3.1. BA Levels and Food Quality Indices in Fermented Food Products, and Univariate Analysis of These Analytical Data
Mean ± SEM values for the individual and total BA contents of the FF products investigated are provided in
Table 2. The major contributors towards the relatively high BA levels observed in fermented cheese samples were cadaverine (mean 60% of total) and tyramine (mean 21.5% of total). Although three of the cheese products analyzed had total BA concentrations of 30–63 ppm, two of them were found to be as high as 666 and 780 ppm, which were markedly above the recommended 200 ppm content limit. The ANOVA Welch test demonstrated that there were highly significant differences between these total BA values (
Table 3), as expected (
p = 2.84 × 10
−4); such differences were largely explicable by those observed between the cheese and wine/vinegar product classifications investigated.
Hence, characteristic “markers” of fermented cheese samples appeared to be cadaverine and tyramine, which had contents markedly elevated over those of the other fermented food products evaluated, although there were very high intra-fermented food classification variances for these estimates.
Univariate statistical analysis performed by ANOVA (robust Welch test derivative), and also
post-
hoc Bonferroni test values, demonstrated that the mean values of each food classification examined were significantly or highly significantly different for 7 and 9 of the marker index variables respectively (
p values ranging from <0.0003 to 0.04 for the former test,
Table 3).
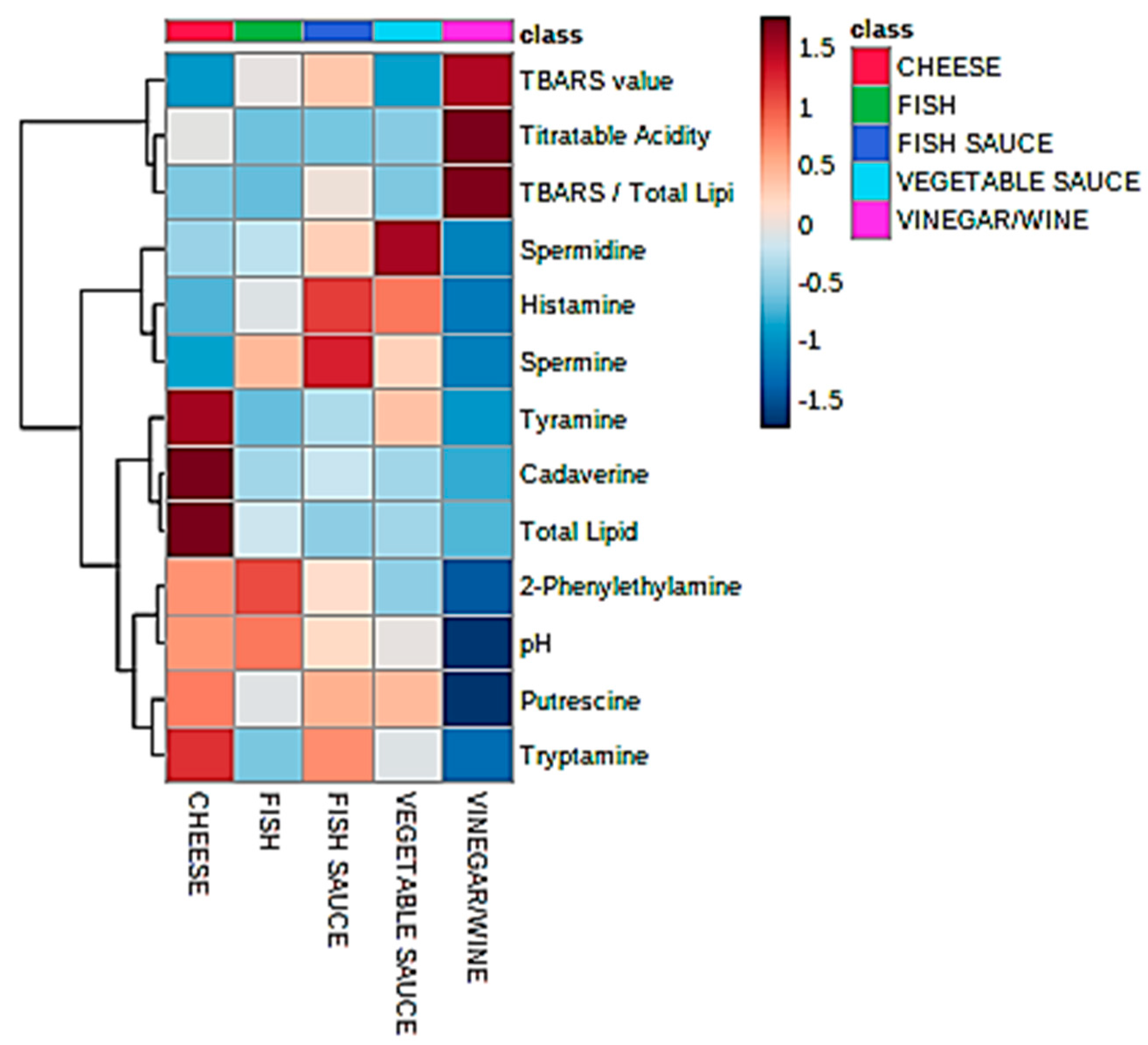
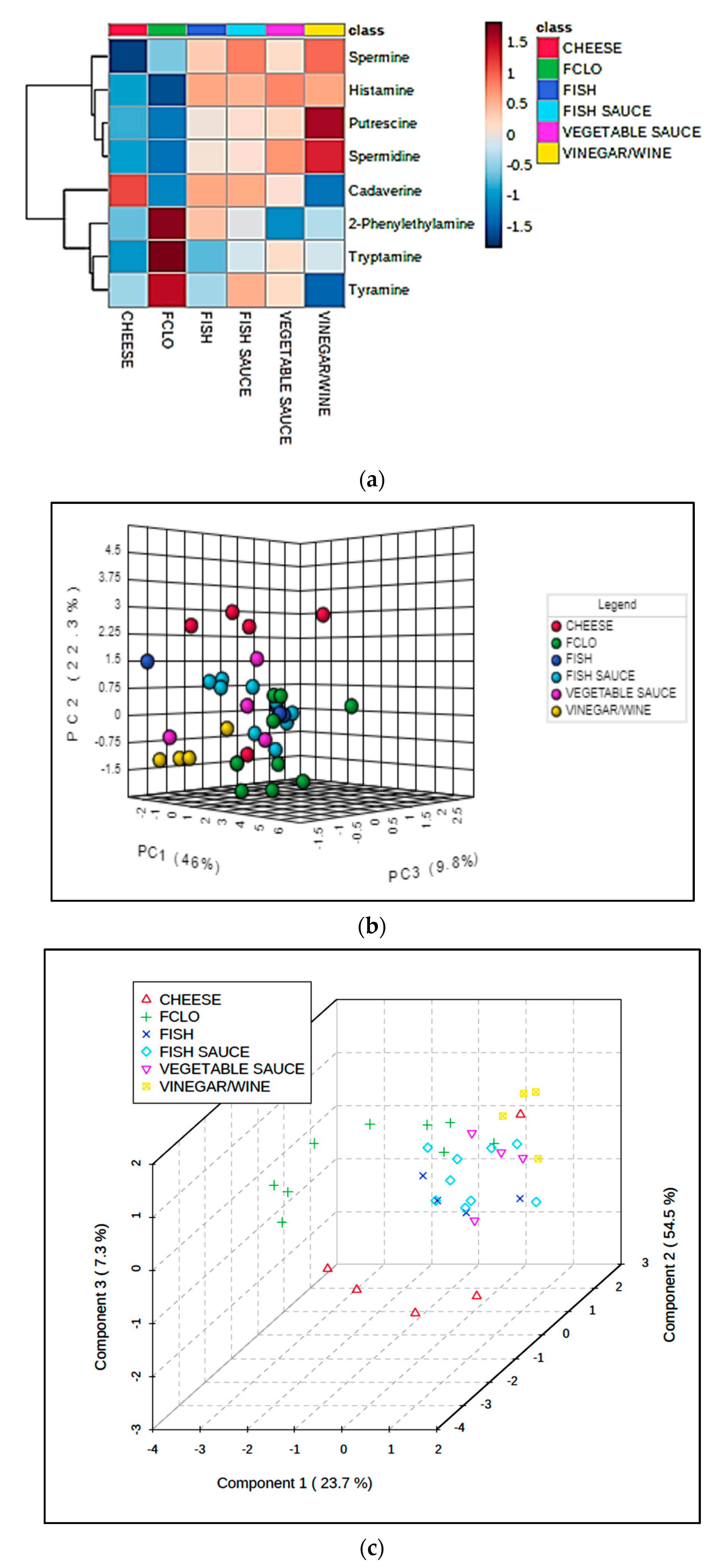
Figure 1 shows a heatmap of the mean BA contents, and further variables included in this analysis; this clearly displays significantly higher tyramine, cadaverine, putrescine, and tryptamine levels in the fermented cheese products; higher histamine concentrations in the fish sauces/pastes explored, as expected (although vegetable sauces also had quite high levels of this BA); and also greater spermine contents in the fish paste/sauce products (
ca. 1.5-fold greater than the mean value found for the fish classification, the next highest concentration). The vegetable sauce products had the highest mean spermidine levels, whereas the fermented fish group contained the largest amounts of 2-phenylethylamine detectable.
