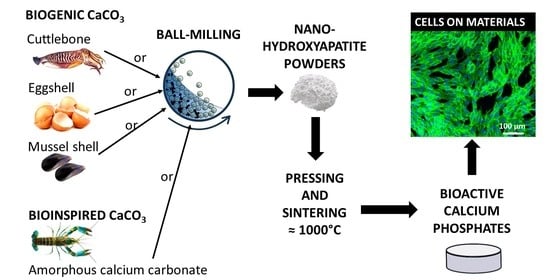
Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Materials Characterization
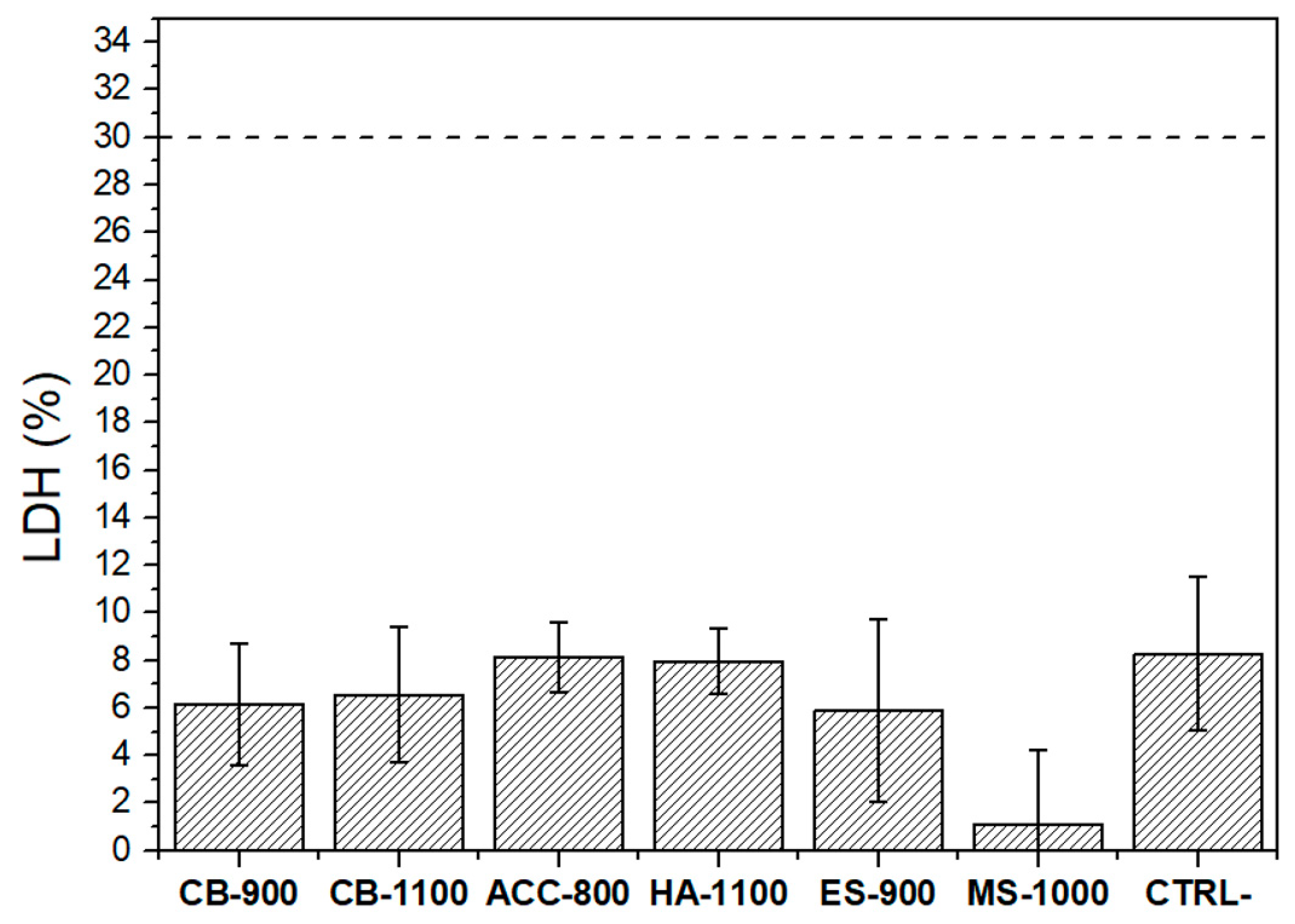
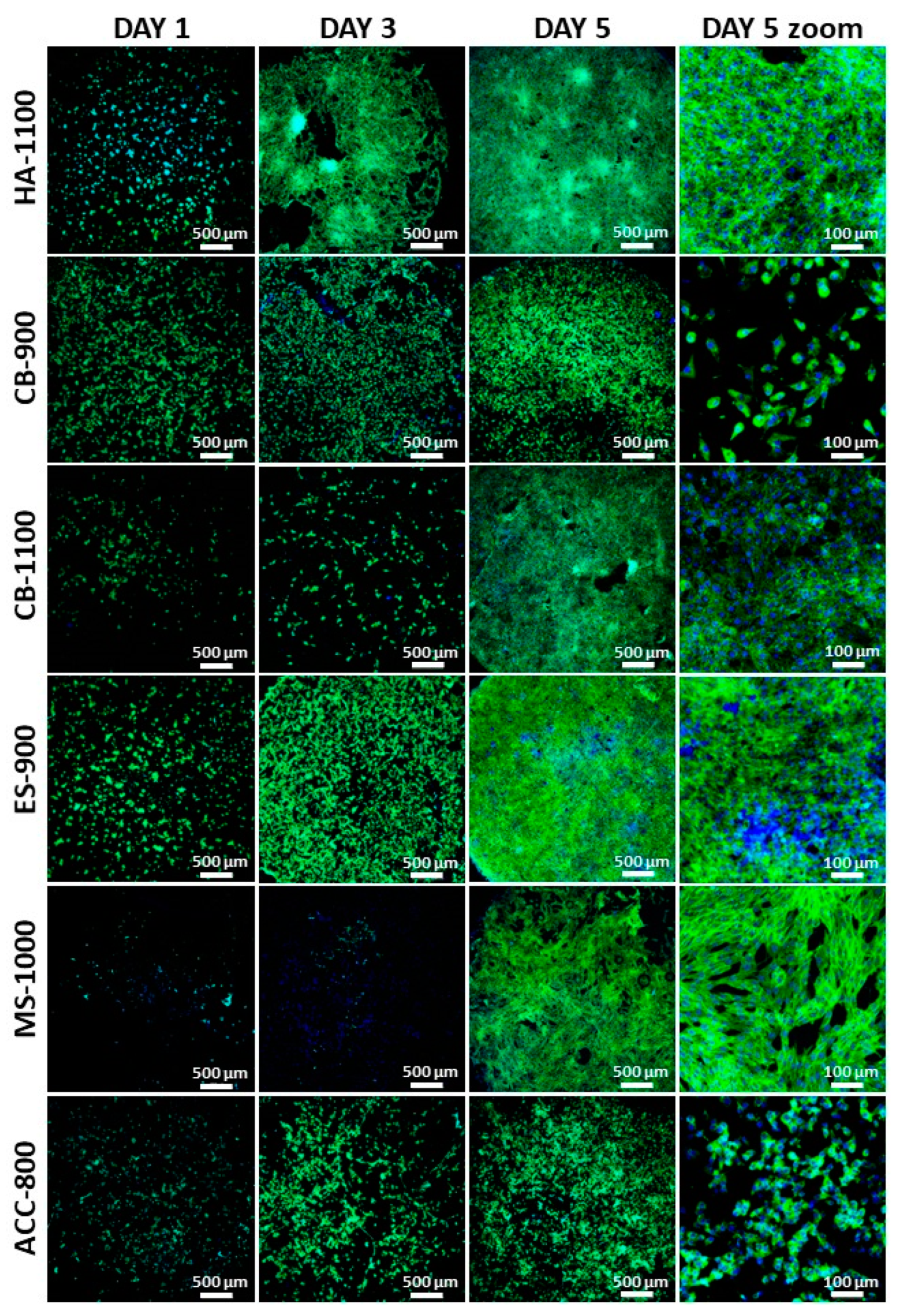
3.2. In Vitro Biological Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeGeros, R.Z.; LeGeros, J.P. Phosphate Minerals in Human Tissues. Photosphate Miner. 1984, 351–385. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Clarke, S.; Walsh, P. Marine Organisms for Bone Repair and Regeneration; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 294–318. [Google Scholar]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Landi, E.; Uggeri, J.; Medri, V.; Guizzardi, S. Sr, Mg cosubstituted HA porous macro-granules: Potentialities as resorbable bone filler with antiosteoporotic functions. J. Biomed. Mater. Res. Part A 2013, 101, 2481–2490. [Google Scholar] [CrossRef]
- Sader, M.S.; LeGeros, R.Z.; Soares, G.A. Human osteoblasts adhesion and proliferation on magnesium-substituted tricalcium phosphate dense tablets. J. Mater. Sci. Mater. Med. 2009, 20, 521–527. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Supová, M. Isolation and preparation of nanoscale bioapatites from natural sources: A review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.C.; Yoon, Y.S. Characteristics of calcium phosphate powders synthesized from cuttlefish bone and phosphoric acid. J. Ceram. Process. Res. 2007, 8, 427–430. [Google Scholar]
- Ivankovic, H.; Tkalčec, E.; Orlić, S.; Ferrer, G.G.; Schauperl, Z. Hydroxyapatite formation from cuttlefish bones: Kinetics. J. Mater. Sci. Mater. Electron. 2010, 21, 2711–2722. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic, H.; Ferrer, G.G.; Tkalcec, E.; Orlic, S. Preparation of highly porous hydroxyapatite from cuttlefish bone. J. Mater. Sci. Mater. Med. 2009, 20, 1039–1046. [Google Scholar] [CrossRef]
