3.1. Synthesis of Zeolite from Geothermal Solid Waste
Zeolite catalyst was synthesized using geothermal solid waste from PT. Geo Dipa, Dieng-Wonosobo, Central Java. The synthesis was carried out by hydrothermal and calcination methods. Before use, the impurities in the waste were removed using distilled water. The clean waste content was analyzed using EDX. The metal oxide content of the waste is presented in
Table 2.
Two main elements, SiO
2 and Al
2O
3, are parameters for the feasibility of utilizing geothermal waste in the zeolite synthesis process [
23]. The synthesis of zeolite is very sensitive to feedstock properties, especially from silica and alumina sources. Silica can influence many aspects of zeolite crystallization, including crystal growth kinetics and product properties. The application of different silica sources greatly affects the results of the synthesis experiment [
24]. In this research, 35%wt of zeolite was obtained from geothermal waste. Based on chemical composition data, geothermal solid waste has the potential to be synthesized into zeolites.
The elemental composition of the geothermal waste and synthesized zeolite has been analyzed using X-Ray Fluorescence (XRF) and the results are presented in
Table 3.
Based on the results of XRF analysis, the highest impurity in geothermal waste was Fe, while other elements were concluded to be negligible due to the very small amount detected. Iron (Fe) is a common metal found in the geothermal waste and was present partly due to dissolved iron from carbon steel piping and brine handling equipment in the geothermal plant [
25]. The presence of iron in the waste would not affect the zeolite synthesis, as it would be irreversibly self-reduced into Fe
2+ atoms when zeolite activation (calcination) took place later in the reaction [
26].
The XRD characterization used to determine the crystallinity and purity levels of the geothermal waste and synthesized zeolite is shown in
Figure 1.
As shown in
Figure 1, the geothermal waste used in this research was amorphous, indicated by the lack of sharp peaks present in the diffractogram result. In contrast, the synthesized zeolite catalyst diffractogram shows that the position of the peak intensity point is observed at a certain 2θ. The synthesized zeolite has many sharp peaks as shown on the diffractogram, indicating that it was more crystal than to the geothermal waste. It could also be concluded that the zeolite has been successfully synthesized from the geothermal waste, due to the significant increase in crystallinity from amorphous to crystalline and complete change of the peaks and patterns between them.
Based on the diffractogram pattern and the use of match software, the highest peak intensities of the synthesized zeolite were observed at 15.977, 26.123, and 30.717°. It indicates that the catalyst has a tetragonal crystal system. All of the highest peaks showed an analcime (Al1.9Na1.86O12Si4) diffraction pattern (The International Centre for Diffraction Data (ICDD) No. 00-044-01030).
Other information obtained from the above diffractogram, based on the analysis using match software: analcime crystals have cell parameters a = 13.6251 Å and c = 13.5870 Å and a density of 2.112 g/mL with a tetragonal crystal system. The synthetic zeolite structure has the general formula M
x/n [(AlO
2)
x(SiO
2)
y].
wH
2O [
27], where M is an alkaline or alkaline earth cation, n is the number of valence cations, w is the number of water molecules per unit cell, x and y is the amount of tetrahedra alumina silicate in the unit cell, while the ratio of y/x or SiO
2/Al
2O
3 is the acidity of zeolite. Based on the XRF analysis results in
Table 3, the Si/Al ratio obtained is 2.39. This result is in accordance with the study conducted by Chen et al. [
28] who obtained the Si/Al ratio of 2.0.
Compared to the diffractogram pattern of analcime zeolite catalysts synthesized from the stem of sorghum Halepenesic ash extract [
29], the highest peak intensity points were observed at 16.0, 26.0, and 30.5°. It can be concluded that the zeolite characterization of the diffractogram pattern does not have a significant difference. Therefore, according to the experimental results, the zeolite synthesized from geothermal solid waste is analcime zeolite.
