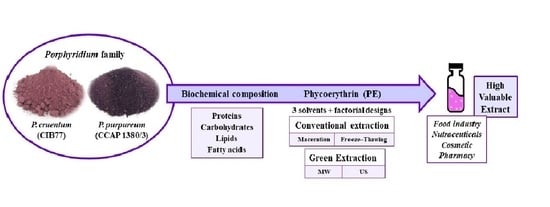
Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass and Chemicals
2.2. Macronutrient Analysis
2.3. Fatty Acid Composition
2.4. Phycoerythrin (PE)
2.5. Conventional Extraction of Phycoerythrin
2.5.1. Maceration
2.5.2. Freeze–Thaw
2.6. Green Extraction Design for Phycoerythrin
2.6.1. Microwave (MW)
2.6.2. Ultrasound (US)
2.7. Statistical Analyses
3. Results and Discussion
3.1. Biochemical Composition and Phycoerythrin Extraction Yield from Porphyridium spp.
3.2. Microwave Extraction of Phycoerythrin from Porphyridium spp.
3.3. Ultrasound Extraction of Phycoerythrin from Porphyridium spp.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernaerts, T.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, J.; Wu, H.; Jiang, P.; Chen, Z.; Xiang, W. Growth and biochemical composition of Porphyridium purpureum SCS-02 under different nitrogen concentrations. Mar. Drugs 2019, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Global Forecast to 2025. Algal Pigments Market by Type (Beta Carotene, Astaxanthin, Fucoxanthin, Phycocyanin, Phycoerythrin), Application (Food and Beverages, Nutraceuticals, Aquaculture, Cosmetics, Pharmaceuticals), and Geography MRFB-104360; Meticulous Market Research Pvt. Ltd.: Maharashtra, India, 2020; p. 150. [Google Scholar]
- Ott, F.D. A brief review of the species of Porphyridium with additional records for the rarely collected alga Porphyridium sordidum Geitler, 1932 (rhodophycophyta, porphyridiales). Arch. Protistenkd. 1987, 134, 35–41. [Google Scholar] [CrossRef]
- Guiry, M. Macroalgae of Rhodophycota, Phaeophycota, Chlorophycota, and two genera of Xanthophycota. Collect. Patrim. Nat. 2001, 50, 20–38. [Google Scholar]
- Bhattacharya, D.; Price, D.C.; Chan, C.X.; Qiu, H.; Rose, N.; Ball, S.; Weber, A.P.M.; Cecilia Arias, M.; Henrissat, B.; Coutinho, P.M.; et al. Genome of the red alga Porphyridium purpureum. Nat. Commun. 2013, 4, 1941. [Google Scholar] [CrossRef]
- Barozai, M.Y.K.; Qasim, M.; Din, M.; Achakzai, A.K.K. An update on the microRNAs and their targets in unicellular red alga Porphyridium cruentum. Pak. J. Bot. 2018, 50, 817–825. [Google Scholar]
- Neushul, M. A Freeze-Etching Study of the Red Alga Porphyridium. Am. J. Bot. 1970, 57, 1231–1239. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Characterization and comparison of Porphyridium sordidum and Porphyridium purpureum concerning growth characteristics and polysaccharide production. Algal Res. 2020, 49, 101931. [Google Scholar] [CrossRef]
- Román, R.B.; Alvarez-Pez, J.; Fernández, F.A.; Grima, E.M. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.M.; Levy-Ontman, O.; Zhang, W.; Tekoah, Y.; Glaser, R. Isolation and characterization of poly-and oligosaccharides from the red microalga Porphyridium sp. Carbohydr. Res. 2009, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Shirai, A.; Ohtaguchi, K.; Koide, K. Carbon dioxide fixation and polyunsaturated fatty acid production by the red alga porphyridium cruentum. Appl. Biochem. Biotechnol. 1998, 73, 269–278. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Gantt, E.; Lipschultz, C.A. Phycobilisomes of Porphyridium cruentum. Pigment analysis. Biochemistry 1974, 13, 2960–2966. [Google Scholar] [CrossRef]
- Viskari, P.J.; Colyer, C.L. Rapid extraction of phycobiliproteins from cultured cyanobacteria samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Yang, H.; Jin, X.; Lam, C.W.K.; Yan, S.-K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef]
- Khozin, I.; Adlerstein, D.; Bigongo, C.; Heimer, Y.M.; Cohen, Z. Elucidation of the biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum (II. Studies with radiolabeled precursors). Plant Physiol. 1997, 114, 223–230. [Google Scholar] [CrossRef]
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gökpinar, Ş.; Işik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227. [Google Scholar] [CrossRef]
- Bergé, J.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z. The production potential of eicosapentaenoic and arachidonic acids by the red alga Porphyridium cruentum. J. Am. Oil Chem. Soc. 1990, 67, 916–920. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; van-Moppes, D.; Grossman, S.; Arad, S.M. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Setyaningsih, I.; Prasetyo, H.; Agungpriyono, D.R.; Tarman, K. Antihyperglycemic activity of Porphyridium cruentum biomass and extra-cellular polysaccharide in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2020, 156, 1381–1386. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Khan, M.K.; Chemat, F. Application of Low and High Power Ultrasound in Food Analysis. Anal. Chem. Lett. 2011, 1, 103–114. [Google Scholar] [CrossRef]
- Mason, T.; Chemat, F.; Vinatoru, M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- CIBNOR. Available online: https://www.cibnor.gob.mx/ (accessed on 30 September 2020).