- Tkalčec, E.; Popović, J.; Orlić, S.; Milardović, S.; Ivanković, H. Hydrothermal synthesis and thermal evolution of carbonate-fluorhydroxyapatite scaffold from cuttlefish bones. Mater. Sci. Eng. C 2014, 42, 578–586. [Google Scholar] [CrossRef]
- Reinares-Fisac, D.; Veintemillas-Verdaguer, S.; Fernández-Díaz, L. Conversion of biogenic aragonite into hydroxyapatite scaffolds in boiling solutions. CrystEngComm 2016, 19, 110–116. [Google Scholar] [CrossRef]
- Venkatesan, J.; Rekha, P.D.; Anil, S.; Bhatnagar, I.; Sudha, P.N.; Dechsakulwatana, C.; Kim, S.-K.; Shim, M.S. Hydroxyapatite from Cuttlefish Bone: Isolation, Characterizations, and Applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Milovac, D.; Ferrer, G.G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C 2014, 34, 437–445. [Google Scholar] [CrossRef]
- Rocha, J.; Lemos, A.; Agathopoulos, S.; Valério, P.; Kannan, S.; Oktar, F.; Ferreira, J.M. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.; Agathopoulos, S.; Ferreira, J. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007, 3, 243–249. [Google Scholar] [CrossRef]
- Rivera, E.M.; Araiza, M.; Brostow, W.; Castaño, V.M.; Díaz-Estrada, J.; Hernández, R.; Rodríguez, J. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999, 41, 128–134. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S. Fabrication of calcium phosphate bioceramics by using eggshell and phosphoric acid. Mater. Lett. 2003, 57, 4570–4574. [Google Scholar] [CrossRef]
- Balázsi, C.; Wéber, F.; Kövér, Z.; Horváth, E.; Németh, C. Preparation of calcium–phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 2007, 27, 1601–1606. [Google Scholar] [CrossRef]
- Sanosh, K.; Chu, M.-C.; Balakrishnan, A.; Kim, T.-N.; Cho, S.-J. Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Mater. Lett. 2009, 63, 2100–2102. [Google Scholar] [CrossRef]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Kashkarov, V.; Rumyantseva, N.; Domashevskaya, E.P.; Lenshin, A.; Agapov, B.; Domashevskaya, E. Synthesis of nanocrystalline hydroxyapatite by precipitation using hen’s eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Ramesh, S.; Natasha, A.; Tan, C.; Bang, L.; Ching, C.; Chandran, H. Direct conversion of eggshell to hydroxyapatite ceramic by a sintering method. Ceram. Int. 2016, 42, 7824–7829. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.K.; Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kumar, T.; Shantha, K.; Rao, K. Development of hydroxyapatite derived from Indian coral. Biomaterials 1996, 17, 1709–1714. [Google Scholar] [CrossRef]
- Jinawath, S.; Polchai, D.; Yoshimura, M. Low-temperature, hydrothermal transformation of aragonite to hydroxyapatite. Mater. Sci. Eng. C 2002, 22, 35–39. [Google Scholar] [CrossRef]
- Hu, J.; Russell, J.J.; Bennissan, B.; Vago, R. Production and analysis of hydroxyapatite from Australian corals via hydrothermal process. J. Mater. Sci. Lett. 2001, 20, 85–87. [Google Scholar] [CrossRef]
- Walsh, P.; Buchanan, F.; Dring, M.; Maggs, C.; Bell, S.; Walker, G. Low-pressure synthesis and characterisation of hydroxyapatite derived from mineralise red algae. Chem. Eng. J. 2008, 137, 173–179. [Google Scholar] [CrossRef]
- Lemos, A.; Rocha, J.; Quaresma, S.; Kannan, S.; Oktar, F.; Agathopoulos, S.; Ferreira, J. Hydroxyapatite nano-powders produced hydrothermally from nacreous material. J. Eur. Ceram. Soc. 2006, 26, 3639–3646. [Google Scholar] [CrossRef]
- De Paula, S.M.; Huila, M.; Araki, K.; Toma, H.E. Confocal Raman and electronic microscopy studies on the topotactic conversion of calcium carbonate from Pomacea lineate shells into hydroxyapatite bioceramic materials in phosphate media. Micron 2010, 41, 983–989. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, Y.-N.; Ho, W.-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Mater. Charact. 2011, 62, 1180–1187. [Google Scholar] [CrossRef]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of Hydroxyapatite from Oyster Shell via Precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149–150, 607–616. [Google Scholar] [CrossRef]