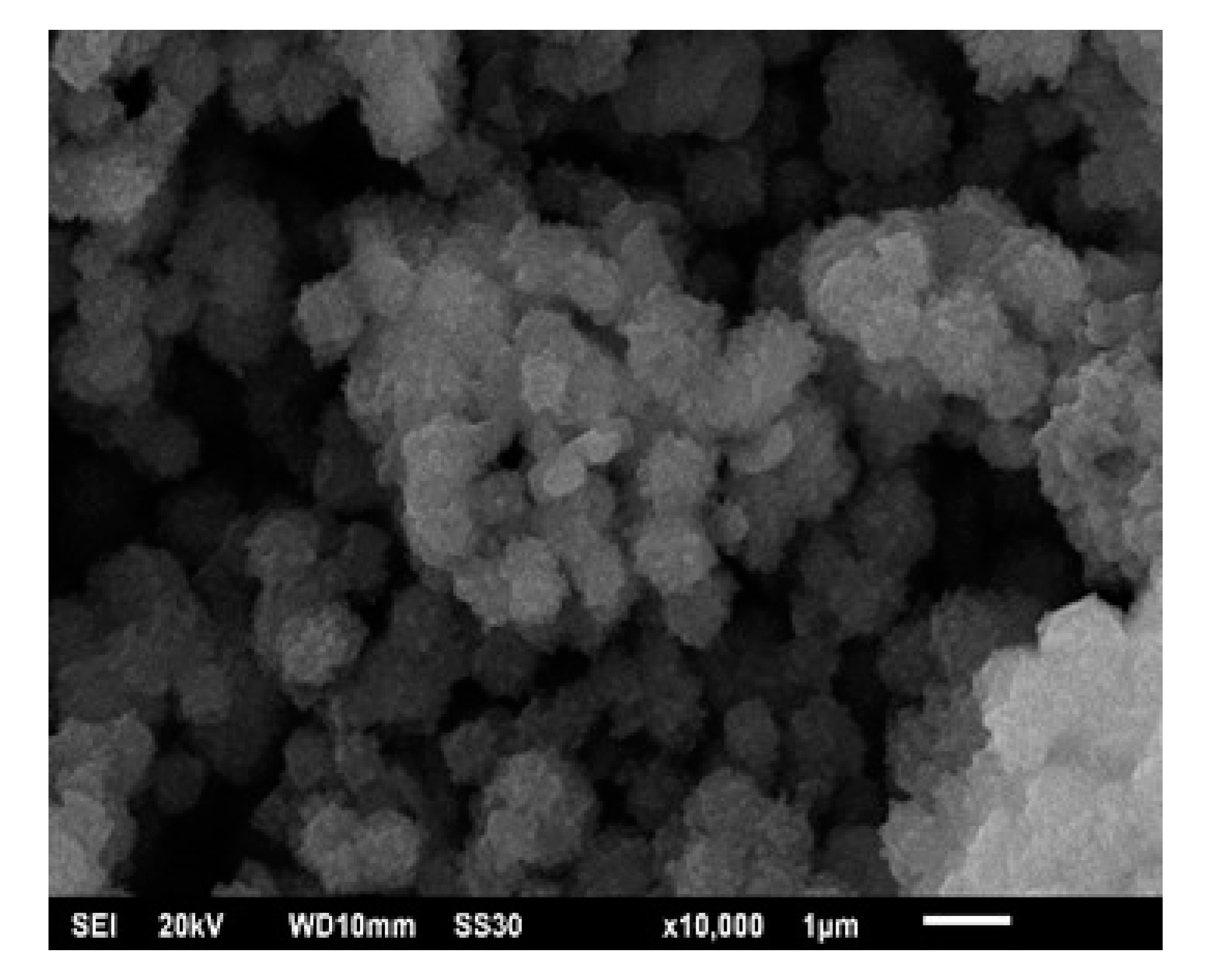
Morphology plays an important role in catalyst activity and selectivity.