As expected, mean TA values were significantly greater for the wine/vinegar products than they were for all the other fermented food classes investigated, and correspondingly the mean pH value for the former group was significantly lower than those of all the other fermented foods. Of course, the mean total lipid content of the cheese group (23.3%) was significantly greater than all other food classifications tested (
p ca. 10
−3), although no significant differences were found for the secondary lipid peroxidation TBARS marker. However, an examination of the mean ratio of TBARS index to total lipid content revealed that this value was markedly greater for the wine/vinegar group than that of all other food product types (Bonferroni-corrected
post-
hoc ANOVA tests), and significantly so over that of the cheese samples analyzed, as might be expected in view of the very low fat contents of fermented wine/vinegar samples (for example, it varies from 0.15–0.44% (
w/
v) in Zhenjiang aromatic vinegar samples [
32]), and potentially substantially inflated TBARS levels resulting from quite high levels of TBA-reactive acetaldehyde and acrolein, amongst other aldehydes, present in such fermented products [
33,
34,
35,
36]. Indeed, many other aldehydes are reactive towards the TBA reagent, and also form chromophoric products on reaction with it [
28]. Estimates for acetaldehyde in vinegar products can be as high as 1.0 g/kg respectively [
33], but such levels are highly variable, with much lower levels being found, e.g., 2.6 mg/L (
ca. 60 µmol/L) [
37].
Acetaldehyde, a volatile flavor component of a variety of foods and beverages such as cheese, yoghurt, and wines [
34], represents one of the most abundant carbonyl compounds detectable in wine, and typically accounts for
ca. 90% of the total aldehydes present; its concentrations therein usually range from 10 to 200 mg/L (predominantly, it is generated as a yeast by-product during alcoholic fermentation processes [
35], or from the chemical oxidation of ethanol [
36]). However, very high levels of the unsaturated aldehyde acrolein are also present in red wine products [