- Pal, A.; Maity, S.; Chabri, S.; Bera, S.; Chowdhury, A.R.; Das, M.; Sinha, A. Mechanochemical synthesis of nanocrystalline hydroxyapatite from Mercenaria clam shells and phosphoric acid. Biomed. Phys. Eng. Express 2017, 3, 015010. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K.; Venkatesh, M.; Karunakaran, G.; Kolesnikov, E.; Kuznetsov, D. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram. Int. 2017, 43, 3457–3461. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Girija, E.K. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater. Lett. 2012, 76, 198–200. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Albéric, M.; Stifler, C.A.; Zou, Z.; Sun, C.-Y.; Killian, C.E.; Valencia, S.; Mawass, M.-A.; Bertinetti, L.; Gilbert, P.U.; Politi, Y. Growth and regrowth of adult sea urchin spines involve hydrated and anhydrous amorphous calcium carbonate precursors. J. Struct. Biol. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zoo L. 2002, 293, 478–491. [Google Scholar] [CrossRef]
- Jia, S.; Guo, Y.; Zai, W.; Su, Y.; Yuan, S.; Yu, X.; Li, G. Preparation and characterization of a composite coating composed of polycaprolactone (PCL) and amorphous calcium carbonate (ACC) particles for enhancing corrosion resistance of magnesium implants. Prog. Org. Coat. 2019, 136, 105225. [Google Scholar] [CrossRef]
- Myszka, B.; Schüßler, M.; Hurle, K.; Demmert, B.; Detsch, R.; Boccaccini, A.R.; Wolf, S.E. Phase-specific bioactivity and altered Ostwald ripening pathways of calcium carbonate polymorphs in simulated body fluid. RSC Adv. 2019, 9, 18232–18244. [Google Scholar] [CrossRef] [Green Version]