Figure 2 presents SEM analysis results in the form of zeolite catalyst morphology with a magnification of 10,000×.
Figure 2 shows the morphology of stacked sheet-like structures, repetitive, and having a book with a tetragonal crystal shape. According to Verboekend et al. [
30], zeolite crystal formation occurs in hydrothermal processes. In this research, the hydrothermal process was conducted by adding heat at 150 °C for 8 h to form a more regular arrangement.
Based on the SEM analysis, it has been established that zeolites are aluminosilicate crystals. Besides being influenced by Si-Al and OH content, the formation of aluminosilicate crystals in this study was also carried out by adding 3 M NaOH solution. The addition of NaOH solution functions to reduce the amorphous phase and increase the crystal phase. In addition, it also acts as an activator by activating the silica and aluminum components found in geothermal waste to form water-soluble aluminates and silicate salts, which then play a role in the formation of zeolites during the hydrothermal process [
31]. The addition of NaOH solution made the solution alkaline (pH > 12) to polymerize zeolite to form ions. The zeolite skeletal ions are Si(OH)
4− as crystalline silica and anion Al(OH)
4−, which is derived from the alumina source.
Figure 2 also shows that calcination of the silica-alumina at a temperature of 550 °C for 5 h affects the morphology of the catalyst. Si and Al can be evenly dispersed on the surface because water and impurities on the surface can be removed. Calcination is the physical activation of the catalyst by hot air or a vacuum system to release water molecules.
Table 4 presents the results of the BET analysis regarding the specific surface area of the synthesized zeolite, before and after calcination.
In this study, calcination at a temperature of 550 °C for 5 h affects the surface area of the catalyst produced because it can remove water and unwanted liquid (impurities) in the zeolite, which is left in the pores of the material, thereby increasing the surface area of the zeolite [
32].
Table 4 shows that if the catalyst were calcined at a higher temperature (550 °C) it would increase the surface area quite significantly.
3.2. Conversion of Used Cooking Oil to Biodiesel
Biodiesel is produced from used cooking oil. Used cooking oil, before it is used as raw material, is filtered first to remove impurities. Used cooking oil, cleaned from impurities, was analyzed by GCMS to determine the components of its constituent compounds. The composition of used cooking oil as a result of the analysis was then compared with the results of an experiment conducted by Syam et al. [
33] and Banani et al. [
34], and is presented in
Table 5.
The results of the GCMS analysis in
Table 5 show the differences in fatty acid composition. The amount of fatty acid composition in used cooking oil depends on the frequency of use and the type of material fried in the oil.
Table 5 shows that the GCMS analysis results of used cooking oil in this study have the highest acid content, namely 9-Octadecenoic acid, which is included in oleic acid. The used cooking oil used in this study is a vegetable oil derived from CPO. Generally, vegetable oils contain 90–98% triglycerides [
35]. It means that used cooking oil has the potential as a raw material for biodiesel production. The characteristics of used cooking oil include density and viscosity, 944 kg/m
3 and 30.182 mm
2/s, respectively.
Biodiesel production is conducted by an esterification–transesterification process using a zeolite catalyst. The biodiesel content produced in this study is shown in
Table 6.
According to the International Agency for Research on Cancer (IARC) [
36], diesel fuel consists of hydrocarbons with a C
9–C
20 carbon chain. Based on
Table 6, the hydrocarbons formed are types of diesel oil (C
9–C
20) and heavy hydrocarbons (>C
20).
Table 6 shows that the percentage of biodiesel containing hydrocarbons > C
20 is very small, namely 0.54% in the form of Hexadecanoic acid, 2-hydroxy-1,3-propannediyl ester. The other, which is the main component, is diesel oil products (C
9–C
20). The compound of Hexadecanoic acid, 2-hydroxy-1,3-propanediol ester (C
35H
68O
5) is an ester bonding compound with an OH group in the second chain.