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: Berlin/Heidelberg, Germany, 1975; pp. 29–60. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, 6. [Google Scholar] [CrossRef]
- ISO-5508. Animal and Vegetable Fats and Oils-Analysis by Gas Chromatography of Methyl esters of Fatty Acids; ISO 5508: 1990; International Organization for Standardization: Geneve, Switzerland, 1990. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Assunção, M.F.; Varejão, J.M.; Santos, L.M. Nutritional characterization of the microalga Ruttnera lamellosa compared to Porphyridium purpureum. Algal Res. 2017, 26, 8–14. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; del Pulgar, J.S.; Aguzzi, A.; Caproni, R.; Gabrielli, P.; Lombardi-Boccia, G. Chemical characterization and nutritional evaluation of microalgal biomass from large-scale production: A comparative study of five species. Eur. Food Res. Technol. 2020, 246, 323–332. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Khatoon, H.; Kok Leong, L.; Abdu Rahman, N.; Mian, S.; Begum, H.; Banerjee, S.; Endut, A. Effects of different light source and media on growth and production of phycobiliprotein from freshwater cyanobacteria. Bioresour. Technol. 2018, 249, 652–658. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid Green Extractions of C-Phycocyanin from Arthrospira maxima for Functional Applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; C Alves, R.; Oliveira, M. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B.; Rao, X.; Huang, J.; Li, M. Optimization of culturing conditions of Porphyridium cruentum using uniform design. World J. Microbiol. Biotechnol. 2007, 23, 1345–1350. [Google Scholar] [CrossRef]