- Cozza, N.; Monte, F.; Bonani, W.; Aswath, P.; Motta, A.; Migliaresi, C. Bioactivity and mineralization of natural hydroxyapatite from cuttlefish bone and Bioglass ® co-sintered bioceramics. J. Tissue Eng. Regen. Med. 2018, 12, e1131–e1142. [Google Scholar] [CrossRef] [Green Version]
- Milovac, D.; Gamboa-Martínez, T.C.; Ivankovic, M.; Ferrer, G.G.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: In vitro cell culture studies. Mater. Sci. Eng. C 2014, 42, 264–272. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of sea urchin spines to Mg-substituted tricalcium phosphate for bone implants. Acta Biomater. 2007, 3, 785–793. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; Lee, J. A polycaprolactone/cuttlefish bone-derived hydroxyapatite composite porous scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 943–951. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kang, H.J.; Yang, S.-S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Mechanism of the Solid-State Transformation of a Calcium-Deficient Hydroxyapatite (CDHA) into Biphasic Calcium Phosphate (BCP) at Elevated Temperatures. Chem. Mater. 2002, 14, 4267–4272. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Kim, H.H.; Phan, T.T.V.; Oh, J. Comparative characterization of biogenic and chemical synthesized hydroxyapatite biomaterials for potential biomedical application. Mater. Chem. Phys. 2019, 228, 344–356. [Google Scholar] [CrossRef]
- Cestari, F.; Chemello, G.; Galotta, A.; Sglavo, V.M. Low-temperature synthesis of nanometric apatite from biogenic sources. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Pepponi, G. Combining XRD and XRF analysis in one Rietveld-like fitting. Powder Diffr. 2017, 32, S225–S230. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Zolotoyabko, E.; Caspi, E.N.; Fieramosca, J.S.; Von Dreele, R.B.; Marin, F.; Mor, G.; Addadi, L.; Weiner, S.; Politi, Y. Differences between Bond Lengths in Biogenic and Geological Calcite. Cryst. Growth Des. 2010, 10, 1207–1214. [Google Scholar] [CrossRef]