The results obtained in this study were better than those of other researchers who used zeolites as catalysts. In a study conducted by Hartono et al. [
37], using Banten Bayah natural zeolite modified with potassium hydroxide, the yield of biodiesel was 94%. Meanwhile, Al-Jamal et al. [
38] used zeolite tuff impregnated with KOH for biodiesel production and provided a maximum biodiesel yield of 96.7%. Ramos et al. [
39] used zeolite catalysts (mordenite, beta, and X) to produce biodiesel from sunflower oil. The yield of methyl esters ranged between 93.5 and 95.1%. The use of Fe/zeolite catalyst from geothermal waste was carried out by Muna et al. [
40]. The highest biodiesel yield was obtained at 87.8%. The yield of biodiesel produced in this study was 98.29%. This result is higher than that of biodiesel produced by several other researchers. In addition, the advantage of this research is that the resulting product does not contain glycerol, so that it can produce a high yield and purity of biodiesel.
The density and viscosity of the biodiesel produced from the esterification–transesterification process were measured and compared with the Indonesian National Standard (SNI). The results are presented in
Table 7 and
Table 8.
Based on
Table 7 and
Table 8, the biodiesel density and viscosity obtained have different values for each variable. The biodiesel density and viscosity obtained in this study have good quality because they fulfill the SNI biodiesel standards (SNI No. 7182-2015). According to SNI, the density is 850–890 kg/m
3 and a maximum viscosity of 6 mm
2/s [
41].
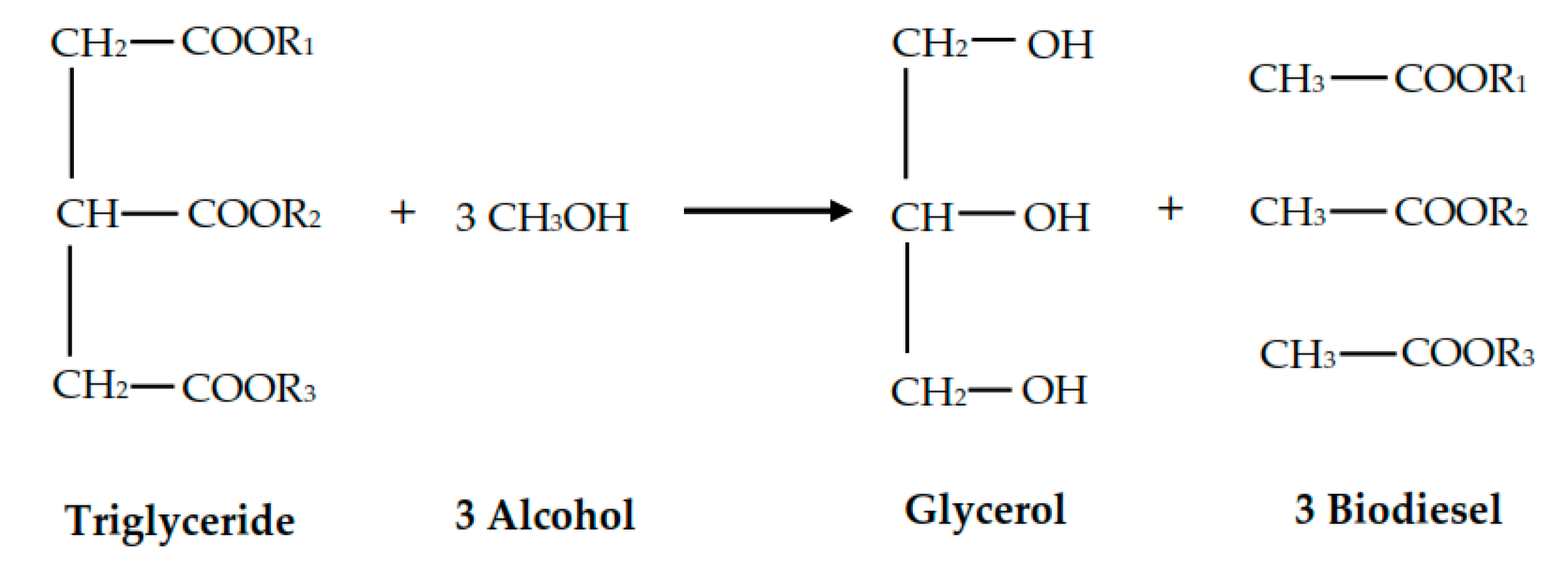
According to Kouzu et al. [
42], the reaction between 1 mole of triglycerides and 3 moles of alcohol will produce three moles of biodiesel and one mole of glycerol, as shown in
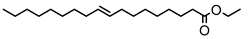
Figure 3. The formation of biodiesel and glycerol from triglycerides requires excess alcohol.
