage | Protective effect against oxidative stress | [254] |
| Seed | CCl4, ethanol70%, dichloromethane, maceration | Streptozocin diabetic rat | 200 mg/kg, 16 days | Carbon tetrachloride extract showed the best and most significant hypoglycemic and hypolipidemic activities with a β-cells-protecting effect from high glucose-induced apoptosis, and also increased insulin level and sensitivity | [255] | |
| Seed | Ethanol 70%, maceration | Streptozocin diabetic rat | 100, 200 mg/kg, 4 weeks | Decreased serum total cholesterol, LDL, increased HDL | [334] | |
| Seed | Methanol 80%, chloroform fraction, maceration | Streptozocin diabetic rat | Methanol: 10, 400 mg/kg Fraction: 400, 600 mg/kg | Securigenin glycosides (isolated) reduced blood glucose equivalent to glibenclamide and elevated insulin level to normal | [256] | |
| Solanum nigrum | Fruit | Water, decoction | Streptozocin diabetic rat | 1 gm/L, 8 weeks | Improved lipid profile, decreased Ca/Mg ratio, decrease vessel atherosclerosis and prevented diabetic vesselcomplications | [306] |
| Teucrium polium | Leaf | Water, decoction | Streptozocin diabetic rats | 100 mg/kg, 3 weeks | No decrease in body weight for diabetic rats | [259] |
| Trigonella foenum-graecum | Whole plant | Water, decoction | High-fructose diet diabetic rat | 10% of extract 8 weeks | The extract improve insulin resistance | [264] |
| Seed | Methanol, maceration | Streptozocin diabetic guinea pigs | 500 mg/kg, 4 weeks | Enhanced GSH content as well as the activity of catalase, decrease in total cholesterol and TG | [247] | |
| Urtica dioica | Whole plant | Aqueous distillate, distillation | Streptozocin diabetic rat | 12.5 mL/kg 1 month | Prevents islet atrophy and/or regenerate pancreatic β-cells | [269] |
| Leaves | Ethanol 70%, maceration | Fructose induced diabetes | 50, 100, 200 mg/kg, 2 weeks | Decreased LDL, leptin, LDL/HDL ratio, FIRI (fasting insulin resistance index), increased serum TG, VLDL, and AST | [335] | |
| Urtica dioica | Whole plant | Water, decoction | High fructose diet diabetic rat | 10% extract, 8 weeks | Urine glucose decreased significantly | [264] |
| Leaves | Water, infusion | Streptozocin diabetic rats, RIN-5F and L6 myotubes cell lines | 6.25 mg/kg, 1.25 g/kg 1 month | Regeneration and less β cell damage, increased insulin secretion in the RIN-5F cells and glucose uptake in the L6 myotubes cells, lower TG and cholesterol | [271] | |
| Vaccinium arctostaphylos | Fruit | Ethanol 95%, maceration | Alloxan diabetic rat | 200, 400 mg/kg, 21 days | Decreased total cholesterol and TG | [266] |
| Vitex agnus-castus | Fruit | Ethanol 70%, maceration | D-galactose-induced aging mouse | 500, 600 mg/kg, twice daily, 7 days | Pancreatic protective effects in natural aged and aging model mice | [267] |
| Zataria multiflora | Leaf | Hydrodistillation | Streptozocin diabetic rats | 50 μL/kg 28 days | Decrease in in plasma ALP, AST, ALT, and significant increase in total protein and insulin | [273] |
| Ziziphus vulgaris | Fruit | Water, maceration | Onreptozocin diabetic rats | 0.25, 0.5, 1, 1.5, 2 g/kg 14 days | Decrease in LDL and TG | [275] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Bauhinia variegate | Mountain ebony, orchid-tree, poor-man’s orchid, camel’s foot, Napoleon’s hat | Fabaceae | Anthelmintic, astringent, bronchitis, diabetes, diarrhea, laxative, leprosy, piles, tumors, tonic, worm infestations | Stem bark, leaves, buds | [18] |
| Momordica charantia | Kerela | Cucurbitaceae | Anthelmintic, hemmoroid, gout, stomachic | Fruit | [345] |
| Rheum ribes | Rawand, Rewas | Polygonaceae | Anthelmintic, diabetes, diarrhea, expectorant, hypertension, obesity, ulcer | Root | [346] |
| Scientific Name | Phytochemcal Constituent | Pharmacological Use | Refernce |
|---|---|---|---|
| Bauhinia variegate | Cardiac glycosides, flavonoids, reducing sugars, saponins, steroids terpenoids, tannins | Anticancer, antioxidant, antimicrobial, anti-inflammatory, antiulcer, hepatoprotective, hypolipidemic, immunomodulating, nephroprotective, molluscicidal and wound healing effects | [347] |