- Xu, Y.; Jiao, K.; Zhong, H.; Wu, S.; Ho, S.-H.; Zeng, X.; Li, J.; Tang, X.; Sun, Y.; Lin, L. Induced cultivation pattern enhanced the phycoerythrin production in red alga Porphyridium purpureum. Bioprocess. Biosyst. Eng. 2020, 43, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-assisted extraction of phycobiliproteins from Porphyridium purpureum. Appl. Biochem. Biotechnol. 2015, 175, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, G.; Chen, F.; Wang, Z.; Wu, J.; Hu, X. Different Effects of Microwave and Ultrasound on the Stability of (all-E)-Astaxanthin. J. Agric. Food Chem. 2006, 54, 8346–8351. [Google Scholar] [CrossRef]
- Sharif, K.; Rahman, M.; Azmir, J.; Mohamed, A.; Jahurul, M.; Sahena, F.; Zaidul, I. Experimental design of supercritical fluid extraction–A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef]
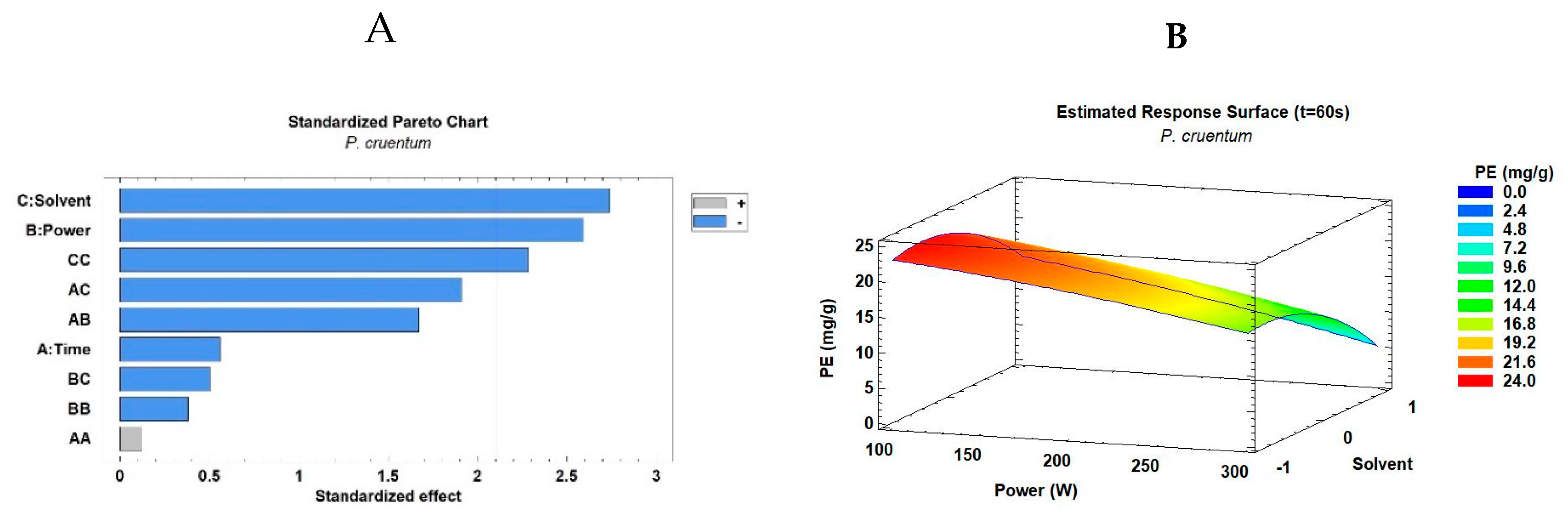
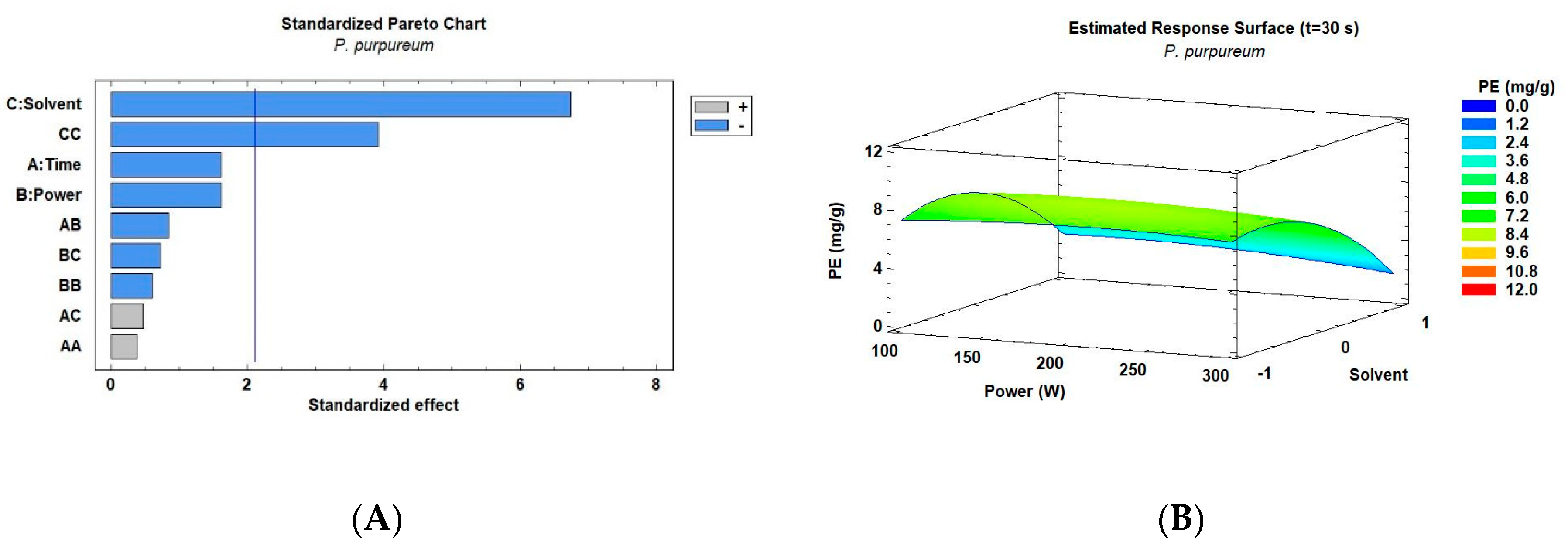


| Exp. | Factors | Response Variable PE Extraction Yield (mg/g) | |||
|---|---|---|---|---|---|
| Extraction-Time (s) | Power (W) | Solvent | P. cruentum | P. purpureum | |
| 1 | 30 | 100 | –1 (PBS) | 10.69 ± 0.52 | 6.60 ± 0.32 |
| 2 | 45 | 100 | –1 | 21.06 ± 0.24 | 5.35 ± 0.21 |
| 3 | 60 | 100 | –1 | 23.94 ± 1.84 | 6.37 ± 0.31 |
| 4 | 30 | 200 | –1 | 20.43 ± 0.56 | 8.54 ± 0.41 |
| 5 | 45 | 200 | –1 | 14.50 ± 0.49 | 6.11 ± 0.30 |
| 6 | 60 | 200 | –1 | 23.20 ± 0.81 | 8.74 ± 0.01 |
| 7 | 30 | 300 | –1 | 17.37 ± 0.50 | 7.36 ± 0.37 |
| 8 | 45 | 300 | –1 | 18.56 ± 0.01 | 6.37 ± 0.20 |
| 9 | 60 | 300 | –1 | 10.64 ±0.46 | 4.35 ± 0.20 |
| 10 | 30 | 100 | 0 (DW) | 19.23 ± 0.73 | 6.80 ± 0.32 |
| 11 | 45 | 100 | 0 | 23.36 ± 0.55 | 10.79 ± 0.49 |
| 12 | 60 | 100 | 0 | 23.94 ± 0.01 | 5.51 ± 0.19 |
| 13 | 30 | 200 | 0 | 23.69 ± 0.47 | 7.39 ± 0.06 |
| 14 | 45 | 200 | 0 | 16.01 ± 0.64 | 6.31 ± 0.30 |
| 15 | 60 | 200 | 0 | 14.65 ± 0.34 | 5.85 ± 0.28 |
