In Situ Deposition of Green Silver Nanoparticles on Urinary Catheters under Photo-Irradiation for Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. In Situ AgNPs Deposition on the Silicone Urinary Catheter
2.2. Characterization of AgNPs
2.3. Investigation of Antibacterial Activity
2.4. Silver Release Analysis
2.5. Statistical Analysis
3. Results and Discussion
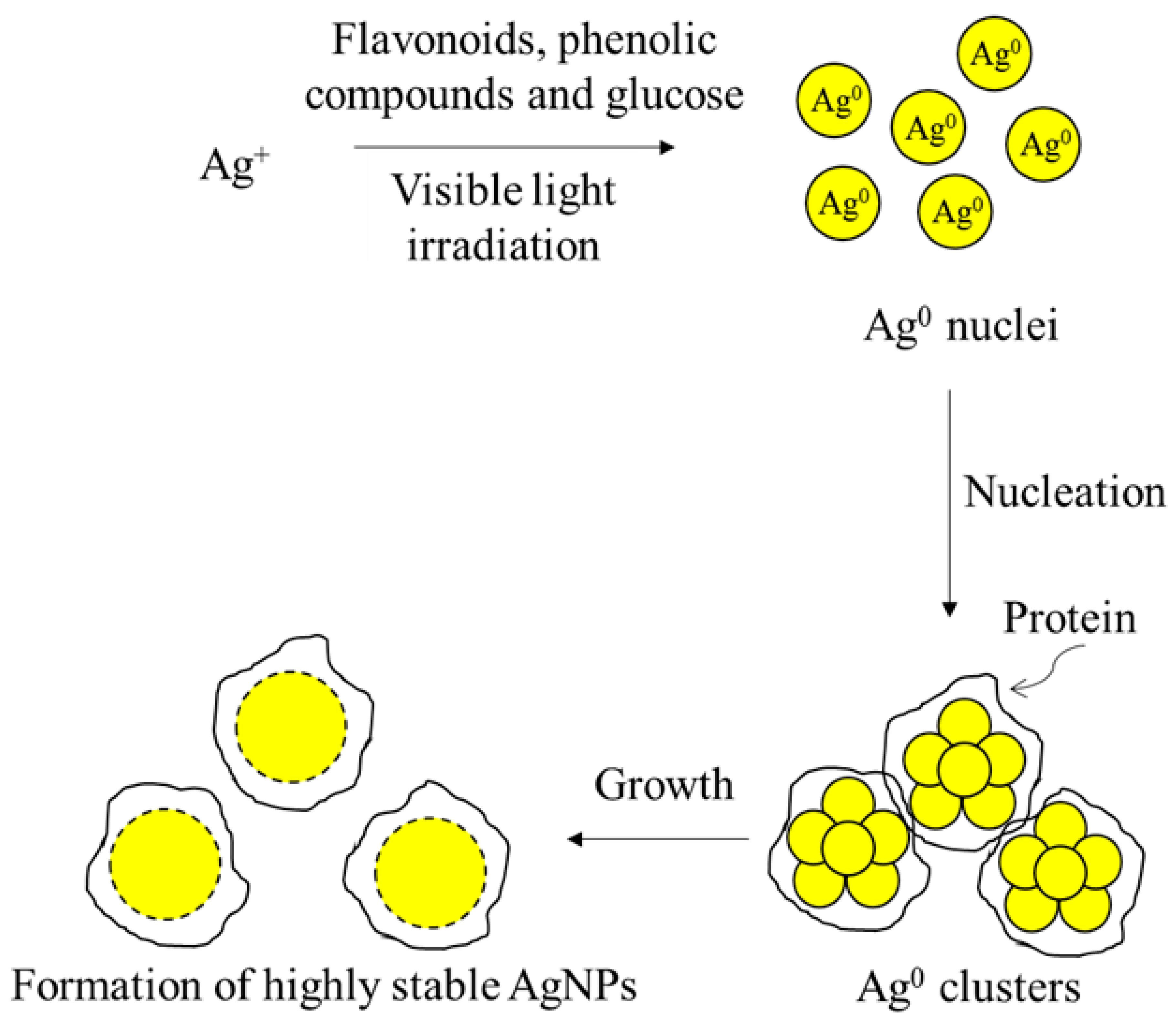
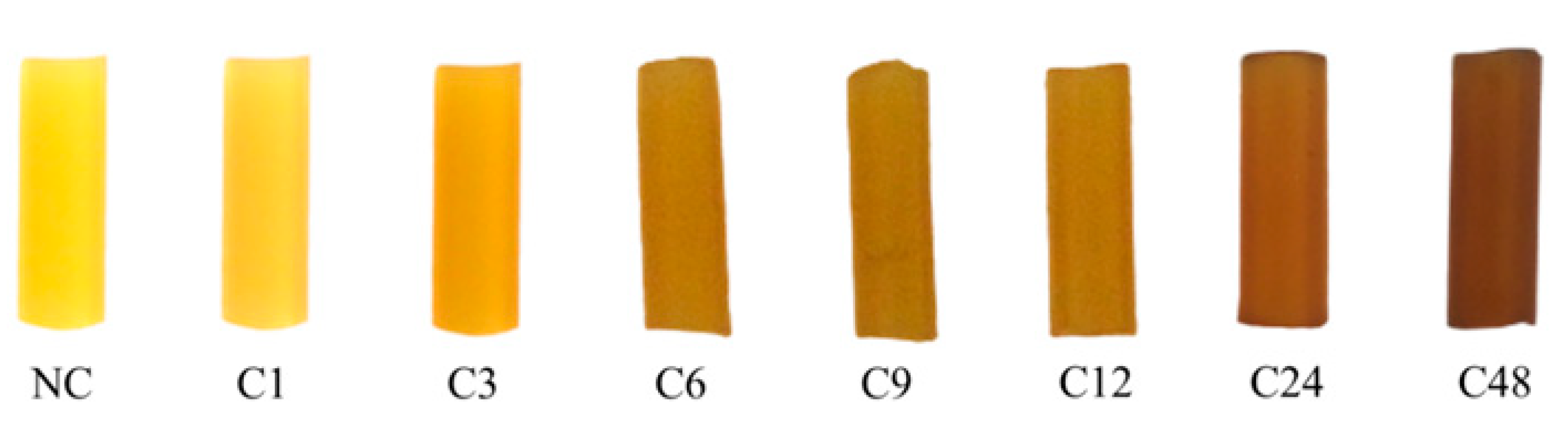
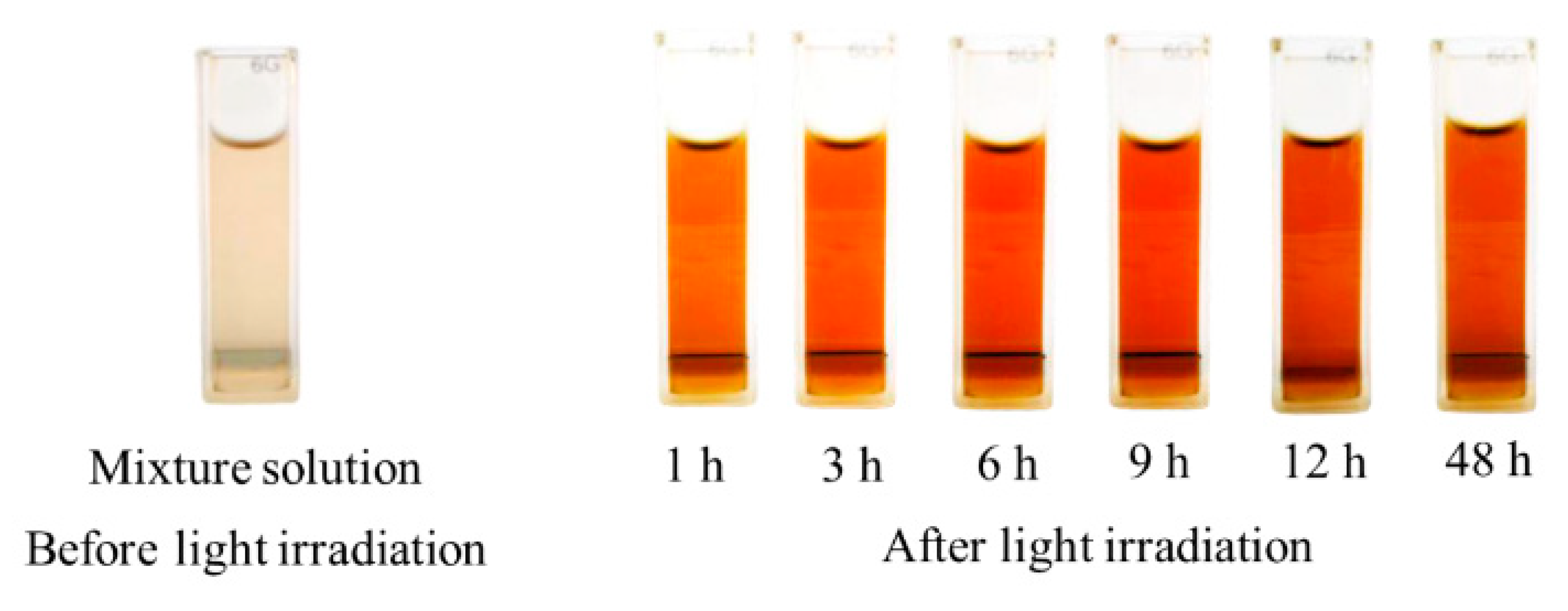
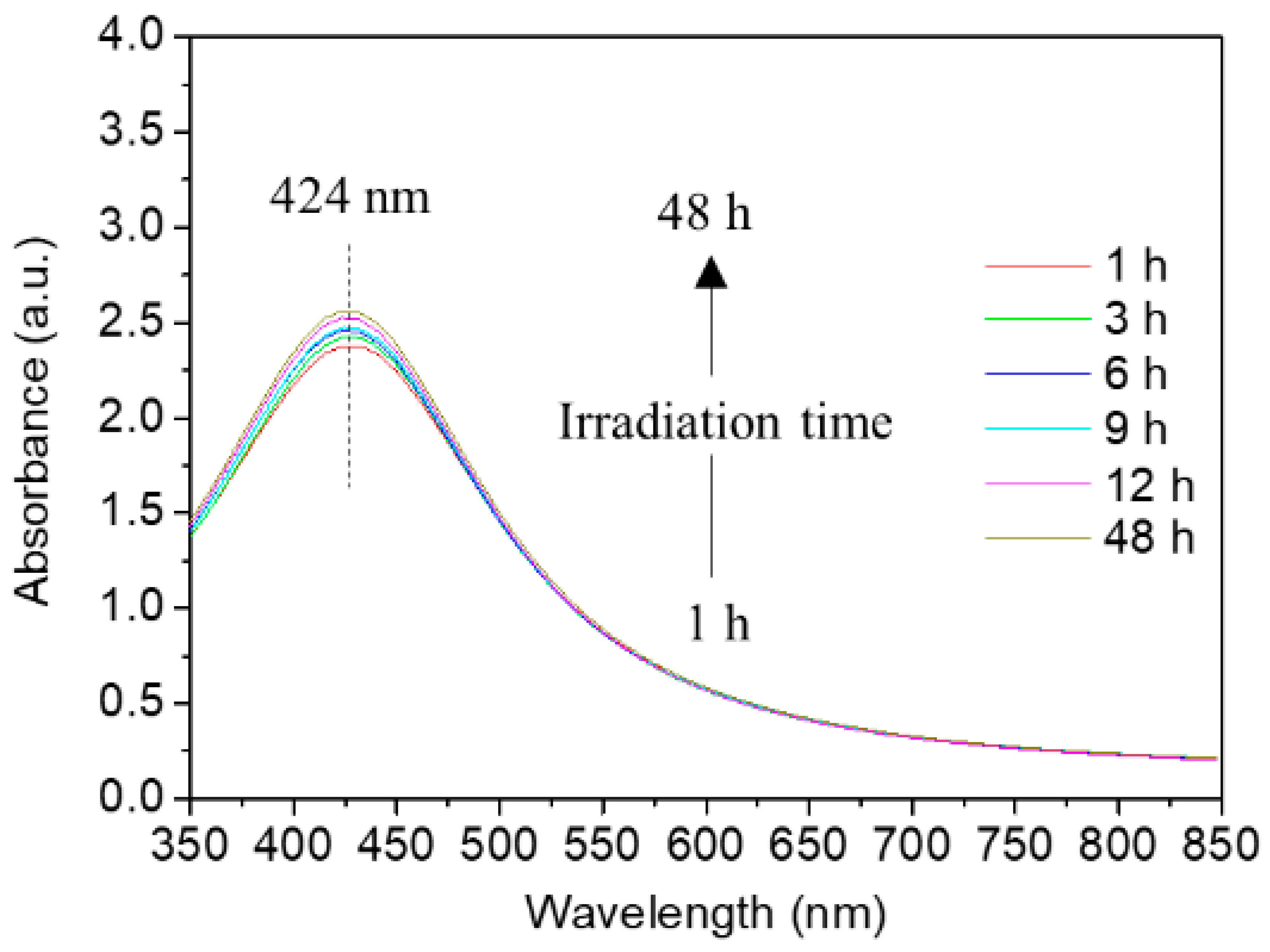
3.1. Effect of the Photo-Irradiation Time on the AgNP Formation
3.2. Bactericidal Activity of Developed Antibacterial Urinary Catheters
3.3. In Vitro Study of Silver Released from Coated Catheter
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, A.S. Preventing Health Care-Associated Infections. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008; Volume 1, pp. 1–29. [Google Scholar]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Anjum, S.; Singh, S.; Benedicte, L.; Roger, P.; Panigrahi, M.; Gupta, B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Glob. Chall. 