| Momordica charantia | Alkaloid, lipid, phenolics, triterpene, saponin, steroid | Diabetes | [339] |
| Rheum ribes | Anthraquinone, flavonoid, phenolic compounds, stilbene, | Antidiabetic, antioxidant | [341,348] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Achillea santolina | Leaf | Water, infusion | Streptozocin diabetic rats | 150, 250 mg/kg, 28 days | Dose-dependent hypoglycemic response | [349] |
| Bauhinia variegate | Leaf | Ethanol 70%, maceration | Dexamethasone diabetic rats | 200 mg/kg, 28 days | Decrease in TC and TG, increase in HDL | [338] |
| Momordica charantia | Seed | Water, maceration | Alloxan diabetic rats | 150 mg/kg, 30 days | Decrease in blood glucose level more than glibenclamide | [340] |
| Rheum ribes | Root | Water, decoction | Alloxan diabetic rats | 200 mg/kg, 30 days | Increased activity of β-cells | [344] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Achillea santolina | Kaisoom, jeaidatelsabian | Asteraceae | Colic, cold, depurative, diabetes, kidney stones, | Aerial parts | [351,366] |
| Artemisia herba alba | Shaih | Asteraceae | Fever, menstrual and nervous problem | Aerial parts, root | [367] |
| Artemisia sieberi | Shaih | Asteraceae | Antispasmodic, antiarthritis, diabetes, pectoral | Foliage | [368] |
| Arum dioscoridis | Louf | Araceae | Anticancer | Leaf | [369] |
| Arum palaestinum | Louf | Araceae | Anticancer, internal bacterial infections, poisoning, disturbances of the circulatorysystem, cooking | Leaf | [369] |
| Crataegus aronia | Zaeroor | Rosaceae | Cardiovascular diseases, diuretic, hypertension, hyperlipidemia, kidney stone, laxative | Leaf | [370,371] |
| Cichorium pumilum | Hendba | Asteraceae | Antiseptic, antidiabetic, eczema | Flower, root | [372] |
| Eryngium reticum | Gersaana | Apiaceae | Scorpion and snake bite | Root | [371] |
| Geranium graveolens | Utryye | Geraniaceae | Diuretic, diabetes, stomachic | Leaf | [351] |
| Pistacia atlantica | Botom | Anacardiacae | Asthma, cough, diabetes, stomach ache, | Fruit, leaf, resin | [373,374] |
| Rheum ribes | Rabbas | Polygonaceae | Diabetes, hypertension, kidney sand and stones, obesity, | Root | [351] |
| Teucrium polium | Ja’deh | Lamiaceae | Abdominal pain, diabetes, urinary tract infection, | Aerial parts, shoot, leaves | [351] |
| Varthemia iphionoides | Qtteileh | Asteraceae | Abdominal pain, diabetes | Shoot, leaf | [351] |
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Achillea santolina | Flavonoids, terpenoids, essential oils | Anti-inflammatory, immunomodulatory, antibacterial | [375] |
| Artemisia herba alba | Sesquiterpene lactones, phenolic compounds, essential oil, flavonoid | Antioxidant, antimicrobial, antivenom, antispasmodic, anthelmintic, diabetes, nematicidal, neurological activity | [376] |
| Artemisia sieberi | Essential oil, flavonoid, polyphenolic compounds | Antioxidant | [353] |
| Arum dioscoridis | Flavonoids, phenolic acids | Antioxidant, antimicrobial | [355,369] |
| Arum palaestinum | Pyrrole alkaloid | Antioxidant, anticancer, antidiabetic | [369,377] |
| Cichorium pumilum | Flavonoids, coumarins, caffeic acid derivatives, sesquiterpene lactones | Antitumor | [378] |
| Eryngium reticum | Coumarine, essential oils, terpenes, sesqui and monoterpenes, sitosterols, tannins, resins | Anti-snake and anti-scorpion venom, antibacterial, antifungal, antimalaria, antileshmania, antioxidant, antimutagenic, antihyperglycemic, cytotoxic | [359] |
| Geranium graveolens | Essential oil | Antiemetic activity, antioxidant, fumigant repellent | [374] |
| Phaseolus vulgaris | Alkaloids, anthocyanin, catechin flavonoids, saponins, tannins, terpenoids | Antioxidant, anticancer | [363] |
| Pistacia atlantica | Flavonoids, phenolic compounds, terpenoids, mono and sesquiterpenoids, volatile oil | Antimicrobial, antiviral, antidiabetic, antitumor, anticholinesterase activity, antioxidant | [374] |