- Caspi, E.N.; Pokroy, B.; Lee, P.L.; Quintana, J.P.; Zolotoyabko, E. On the structure of aragonite. Acta Cryst. Sect. B Struct. Sci. 2005, 61, 129–132. [Google Scholar] [CrossRef]
- Ardanova, L.I.; Get’Man, E.I.; Loboda, S.N.; Prisedskii, V.V.; Tkachenko, T.V.; Marchenko, V.I.; Antonovich, V.P.; Chivireva, N.A.; Chebishev, K.A.; Lyashenko, A.S. Isomorphous Substitutions of Rare Earth Elements for Calcium in Synthetic Hydroxyapatites. Inorg. Chem. 2010, 49, 10687–10693. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Boudin, S.; Grandin, A.; Borel, M.M.; LeClaire, A.; Raveau, B. Redetermination of the β-Ca2P2O7 structure. Acta Cryst. Sect. C Cryst. Struct. Commun. 1993, 49, 2062–2064. [Google Scholar] [CrossRef]
- Fiquet, G.; Richet, P.; Montagnac, G. High-temperature thermal expansion of lime, periclase, corundum and spinel. Phys. Chem. Min. 1999, 27, 103–111. [Google Scholar] [CrossRef]
- Busing, W.R.; Levy, H.A. Neutron Diffraction Study of Calcium Hydroxide. J. Chem. Phys. 1957, 26, 563–568. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2010, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Conforto, E.; Caillard, D.; Müller, F.A. Biomimetic apatite coatings—Carbonate substitution and preferred growth orientation. Biomol. Eng. 2007, 24, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Berndt, C.C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- Bigi, A.; Marchetti, F.; Ripamonti, A.; Roveri, N.; Foresti, E. Magnesium and strontium interaction with carbonate-containing hydroxyapatite in aqueous medium. J. Inorg. Biochem. 1981, 15, 317–327. [Google Scholar] [CrossRef]
- Bigi, A. Isomorphous substitutions in β-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Kannan, S.; Pina, S.; Ferreira, J.M. Formation of Strontium-Stabilized?-Tricalcium Phosphate from Calcium-Deficient Apatite. J. Am. Ceram. Soc. 2006, 89, 3277–3280. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg2+ doping on beta–alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef]