2017, 2, 1700068. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile Coating of Urinary Catheter with Bio-Inspired Antibacterial Coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef]
- Albu, S.; Voidazan, S.; Bilca, D.; Badiu, M.; Truţă, A.; Ciorea, M.; Ichim, A.; Luca, D.; Moldovan, G. Bacteriuria and Asymptomatic Infection in Chronic Patients with Indwelling Urinary Catheter. Medicine (Baltimore) 2018, 97, e11796. [Google Scholar] [CrossRef]
- Mukhit Kazi, M.; Harshe, A.; Sale, H.; Mane, D.; Yande, M.; Chabukswar, S. Catheter Associated Urinary Tract Infections (CAUTI) and Antibiotic Sensitivity Pattern from Confirmed Cases of CAUTI in a Tertiary Care Hospital: A Prospective Study. Clin. Microbiol. 2015, 4, 1000193. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In-vitro Antibacterial and Anti-Encrustation Performance of Silver-Polytetrafluoroethylene Nanocomposite Coated Urinary Catheters. J. Hosp. Infect. 2019, 103, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Marklew, A. Urinary Catheter Care in the Intensive Care Unit. Nurs. Crit. Care 2004, 9, 21–27. [Google Scholar] [CrossRef]
- Gould, C.V.; Umscheid, C.A.; Agarwal, R.K.; Kuntz, G.; Pegues, D.A. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Infect. Control Hosp. Epidemiol. 2010, 31, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W. Catheter-Associated Urinary Tract Infections. Int. J. Antimicrob. Agents 2001, 17, 299–303. [Google Scholar] [CrossRef]
- Fernandez, R.S.; Griffiths, R.D. Duration of Short-Term Indwelling Catheters: A Systematic Review of the Evidence. J. Wound Ostomy Cont. Nurs. 2006, 33, 145–153. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sujitha, P. Green Synthesis of Kocuran-Functionalized Silver Glyconanoparticles for Use as Antibiofilm Coatings on Silicone Urethral Catheters. Nanotechnology 2014, 25, 325101. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W.; Darouiche, R.O. Catheter-Associated Infections: Pathogenesis Affects Prevention. Arch. Intern. Med. 2004, 164, 842–850. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.