| Tecoma stans | Alkaloid, chlorogenic acid | Diabetes, lower cholesterol, | [358,379] |
| Teucrium polium | Essential oil, flavonoid, Iridoids, steroidal compounds | Antioxidant, anti-inflammatory, anticancer antihyperlipidemic, antimicrobial, antimutagenic, antiulcer, antispasmodic, antinociceptive, hepatoprotective, diabetes | [258] |
| Varthemia iphionoides | Sesquiterpene, essential oil Flavonoids | Antiplatelet, antioxidant, antitumor, antibacterial, antifungal | [375] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Achillea santolina | Aerial parts | Water, reflux | Starch loaded rats | 125, 500 mg/kg | Enhanced oral glucose tolerance | [343] |
| Aerial parts | Water, reflux | MIN6 β-cell line | 0.05–1 mg/mL | Dose dependent pancreatic β-cell proliferation | [350] | |
| Artemisia herba alba | Aerial parts | Water, maceration | Alloxan diabetic rabbits | 1 day | Significant hypoglycemic effect | [380] |
| Artemisia sieberi | Aerial parts | Essential oil, hydrodistillation | Alloxan diabetic rats | 80 mg/kg, 30 days | LD50 800 mg/kg (body weight) | [353] |
| Arum dioscoridis & Arum palaestinum | Aerial parts | Water, ethanol, reflux | Fasted rats | 125, 250, 500 mg/kg | Concentration-dependent pancreatic lipase inhibition | [355] |
| Crataegus aronia | Flower, leaf | Water, reflux | Highcholesterol diet fed rats | 100, 200, 400 mg/kg, 10 weeks | Potent antiobesity, marked triacylglycerol-reducing efficacy | [357] |
| Cichorium pumilum | Leaf | Ethanol, percolation | Alloxan diabetic rats | 1 gm/kg | Reduced bloodglucose after 3 h of administration | [358] |
| Eryngium creticum | Aerial parts | Water, reflux | MIN6 β-cell line | 0.1, 0.5, 1 mg/mL | Dose-dependent highly significant pancreatic β-cell proliferation | [350] |
| Aerial parts | Water, reflux | Starch treated rats | 125, 250, 500 mg/kg | Lack of effect on enzymatic starch digestion, no overall glycemicexcursion, no improvement in OGTT | [360] | |
| Geranium graveolens | Leaf | Water reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Augmented β-cell mass expansion, dose-dependent dual inhibition of α-amylase and α-glucosidase | [360,361] |
| Phaseolus vulgaris | Fresh pods | Ethanol, percolation | Alloxan diabetic rats | 1 gm/kg | Significantly reduced blood glucose after 3 h of administration | [358] |
| Pistacia atlantica | Aerial parts | Water, reflux | Starch-loaded rat | 125 mg/kg | Hypoglycaemic effect reversed at higher doses for the extract | [343] |
| Aerial parts | Water, reflux | MIN6 β-cell line | 0.01, 0.1, 0.5 mg/mL | Augmented acute β-cell insulin secretory efficacy, preserved β-cell integrity | [350] | |
| Rheum ribes | Root, rhizome | Water, reflux | Pancreatic β-cells MIN6 | 0.01, 0.05, 0.1 and 0.5 mg/mL | Acute insulin secretion, augmented β-cell mass expansion | [260] |
| Sarcopoterium spinosum | Aerial parts | Water, reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Insulinotropic and proliferative effects in the pancreas, dose-dependent dual inhibition of α-amylase and α-glucosidase | [343,361] |
| Tecoma stans | Leaf | Ethanol, percolation | Alloxan diabetic rats | 1gm/kg | Significantly reduced bloodglucose after 3 h of administration | [358] |
| Teucrium polium | Aerial parts | Ethanol, percolation | Alloxan diabetic rats | 1gm/kg | Reduced blood glucose after 3 h of administration | [358] |
| Varthemia iphionoides | Aerial parts | Water, reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Augmented β-cell mass expansion | [361] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Centaurea horrida | Not mentioned | Asteraceae | Diarrhea, diabetes, hypertension | Not mentioned | [387] |
| Inula viscosa, Inula vulgaris | Tayyoun, ‘Ergel-tayyoun | Asteraceae | Rheumatoisim | Whole plant | [388] |
| Psoralea bituminosa | Homan Homri | Fabaceae | Iintestinal ailments, gastric ulcers | Leaf, fruit | [389] |