33]. Furthermore, a wide range of further aldehydes have been found to serve as major flavor constituents of traditional Chinese rose vinegar, and these include aliphatic
n-alkanals such as heptanal, hexanal, nonanal, and dodecanal (ranging from 6–147 µg/kg), with larger amounts of benzaldehyde (851 µg/kg) [
38].
Hence, overall these data clearly demonstrated that, in a univariate context, there were indeed significant differences between the mean contents of BAs and further parameters considered for the five classes of fermented food products studied.
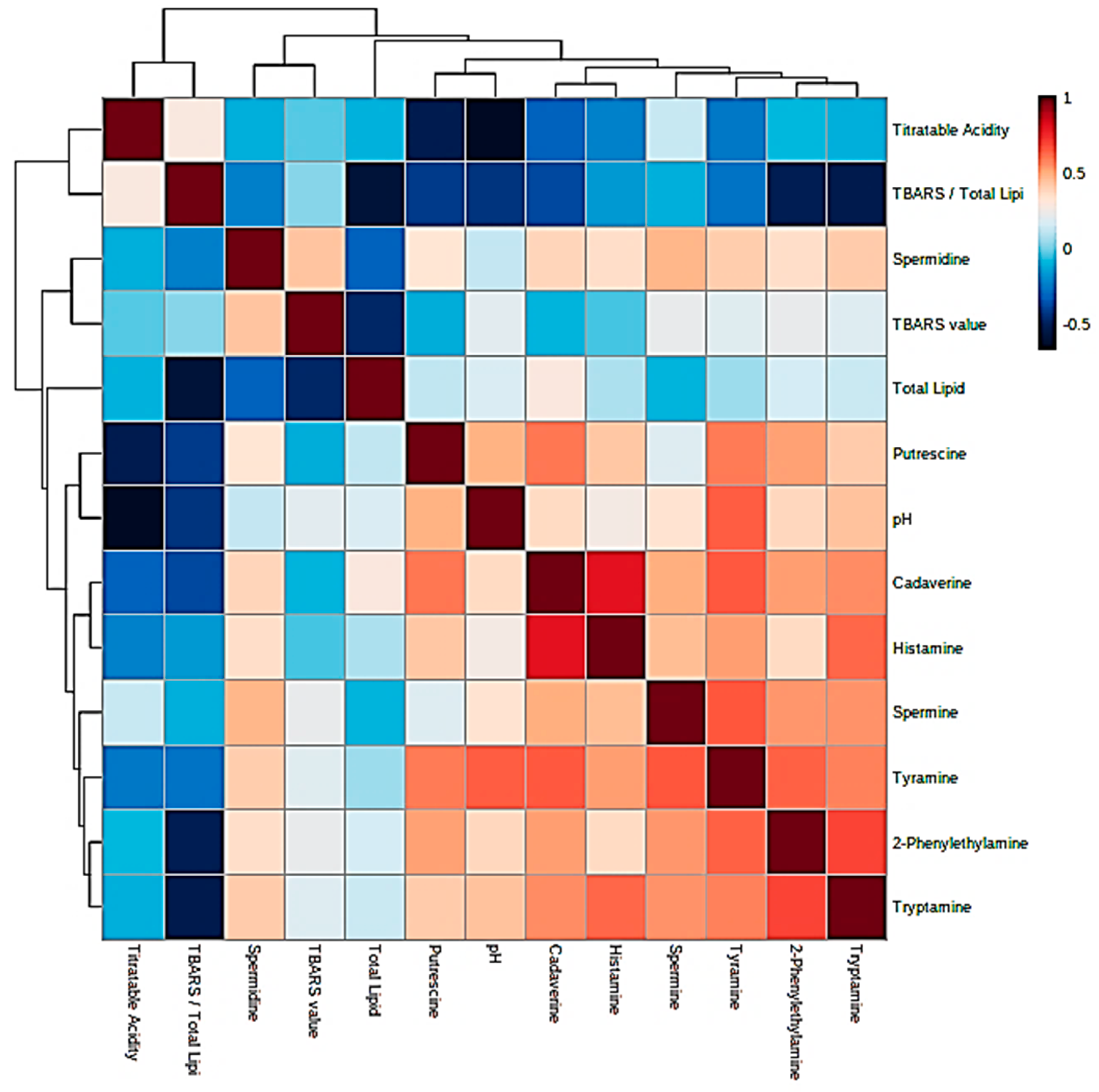
Prior to the performance of MV statistical analysis of the dataset acquired, simple Pearson correlations were explored between all explanatory variables considered, and
Figure 2 shows a correlation heatmap for these relationships. Clearly, there were moderate to strong positive correlations observed between all fermented food BAs present, the strongest observed between 2-phenylethylamine and tyramine (both aromatic BAs), tryptamine and spermine, and most notably, between cadaverine and histamine. Food pH values were found to have the strongest positive correlations with tyramine > putrescine > tryptamine, although spermidine was predominantly uncorrelated with this index. Moreover, as anticipated, TA was strongly negatively correlated with pH value > putrescine > tyramine ≈ histamine contents in that order. TBARS level, however, was largely independent of all BAs and their concentrations, with the exception of spermidine, which exhibited a weak positive relationship with this variable. Similarly, total lipid level was also mainly uncorrelated with all BA contents but was quite strongly anti-correlated with (TBARS):(total lipid) ratio and non-lipid-normalized TBARS value (both expected). The (TBARS):(total lipid) ratio was either strongly or moderately anti-correlated with all BA levels, and this may provide an indication of their potential antioxidant functions. In view of the complexity of these inter-relationships, the MV PCA and PLS-DA techniques were employed to explore them further.