In this study, glycerol compounds were not produced, but instead, there was the formation of a hexadecanoic acid, 2-hydroxy-1,3-propanediol ester (C
35H
68O
5), which is an ester bond compound with the OH group in the second chain. It can be interpreted that the reaction mechanism that occurs between triglycerides and alcohol does not follow
Figure 3. In this study, the reactions that occur follow other reaction mechanisms, as shown in
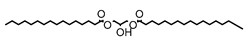
Figure 4.
The proposed mechanism was the basic active site of CH3O− directly attacked the triglyceride’s second palmitic chain, causing the double bond to break and formed an ester bond. Next, the second palmitic chain will be broken down due to the nucleophilic attack on the carbon atom, leaving only the first and third chain of the triglyceride intact. Rearrangement of the chain will take place, followed by the addition of H+ proton forming FAME and diglyceride molecule. Due to the lack of OH− bond formed, glycerol was not completely formed as evidenced by the GCMS results.
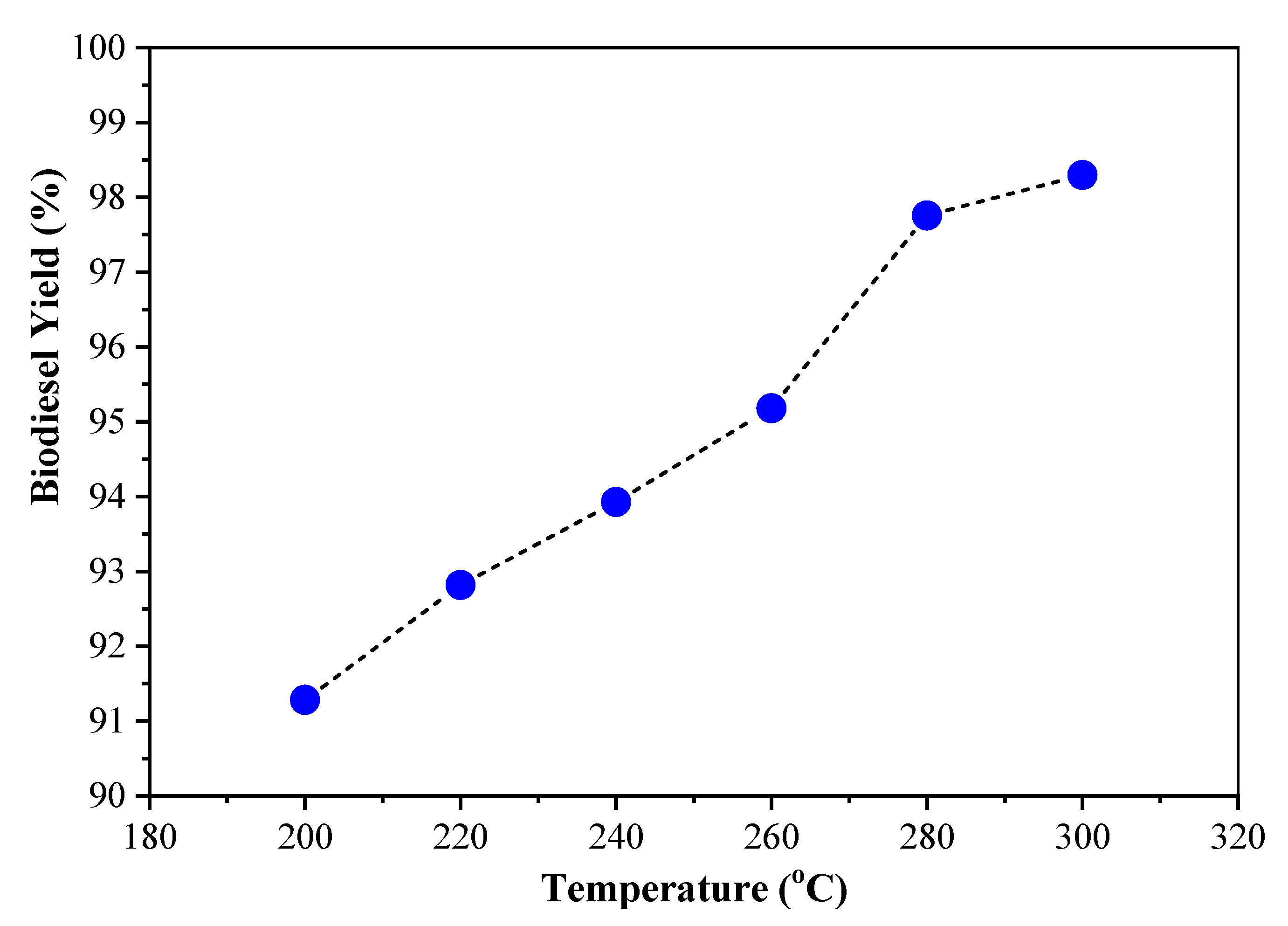