-T.; Chiong, E.; Tambyah, P.A. Surface Modification Strategies for Combating Catheter-Related Complications: Recent Advances and Challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial Coatings on Haemodialysis Catheters by Photochemical Deposition of Silver Nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef]
- Charnley, M.; Textor, M.; Acikgoz, C. Designed Polymer Structures with Antifouling-Antimicrobial Properties. React. Funct. Polym. 2011, 71, 329–334. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial Surface Functionalization of Plastic Catheters by Silver Nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Al-Qahtani, M.; Safan, A.; Jassim, G.; Abadla, S. Efficacy of Anti-Microbial Catheters in Preventing Catheter Associated Urinary Tract Infections in Hospitalized Patients: A Review on Recent Updates. J. Infect. Public Health 2019, 12, 760–766. [Google Scholar] [CrossRef]
- Martins, K.B.; Ferreira, A.M.; Pereira, V.C.; Pinheiro, L.; Oliveira, A.d.; Cunha, M.d.L.R.d.S.d. In vitro Effects of Antimicrobial Agents on Planktonic and Biofilm Forms of Staphylococcus saprophyticus Isolated from Patients with Urinary Tract Infections. Front. Microbiol. 2019, 10, 40. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.; Blaskovich, M.A.T.; Ziora, Z. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-Dependent Antimicrobial Activities of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized Over the Range 5–100 nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Majeed, S.; Joel, E.L.; Hasnain, M.S. Novel Green Approach for Synthesis of Metallic Nanoparticles and its Biomedical Application. Recent Pat. Nanomed. 2018, 8, 177–183. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K.; Limsuwan, P. Photo-Irradiation Induced Green Synthesis of Highly Stable Silver Nanoparticles Using Durian Rind Biomass: Effects of Light Intensity, Exposure Time and pH on Silver Nanoparticles Formation. J. Phys. Commun. 2020, 4, 095015. [Google Scholar] [CrossRef]
- DIN EN 1616:1999. Sterile Urethral Catheters for Single Use; German Institute for Standardisation (Deutsches Institut für Normung): Berlin, Germany, 1999; pp. 1–16. [Google Scholar]
- Chen, Y.; Ming, H. Review of Surface Plasmon Resonance and Localized Surface Plasmon Resonance Sensor. Photonic Sens. 2012, 2, 37–49. [Google Scholar] [CrossRef]
- Rastogi, L.; Arunachalam, J. Sunlight Based Irradiation Strategy for Rapid Green Synthesis of Highly Stable Silver Nanoparticles Using Aqueous Garlic (Allium sativum) Extract and Their Antibacterial Potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Maki, D.G.; Tambyah, P.A. Engineering out the Risk for Infection with Urinary Catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Anitimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, Their Toxicity and Possible Mechanisms of Action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Density. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of Silver Nanoparticle Induced Cell Apoptosis for in vitro Gene Therapy. Nanotechnology 2008, 19, 075104. [Google Scholar] [CrossRef]

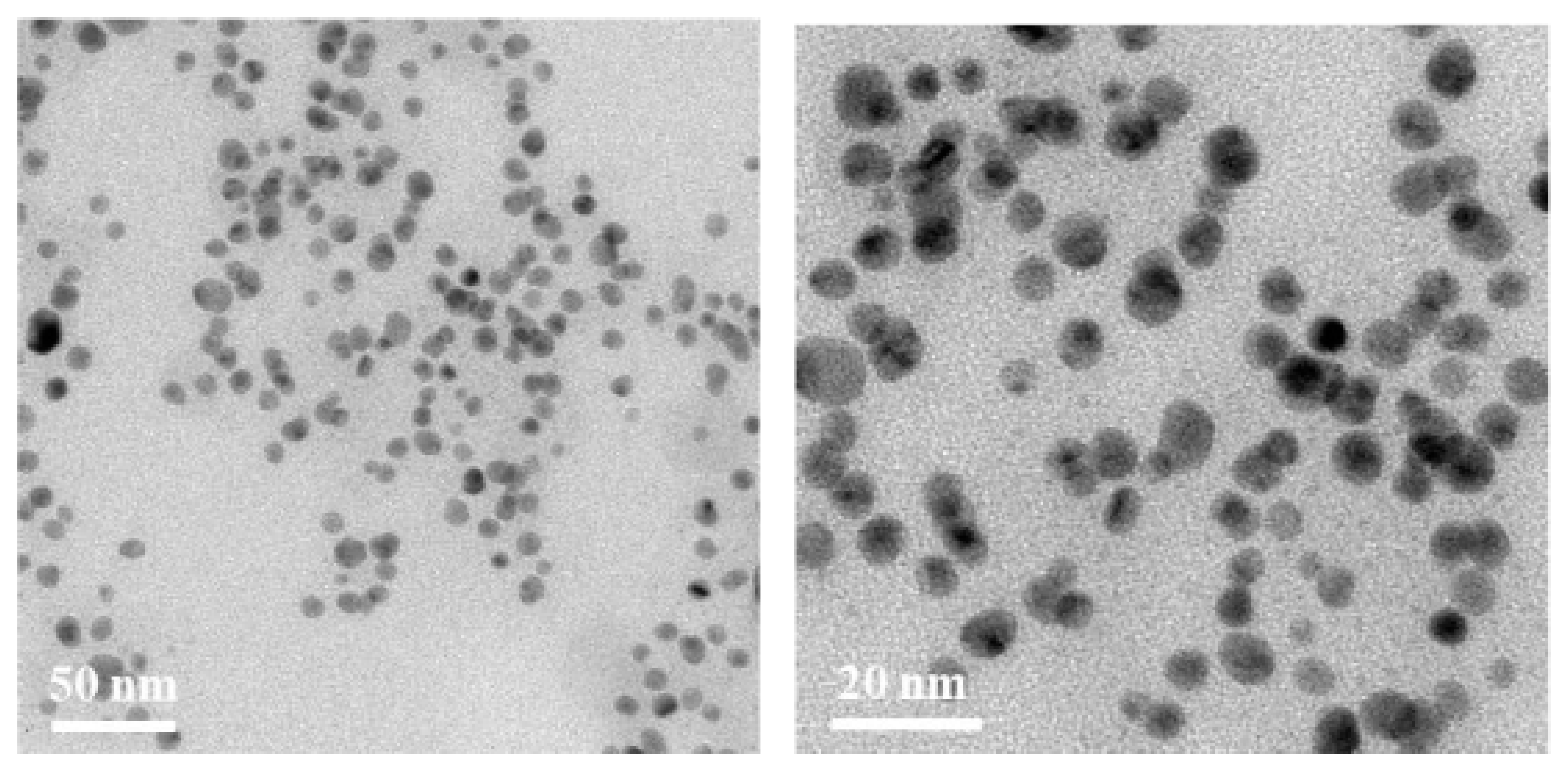

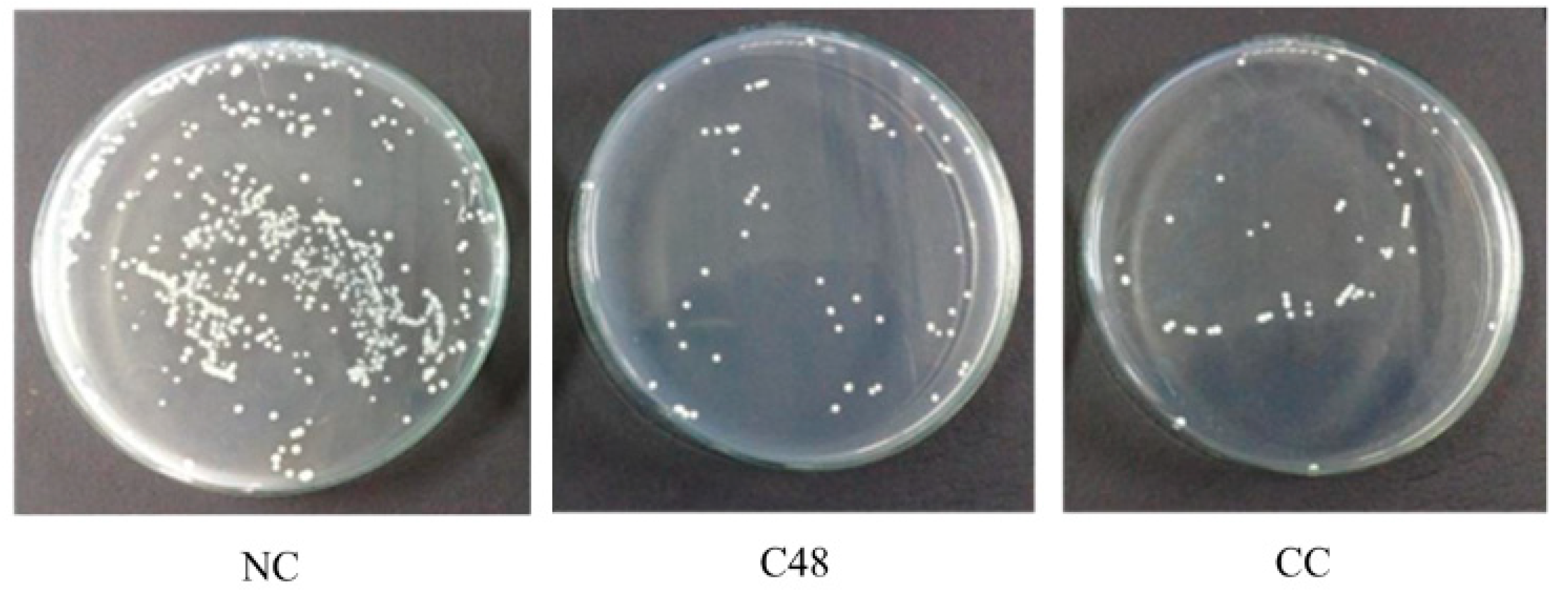


| Sample | Silver Content Coated on the Catheter Surfaces (mg/cm2) | Mortality Rate (%) | Relative Mortality Rate (%/mg∙cm−2) |
|---|---|---|---|
| C3 | 0.549 | 47 | 86 |
| C12 | 0.637 | 82 | 129 |
| C24 | 0.643 | 84 | 130 |
| C48 | 0.712 | 91 | 128 |