3.2. Principal Component Analysis (PCA) of the Multivariate Fermented Food Dataset
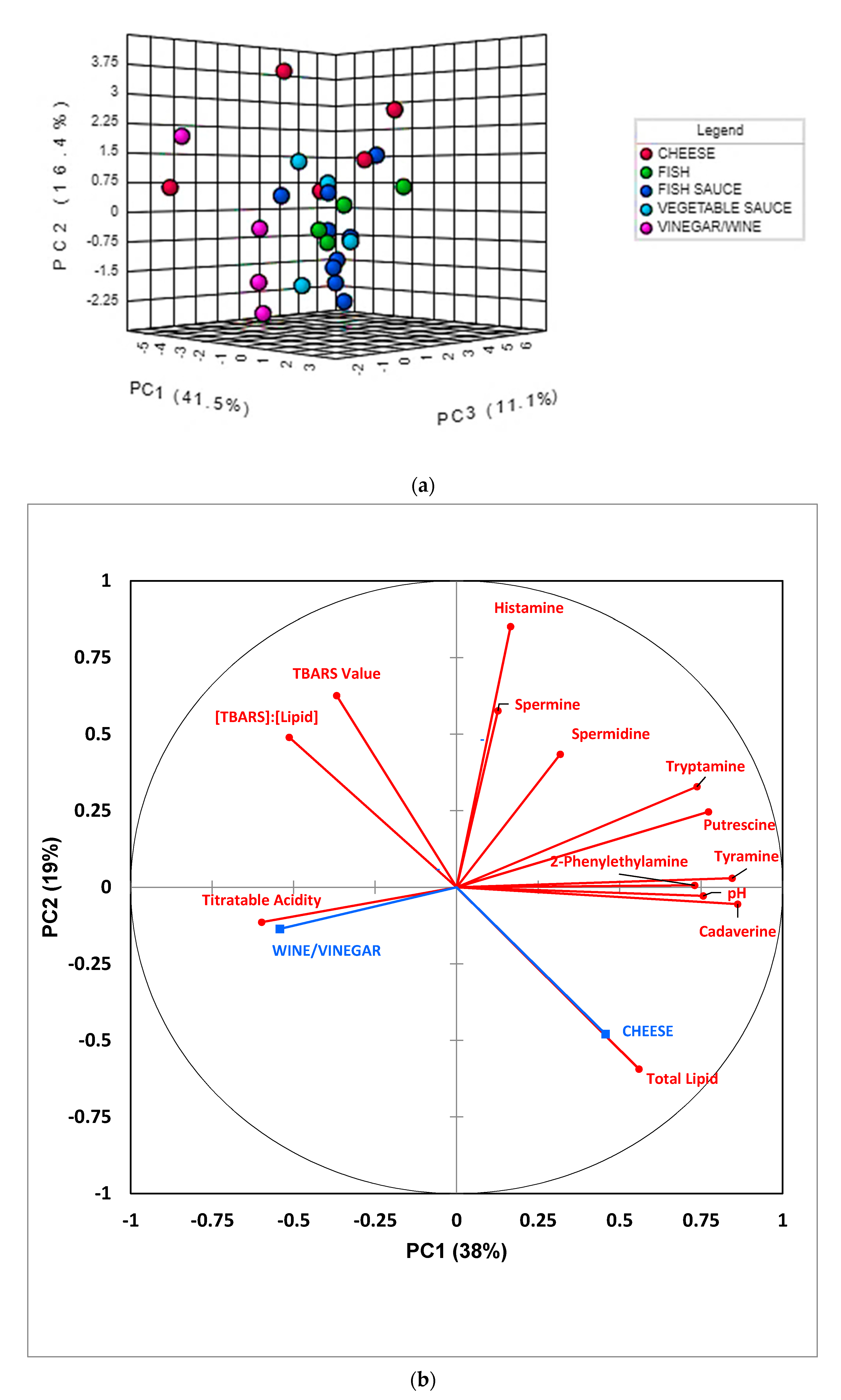
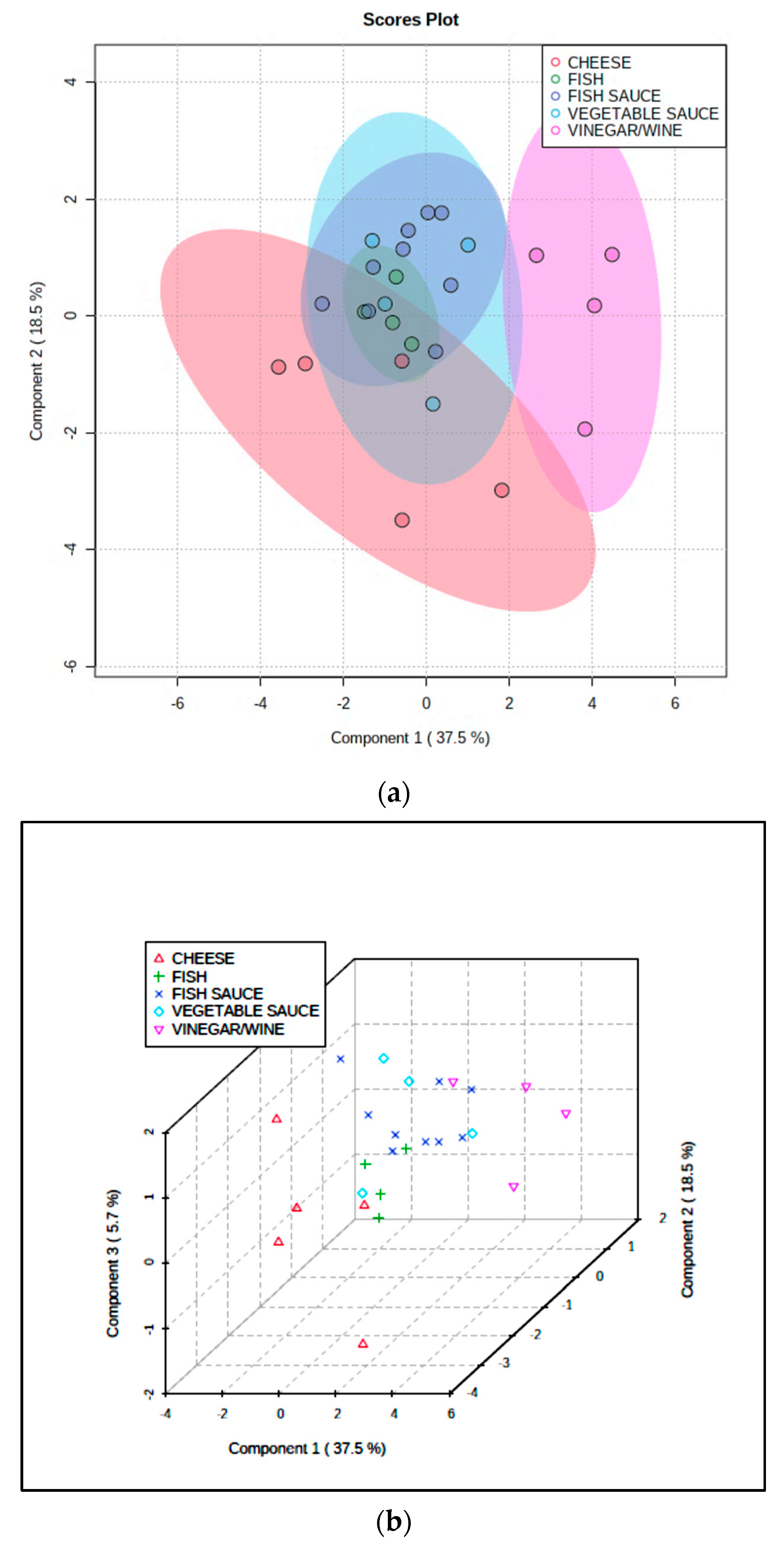
PCA was primarily conducted in order to acquire an overview of the degree of distinctiveness between, i.e., clustering of, the fermented food classifications investigated, and also to identify any potential data outliers. An examination of two-dimensional (2D) scores plots from this analysis demonstrated that no significant outliers were detectable, and that PCs 1, 2, and 3 accounted for 41.5, 16.4, and 11.1% of the total variance respectively for the complete dataset which was glog-transformed and autoscaled. 2D and three-dimensional (3D) scores plots featuring these two most important PCs revealed that there was a reasonable level of distinction between the wine/vinegar and all other food product groups, and also between the cheese and fish classifications (