| Salvia libanotica | Aiza’an Kassiin ‘Ouaiss’e Maryamiyy’e | Lamiaceae | Asthma, arthritis, antiseptic, aphrodisiac, antimicrobial, carminative, constipation carminative, cough, diabetes, expectorant, influenza, hypertension, gastralgia, hepatitis, febrifuge, nephropathy, rheumatism, spasmolytic, stomachic, stimulating memory | Flower, leaf | [390] |
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Centaurea horrida | Flavonoids, lactones, phenolic acids | Antioxidant | [387] |
| Inula viscosa, Inula vulgaris | Costic acid, flavonoid, guaianolide, phenolic compounds, terpenoid | Abortifacient, antibacetraial, anti-implantation, antihypertensive, cytotoxic, hypoglycemic | [383] |
| Psoralea bituminosa | Furanocoumarins, isoflavonoid, meroterpenoids, sesquiterpene, volatile oil | Antioxidant, cytotoxicity | [386] |
| Saliva libanotica | Essential oil | Antioxidant activity, anticholinesterase activity, anticancer | [391] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Centaurea horrida | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, 8 days | Improve peripheral nerve function | [381] |
| Hordeum spontaneum | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, i.p, 8 days | Improve peripheral nerve function | [381] |
| Inula viscosa, Inula vulgaris | Aerial parts | Ethanol, maceration | Alloxan diabetic rats | 12.5, 25 and 50 mg/kg, 8 days | Long treatment led to reverse free radicals activities | [384] |
| Psoralea bituminosa | Aerial parts | Ethanol (80%), ethyl acetate, hexane, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, 8 and 21 days | Significant management of neuropathic pain | [386] |
| Rheum ribes | Rhizome | Water, maceration | Alloxan diabetic rats | 12.5, 25, 50 mg/kg, 8 days | Had a protective effect against diabetes and diabetic neuropathy | [381] |
| Salvia libanotica | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 12.5, 25, 50 mg/kg, 8 days | Improve peripheral nerve function | [381] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Atriplex halimus | Katf | Chenopodiaceae | Diabetes, heart disease | Leaf | [406] |
| Ocimum basilicum | Rehan | Lamiaceae | Diarrhea, kidney stone, carminative, diuretic, dysentery, skin disease, anti-colic. | Whole palnt | [407,408] |
| Sarcopoterium spinosum | Bullan, Natesh | Rosaceae | Anti-inflammatory, toothache, analgesic, for hemorrhoids, antidiabetic, stomach pain, diuretic, renal calculi, stimulant for circulation | Root, fruit, seed | [409] |
| Trigonella foenum-graecum | Hilbeh | Fabaceae | Diabtes, cancer | Seed | [410,411] |
| Withania somnifera | Samoh | Solanaceae | Skin disease, kidney stone, wound healing | Leaves, root, young branches | [412,413] |
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Atriplex halimus | Alkaloid, flavonoid, saponin, sterol, tannin | Antidiabetic, antimicrobial, antioxidant, insecticidal | [392] |
| Ocimum basilicum | Cardiac glycosides, caffeic acid derivatives, essential oil, flavonoid, phenolic acids, saponins, tannins | Antimicrobial, hepatoprotective, hypertension nematicidal | [393,394] |
| Sarcopoterium spinosum | Catechin, epicatechin, essential oil | Antidiabetic | [414] |
| Trigonella foenum-graecum | Alkaloid, flavonoid, polyphenols, saponin, volatile oil | Antidiabetic, antilipidemic, anticarcinogenic, anticataract, antioxidant, immunomodulatory | [399,400,415] |
| Withania somnifera | steroidal alkaloids and lactones, withanolides | Anticancer, immunomodulatory, anti-inflammatory, antistress, adaptogenic, CNS activities, cardiovascular activities | [416] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Atriplex halimus | Leaf, stem | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Increased GLUT4 translocation, EC50 was about 2 mg/mL | [272] |
| Gundelia tournefortii | Aerial parts | Methanol, hexane extract, maceration | Rat L6 muscle cell lines | 0–1 mg/mL | Methanol extract was the most efficient in GLUT4 translocation enhancement in skeletal muscle | [417] |