- Batra, U.; Kapoor, S.; Sharma, S. Influence of Magnesium Ion Substitution on Structural and Thermal Behavior of Nanodimensional Hydroxyapatite. J. Mater. Eng. Perform. 2013, 22, 1798–1806. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci. Mater. Electron. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weiner, S.; Addadi, L. Coexistence of Amorphous and Crystalline Calcium Carbonate in Skeletal Tissues. Connect. Tissue Res. 2003, 44, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lilley, K.J.; Gbureck, U.; Wright, A.J.; Farrar, D.F.; Barralet, J.E. Cement from nanocrystalline hydroxyapatite: Effect of calcium phosphate ratio. J. Mater. Sci. Mater. Med. 2005, 16, 1185–1190. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Paul, W.; Sharma, C.P. Effect of calcium, zinc and magnesium on the attachment and spreading of osteoblast like cells onto ceramic matrices. J. Mater. Sci. Mater. Electron. 2006, 18, 699–703. [Google Scholar] [CrossRef]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: Towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. Part A 2019, 108, 633–644. [Google Scholar] [CrossRef]
| Raw Material | Label | Milling Time | Phosphate Provider | Drying Temperature |
|---|---|---|---|---|
| DENSITY™ | ACC | 30 min | (NH4)2HPO4 | 120 °C |
| Eggshell | ES | 4 h | H3PO4 | 150 °C |
| Mussel shell | MS | 4 h | (NH4)2HPO4 | 150 °C |
| Cuttlebone | CB | 30 min | (NH4)2HPO4 | 120 °C |
| Raw Material | Synthesized Powder | Sintered Pellet | |||
|---|---|---|---|---|---|
| Label | Phase Composition | Label | Phase Composition | Label | Phase Composition |
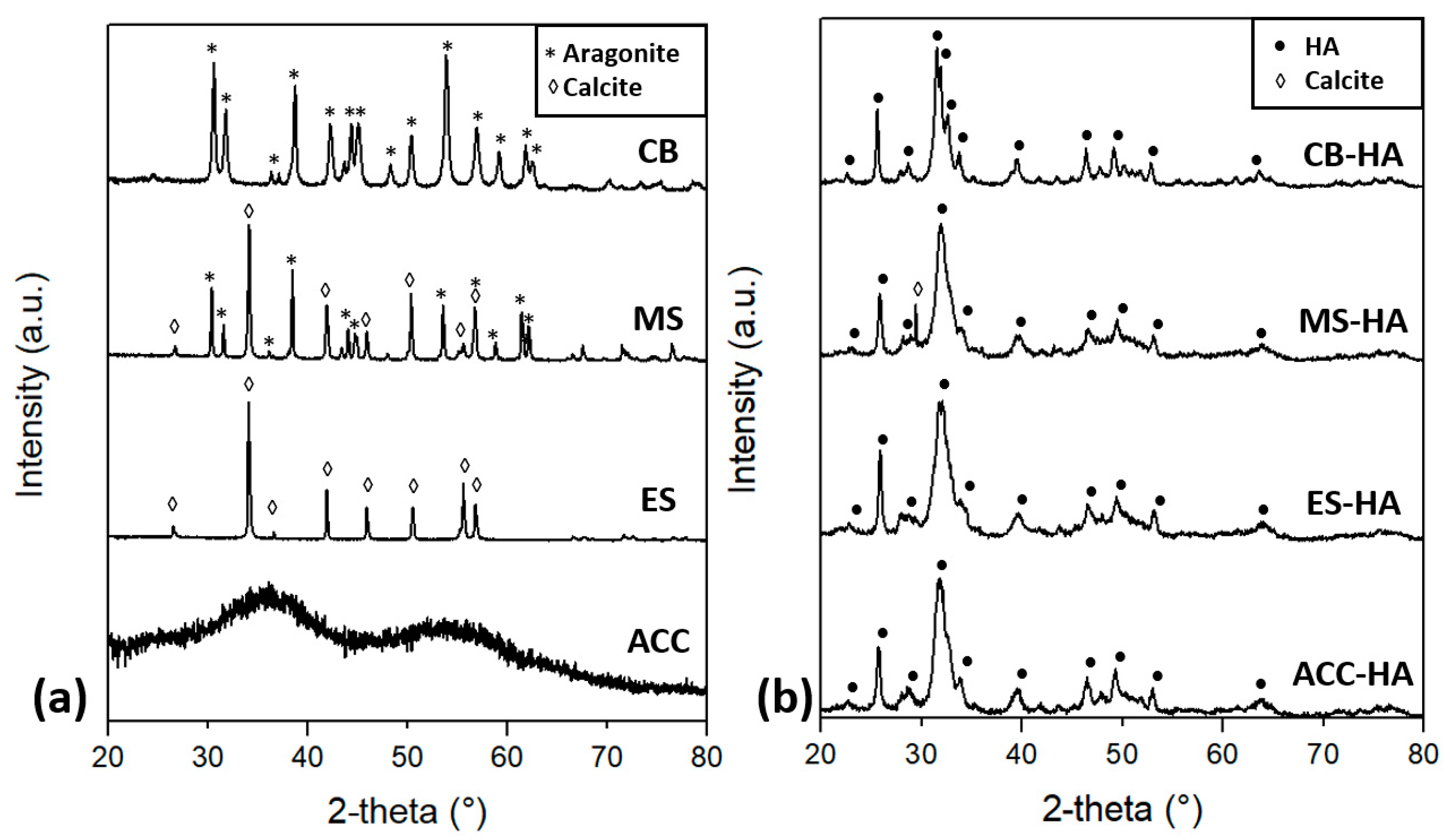
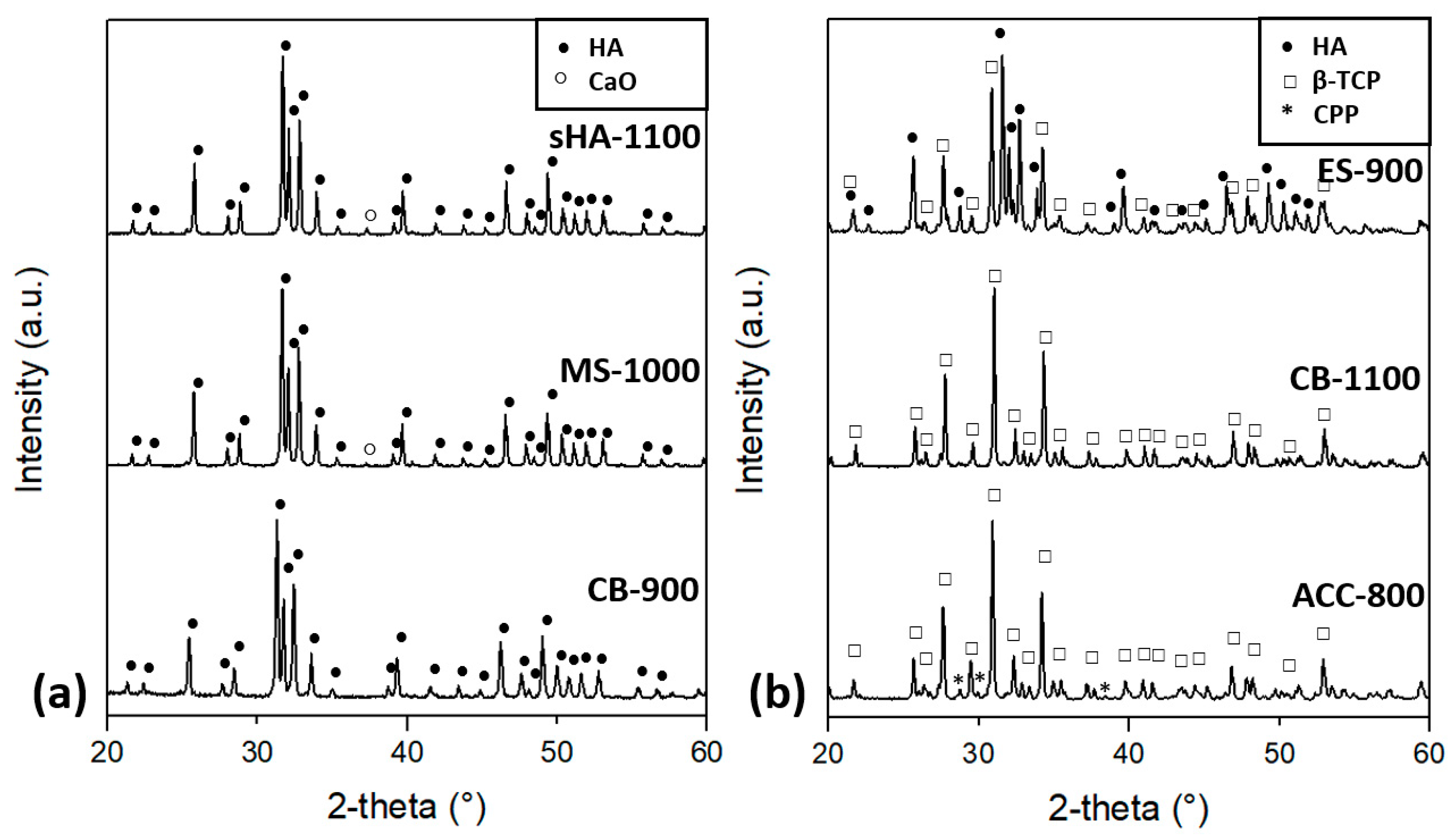
| ACC | 100% amorphous CaCO3 | ACC–HA | 100% HA | ACC-800 | ~85% β-TCP, ~15% CPP |
| ES | 100% calcite | ES–HA | 100% HA | ES-900 | ~50% HA, ~50% β-TCP |