| CC | 5.165 | 91 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutrakulwong, F.; Thamaphat, K.; Tantipaibulvut, S.; Limsuwan, P. In Situ Deposition of Green Silver Nanoparticles on Urinary Catheters under Photo-Irradiation for Antibacterial Properties. Processes 2020, 8, 1630. https://doi.org/10.3390/pr8121630
Chutrakulwong F, Thamaphat K, Tantipaibulvut S, Limsuwan P. In Situ Deposition of Green Silver Nanoparticles on Urinary Catheters under Photo-Irradiation for Antibacterial Properties. Processes. 2020; 8(12):1630. https://doi.org/10.3390/pr8121630
Chicago/Turabian StyleChutrakulwong, Fueangfahkan, Kheamrutai Thamaphat, Sukon Tantipaibulvut, and Pichet Limsuwan. 2020. "In Situ Deposition of Green Silver Nanoparticles on Urinary Catheters under Photo-Irradiation for Antibacterial Properties" Processes 8, no. 12: 1630. https://doi.org/10.3390/pr8121630
APA StyleChutrakulwong, F., Thamaphat, K., Tantipaibulvut, S., & Limsuwan, P. (2020). In Situ Deposition of Green Silver Nanoparticles on Urinary Catheters under Photo-Irradiation for Antibacterial Properties. Processes, 8(12), 1630. https://doi.org/10.3390/pr8121630