| Ocimum basilicum | Aerial part | Methanol, hexane, dichloromethane extracts, reflux | L6 muscle cell line | 0–2 mg/mL | The extracts increased GLUT4 translocation up to 7 times | [394] |
| Olea europaea | Leaf | Ethanol(80%) extract, soxhlet | Streptozocin diabetic rats | 10, 20, 40 mg | The extract inhibited both digestion and absorption (concentration-dependent) | [418] |
| Sarcopoterium spinosum | Root | Aqueous extract, decoction | RIN β-cells, L6 myotubes, 3T3-L1 adipocytes, AML-12 hepatocytes | 0.001–10 mg/mL | Preventive effect for progression in diabetes | [395] |
| Root | Aqueous extract, decoction | KK-a/y mice | 600 mg/kg, 6 weeks | Improved insulin sensitivity, increased glucose uptake | [396] | |
| Root, leaf, fruit | Aqueous extract, decoction | 3 T3-L1 adipocytes cell line | Root: 1 mg/mLFruit: 2 mg/mL | Inhibited α-glucosidase and amylase, induced insulin secretion | [397] | |
| Root | Aqueous extract, decoction | High-fat diet mice, KK-Ay male mice | 70 mg/day, 6 weeks | Improved glucose tolerance and sensitivity | [398] | |
| Trigonella foenum-graecum | Seed | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Significant translocation of GLUT4 | [272] |
| Urtica dioica | Leaf, stem | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Extract almost doubled GLUT4 translocation | [272] |
| Withania somnifera | Leaf, root | Methanol extract, maceration, isolated compound | Pancreatic RIN-5F | 50 mg/mL | Extracts increased glucose uptake in myotubes and adipocytes (dose-dependent). Leaf extract increased insulin secretion, Withaferin A increased glucose uptake. | [403] |
| Scientific name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cichorium intybus | Sakızotu, hindiba, sakızçiçeği | Asteraceae | Cancer, kidney stone, hemorrhoids, urinary disorders, wound keeling | Aerial parts, leaf, root | [202,428] |
| Cinnamomum verum | Tarçın | Lauraceae | Not mentioned | Not mentioned | [429] |
| Haplophyllummyrtifolium | Not mentioned | Rutaceae | Sudanese and Mongolian folk medicin: antipyretic, diarrhea | Not mentioned | [430] |
| Helichrysum graveolens | Olmezcicek or altinotu | Asteraceae | diuretics, as lithagogues, for stomachache, for anti-asthmatic properties, against kidney stones | Not mentioned | [431,432] |
| Hedysarum varium | Fabaceae | [420] | |||
| Laurus nobilis | Define | Lauraceae | Antiseptic, against rheumatic pain | Fruit, seed | [433,434] |
| Mentha pulegium | Filiskin, yarpuz | Lamiaceae | Bronchitis, cold, flatulent dyspepsia, intestinal colic, chlorera, food posioning, sinusitis, tuberculosis | Flowering aerial parts | [425,435,436] |
| Phlomis armeniaca | Calba | Lamiaceae | Colds, stomachache | Not mentioned | [419] |
| plantago lanceolata | Giyamambel | Plantaginaceae | Diabetes, stomach ache | Not mentioned | [419] |
| Onobrychis hypargyrea | Not mentioned | Fabaceae | Cold and flu | Not mentioned | [420] |
| Origanum onites | Kekik’ | Lamiaceae | Abdominal ache, bronchitis, cold, diabetes, dizziness, headache, hypertension, high cholesterol, itching, gastralgia, leukemia, toothache, stomach disorders | Aerial parts flowers, leaves | [424] |
| Salvia limbata | Baresaspi | Lamiaceae | Colds, stomach ache, wounds | Not mentioned | [419] |
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cistus laurifolius | Defne yapraklı | Cistaceae | Diabetes, fever in common cold, rheumatic and inflammatory diseases, peptic ulcer, applied externally in a line of the kidneys for urinary inflammations | Leaf, flower, bud, branch | [437,446] |
| Juniperus oxycedrus | KatranArdıcı | Cupressaceae | Abdominal pain bronchitis, calcinosis, common cold, cough, diabetes, gynecological diseases, fungal infections, hemorrhoids, kidney stone, stomachic disorders, kidney inflammation, wounds | Tar, leaf, fruit, berry | [438,447] |