| MS | ~70% calcite, ~30% aragonite | MS–HA | ~93% HA, ~7% calcite | MS-1000 | HA, <3% CaO |
| CB | 100% aragonite | CB–HA | 100% HA | CB-900 | 100% HA |
| CB-1100 | ~90% β-TCP, ~5% HA, ~5% CaOH | ||||
| sHA | - | - | - | sHA-1100 | HA, <3% CaO |
| P | Ca/P Molar Ratio | Na | K | Mg | Sr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | |
| ACC | 2.0% | 1.28 ± 0.02 | 1.5% | 1.4% | <0.1% | - | - | - | <0.1% | <0.1% |
| MS | <0.2% | 1.76 ± 0.02 | 0.3% | 0.3% | <0.1% | - | 0.1% | 0.1% | 0.1% | 0.1% |
| CB | <0.2% | 1.64 ± 0.02 | 0.7% | 0.9% | 0.1% | 0.1% | <0.1% | 0.1% | 0.2% | 0.2% |
| ES | <0.2% | 1.58 ± 0.02 | 0.1% | 0.1% | 0.1% | 0.1% | 0.4% | 0.3% | <0.1% | <0.1% |
| sHA | - | 1.71 ± 0.02 | - | <0.1% | - | <0.1% | - | <0.1% | - | <0.1% |
| ACC-800 | MS-1000 | CB-900 | CB-1100 | ES-900 | sHA-1100 | |
|---|---|---|---|---|---|---|
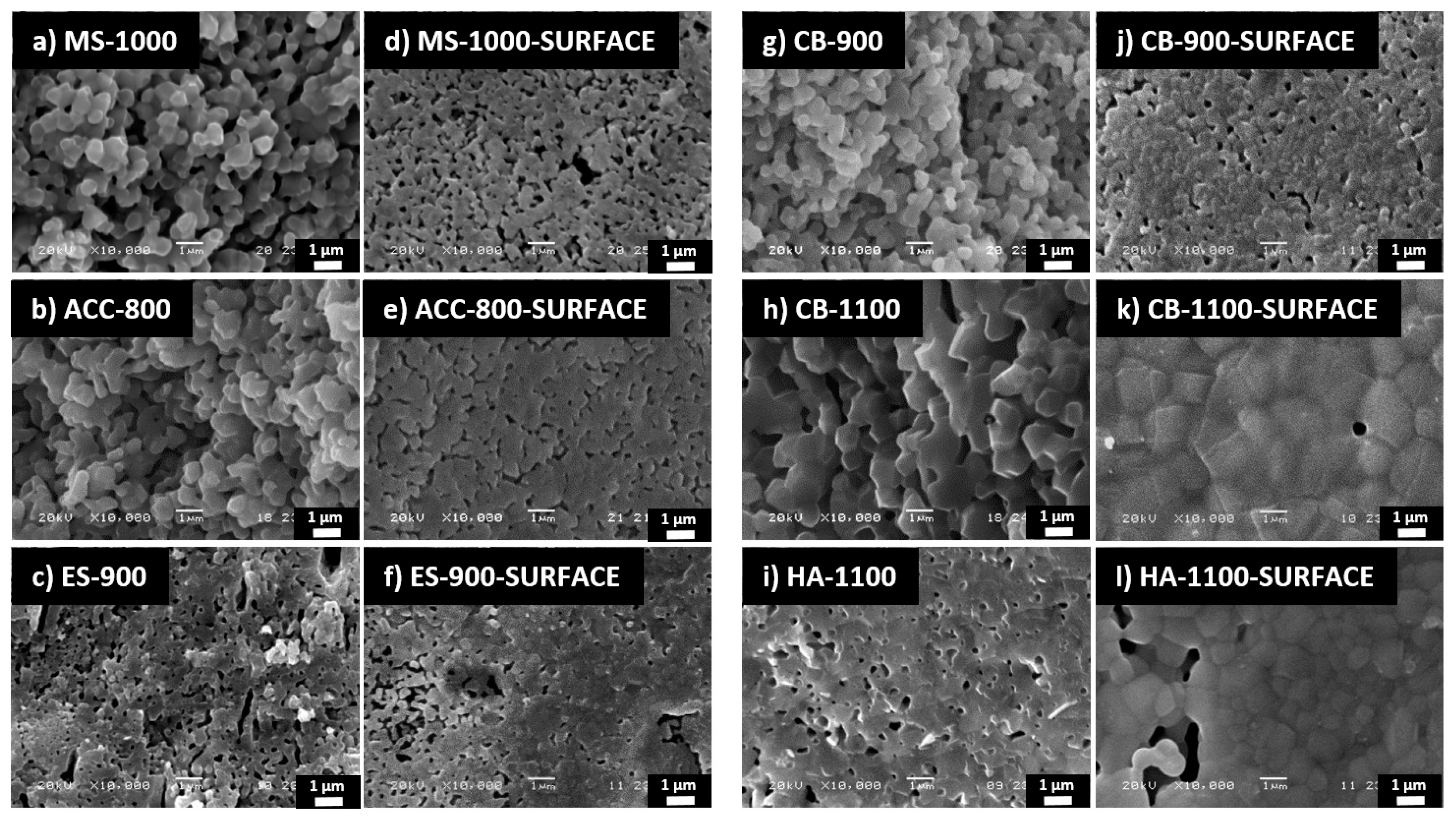
| Bulk density (g/cm3) | 2.19 ± 0.03 | 2.18 ± 0.08 | 2.33 ± 0.03 | 2.78 ± 0.03 | 2.06 ± 0.02 | 2.82 ± 0.08 |
| Relative density (%) | 71 ± 1 | 69 ± 2 | 74 ± 1 | 91 ± 1 | 66 ± 1 | 89 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; M. Sglavo, V. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. https://doi.org/10.3390/nano11020264
Cestari F, Agostinacchio F, Galotta A, Chemello G, Motta A, M. Sglavo V. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials. 2021; 11(2):264. https://doi.org/10.3390/nano11020264
Chicago/Turabian StyleCestari, Francesca, Francesca Agostinacchio, Anna Galotta, Giovanni Chemello, Antonella Motta, and Vincenzo M. Sglavo. 2021. "Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity" Nanomaterials 11, no. 2: 264. https://doi.org/10.3390/nano11020264
APA StyleCestari, F., Agostinacchio, F., Galotta, A., Chemello, G., Motta, A., & M. Sglavo, V. (2021). Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials, 11(2), 264. https://doi.org/10.3390/nano11020264