This can occur from one of the possibilities, namely the addition of a little alcohol or used cooking oil raw materials that are not suitable. The triglycerides are not completely cut and separated into glycerol and biodiesel. Based on the calculation of mass balance with the weight ratio of used cooking oil: alcohol is 4:1, and the yield is 98.29%, then every 0.3 moles of triglycerides will react with 1.5 moles of alcohol. If the triglycerides are used up reacting with excess methanol, it takes 315 mL of used cooking oil and 87 mL of alcohol. In this case, the used cooking oil is not suitable, causing the alcohol to not react completely with the triglycerides. To ascertain the direction of the chemical reactions that occur, further research is needed by carrying out nuclear magnetic resonance (NMR) spectroscopic analysis.
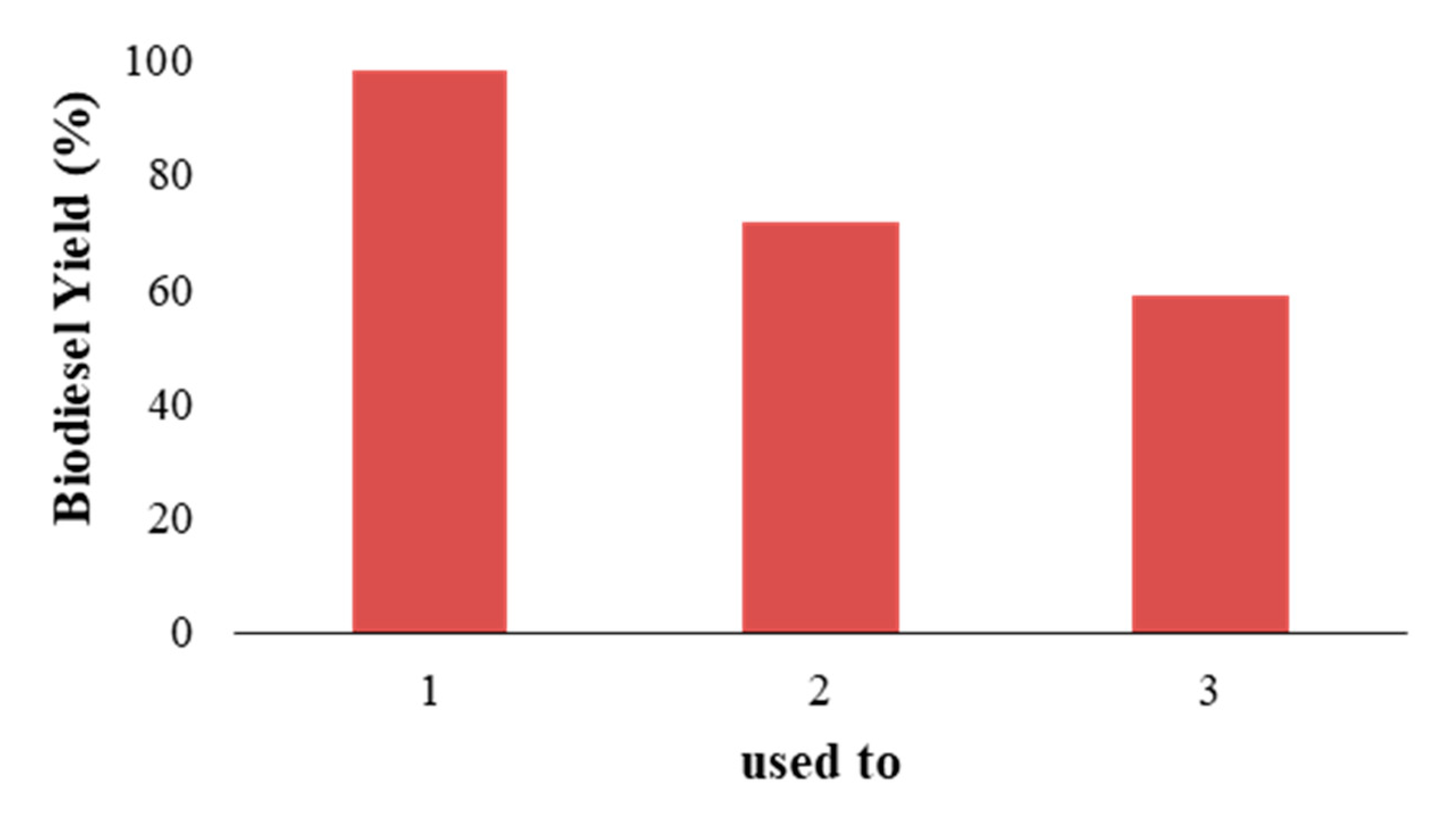
3.5. Reusability Catalyst
The zeolite catalyst was used three times to study the stability and effect of repeated use of the synthesized catalyst. The operating conditions were kept constant, namely the operating temperature of 300 °C, the weight ratio of used cooking oil: alcohol was 4:1, the reaction time of 60 min, and the catalyst concentration of 5% wt. After being used for the reaction, the catalyst was simply washed using n-hexane at room temperature without any treatment. In response, the biodiesel yield will be observed. The biodiesel yield is presented in
Figure 7.
In
Figure 7, it can be seen that, in the first usage, the biodiesel yield is 98.299%, it is 71.56% in the second use, and 58.95% by the third use. This decrease is due to the decreased reactivity or ability of the zeolite catalyst in the esterification–transesterification process with repeated use of the catalyst.
Another result is shown through the analysis of catalyst morphology. As shown in
Figure 8, the catalyst shape changes compared to
Figure 2. Before use, the zeolite catalyst is shaped like a sheet of stacked books, and after three uses, it is shaped like a separate square flake. The change in shape in the morphology of the catalyst after repeated use can be caused by the release of active components [
45]. In this case, it can be concluded that the catalyst cannot be regenerated.