| 16 | 30 | 300 | 0 | 15.93 ± 0.79 | 8.07 ± 0.40 |
| 17 | 45 | 300 | 0 | 15.54 ± 0.37 | 4.64 ± 0.23 |
| 18 | 60 | 300 | 0 | 17.91 ± 0.74 | 4.54 ± 0.19 |
| 19 | 30 | 100 | 1 (CCS) | 19.79 ± 0.54 | 3.69 ± 0.10 |
| 20 | 45 | 100 | 1 | 12.83 ± 0.49 | 1.56 ± 0.03 |
| 21 | 60 | 100 | 1 | 13.27 ± 0.13 | 3.11 ± 0.11 |
| 22 | 30 | 200 | 1 | 12.72 ± 0.50 | 1.58 ± 0.04 |
| 23 | 45 | 200 | 1 | 18.07 ± 0.03 | 2.15 ± 0.05 |
| 24 | 60 | 200 | 1 | 9.16 ± 0.23 | 1.34 ± 0.03 |
| 25 | 30 | 300 | 1 | 14.13 ± 0.70 | 1.51 ± 0.08 |
| 26 | 45 | 300 | 1 | 7.80 ± 0.09 | 1.21 ± 0.05 |
| 27 | 60 | 300 | 1 | 8.18 ± 0.39 | 1.69 ± 0.08 |
| Exp. | Factors | Response Variable PE Extraction Yield (mg/g) | ||
|---|---|---|---|---|
| Extraction-Time (min) | Solvent | P. cruentum | P. purpureum | |
| 1 | 5 | –1 (PBS) | 25.32 ± 1.11 | 10.06 ± 0.32 |
| 2 | 10 | –1 | 28.95 ± 0.78 | 13.06 ± 0.32 |
| 3 | 15 | –1 | 32.63 ± 1.09 | 13.07 ± 0.64 |
| 4 | 5 | 0 (DW) | 26.53 ± 1.01 | 9.13 ± 0.41 |
| 5 | 10 | 0 | 24.74 ± 0.79 | 17.20 ± 0.21 |
| 6 | 15 | 0 | 31.05 ± 0.20 | 19.26 ± 0.25 |
| 7 | 5 | 1 (CCS) | 30.72 ± 0.31 | 4.96 ± 0.28 |
| 8 | 10 | 1 | 26.94 ± 0.99 | 7.55 ± 0.34 |
| 9 | 15 | 1 | 23.02 ± 0.05 | 6.70 ± 0.37 |
| Macronutrient (%, w/w) | P. cruentum | P. purpureum | P. cruentum | P. cruentum | P. purpureum F&M-M |
|---|---|---|---|---|---|
| Proteins | 42.90 ± 1.84 b | 26.30 ± 0.94 a | 28–39 | 35.4 ± 0.9 | 34.2 ± 0.10 |
| Carbohydrates | 13.89 ± 0.17 a | 14.42 ± 0.22 b | 40–57 | 12.5 ± 0.6 | 17.0 ± 1.72 |
| Lipids | 14.67 ± 0.24 a | 17.34 ± 1.35 b | 9–14 | 5.3 ± 0.3 | 13.1 ± 1.12 |
| References | * | * | [45] | [48] | [49] |
| Fatty Acids Profile (%, Total FA) | P. cruentum | P. purpureum | P. cruentum | P. purpureum | P. purpureum |
| C16:0, Palmitic | 41.02 ± 1.75 b | 29.01 ± 0.94 a | 34.11 | 33.81 ± 0.09 | 25 |