| Origanum minutiflorum | Toga kekik, mountain kekik, | Lamiaceae | Herbal tea | Leaf | [444,448] |
| Rhus coriaria | Sumac | Anacardiacea | Diabetes, toothache, eye diseses | Not mentioned | [449,450] |
| Salvia triloba | AnadoluAdacay | Lamiaceae | Blood, skin andinfectious, ailments of the digestive, circulatory and respiratory systems | Leaf | [445] |
| Thymus praecox | Kekik | Lamiaceae | Herbal tea, condiment | Herbal parts | [451,452] |
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Cistus laurifolius | Flavonoids, phenolic acids | Antiulcerogenic, analgesic, antioxidant, hepatoprotective | [437] |
| Juniperus oxycedrus | Lignans, abietane, sesquiterpenes, diterpenes, biflavonols, tannins, coumarins, flavonoids, sterols, terpenes, mono, sesqui and diterpenes, alkanes, fatty acids, waxes | Antioxidant, antiseptic, antiviral, anti-inflammatory, analgesic, anticancer, antidiabetic, neuroprotective | [438,447,453] |
| Origanum minutiflorum | Essential oils | Antioxidant, antibacterial, antiviral, antifungal | [444] |
| Rhus coriaria | Anthocyanins, flavonoids, isoflavonoids, tannins, terpenoids | Antioxidant, antiseptic, antifungal, antibacterial, anti-ischemic, antifibrogenic, antitumourigenic activities, hypoglycemic, non mutagenic, fever, DNA protective, hypouricaemic, hepatoprotective properties | [449,454] |
| Salvia triloba | Essential oil, flavonoids, flavone, terpene, sesqui, di and triterpenes, steroidal compounds | Antimicrobial | [452,455] |
| Thymus praecox | Essential oil | Anti-inflammatory, antifungal, antiviral, antioxidant, anticancer, antidiabetic effects | [445,452] |
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Cistus laurifolius | Leaf | Water, ethanol, maceration | Streptozocin diabetic rats | 250, 500 mg/kg | Inhibited of α-glucosidaseand α-amylase | [437] |
| Heracleum persicum | Whole plant | Water, maceration | Streptozocin diabetic rats | 100, 200, 400 mg/kg 21 days | Decreased HbA1c, restored diabetic complications parameters towards normal, increased AST and ALT significantly | [320] |
| Juniperus oxycedrus | Berries | Water, maceration isolated compound | Streptozocin diabetic rats | 500, 1000 mg/kg Shikimic acid: 15 and 30 mg/kg, 8 days | The effect of shikimic acid was more effective than the reference antidiabetic drug glipizide, TG and enzyme levels were reduced significantly | [438] |
| Leaf | Water, ethanol, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg | Fatty acids were found as the major compounds | [440] | |
| Fruit, leaf | Ethanol 80%, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg, 10 days | Augment Zn level in liver | [441] | |
| Juniperus foetidissima & Juniperus sabina | Leaf, fruit | Ethanol 80%, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg, 8 days | Leaf extract caused death at both doses | [439] |
| Origanum minutiflorum | Whole plant | Water, decoction | Streptozocin diabetic rats | 150 mg/kg, 30 days | Decreased ALT and AST levels | [444] |
| Salvia triloba | Aerial parts | Methanol 80%, soxhlet | Streptozocin-nicotinamide diabetic rats | 100, 200 mg/kg, 21 days | Little weight loss | [445] |
| Thymus praecox | Aerial parts | Methanol, maceration | Streptozocin-nicotinamide diabetic rats | 100, 200 mg/kg, 21 days | Little weight loss | [445] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Odeh, A.M.; Talib, W.H. Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules 2021, 26, 742. https://doi.org/10.3390/molecules26030742
Abu-Odeh AM, Talib WH. Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules. 2021; 26(3):742. https://doi.org/10.3390/molecules26030742
Chicago/Turabian StyleAbu-Odeh, Alaa M., and Wamidh H. Talib. 2021. "Middle East Medicinal Plants in the Treatment of Diabetes: A Review" Molecules 26, no. 3: 742. https://doi.org/10.3390/molecules26030742
APA StyleAbu-Odeh, A. M., & Talib, W. H. (2021). Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules, 26(3), 742. https://doi.org/10.3390/molecules26030742