| C16:1, Palmitoleic | 5.22 ± 0.16 | n.d. | 2.63 | n.d. | n.d. |
| C18:0, Stearic | 1.35 ± 0.04 a | 50.02 ± 1.72 b | 0.87 | 52.78 ± 2.82 | n.d. |
| C18:1, Oleic | 4.23 ± 0.11 a | 5.70 ± 0.15 b | 2.32 | 2.64 ± 0.96 | n.d. |
| C18:2, Linoleic | 9.19 ± 0.35 b | 6.49 ± 0.23 a | 10.54 | 1.10 ± 0.45 | 23 |
| C20:0, Arachidic | 11.23 ± 0.41 b | 5.90 ± 0.15 a | n.d. | 0.96 ± 0.32 | n.d. |
| C20:4ω-6, ARA | 18.02 ± 0.81 b | 1.98 ± 0.07 a | 29.12 | 2.66 ± 0.57 | 39 |
| C20:5ω-3, EPA | 9.74 ± 0.27 b | 0.90 ± 0.02 a | 15.88 | 0.54 ± 0.46 | 13 |
| Others FA | n.d. | n.d. | 5.43 | 5.51 | n.d. |
| References | * | * | [50] | [46] | [51] |
| Solvent/Microalgae | Maceration | Freeze–Thawing | ||
|---|---|---|---|---|
| P. cruentum | P. purpureum | P. cruentum | P. purpureum | |
| PBS | 15.71 ± 0.59 b | 5.61 ± 1.17 b | 16.01 ± 0.72 a | 5.20 ± 0.67 b |
| DW | 15.93 ± 0.19 b | 5.19 ± 0.69 b | 16.08 ± 0.35 a,b | 5.49 ± 0.96 b |
| CCS | 12.01 ± 0.64 a | 0.86 ± 0.04 a | 17.66 ± 1.18 b | 1.85 ± 0.70 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. https://doi.org/10.3390/pr8121628
Ardiles P, Cerezal-Mezquita P, Salinas-Fuentes F, Órdenes D, Renato G, Ruiz-Domínguez MC. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes. 2020; 8(12):1628. https://doi.org/10.3390/pr8121628
Chicago/Turabian StyleArdiles, Paula, Pedro Cerezal-Mezquita, Francisca Salinas-Fuentes, Daniel Órdenes, Gabriel Renato, and Mari Carmen Ruiz-Domínguez. 2020. "Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies" Processes 8, no. 12: 1628. https://doi.org/10.3390/pr8121628
APA StyleArdiles, P., Cerezal-Mezquita, P., Salinas-Fuentes, F., Órdenes, D., Renato, G., & Ruiz-Domínguez, M. C. (2020). Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes, 8(12), 1628. https://doi.org/10.3390/pr8121628