Figure 3a); however, distinctions between the fish, fish sauce/paste, and vegetable sauce groups were not found, there being a significant degree of overlap between them. Notwithstanding, the sample sizes of the fermented fish and vegetable sauce groups involved were quite limited. A corresponding preliminary correlation circle diagram is shown in
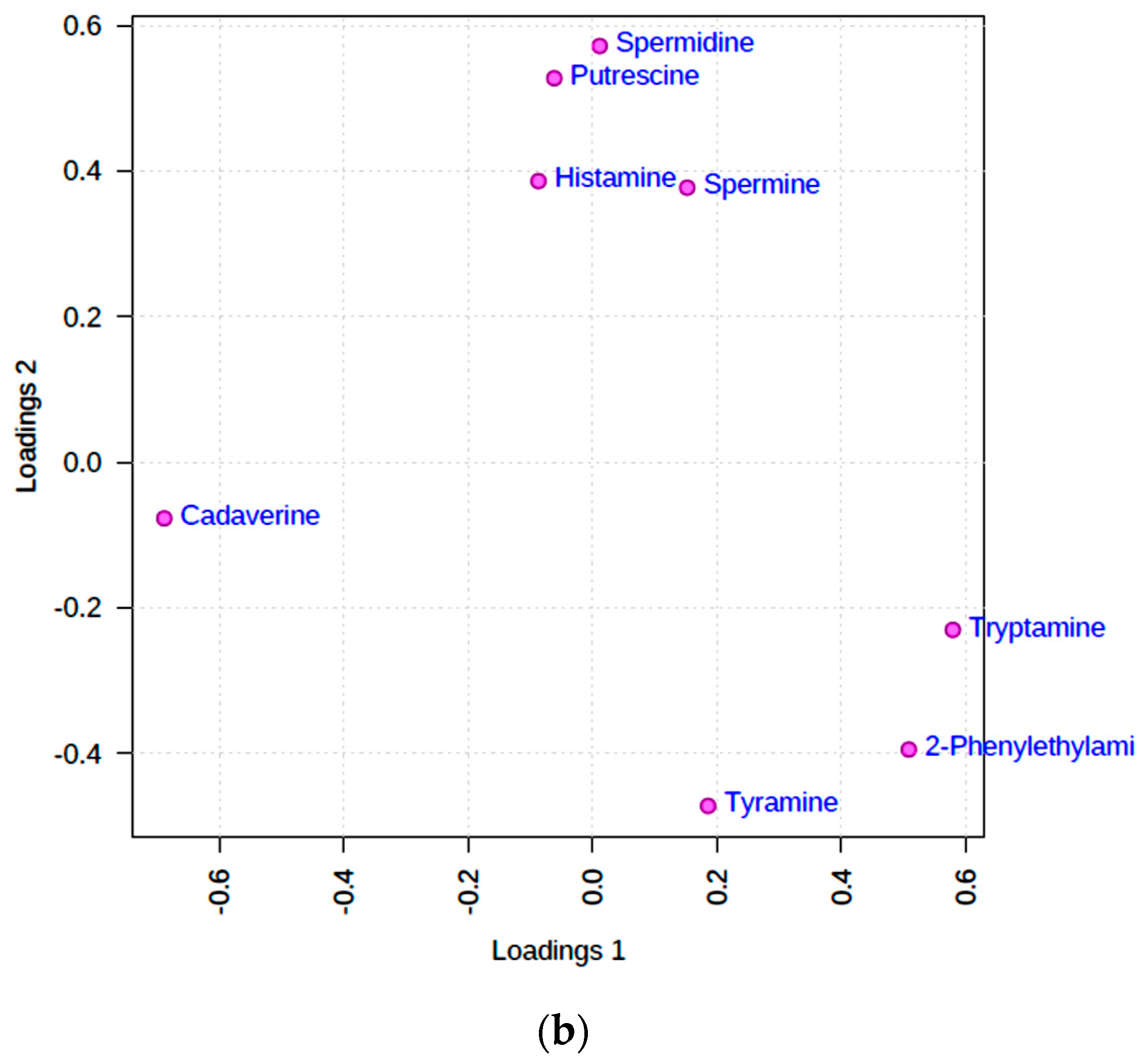
Figure 3b. Clear observations from this diagram are that (1) 2-phenylethylamine, tyramine, and cadaverine, and to a lesser extent, putrescine and tryptamine, are all correlated with PC1, and this observation indicates their communality in this model; (2) food pH values are also strongly correlated to PC1, and this indicates that higher values of this parameter may arise from the basicity of the above BAs (gas-phase primary amine basicity values increase with the length of its carbon chain substituents in view of their electron-donating positive charge-stabilizing effects—such values also increase with progression from primary to secondary to tertiary alkylamines [
39]); (3) an at least partial correlation of histamine contents with PC2, which indicates distinction of this BA from those aligned with PC1; (4) an inverse correlation (anti-correlation) of total lipid level with the (TBARS):(total lipid) ratio index, as might be expected; and (5) a strong anti-correlation of TA value with BA levels, particularly tryptamine and putrescine, and this suggests that these amines serve to offer neutralization potential against acidic fermented food products. Also notable from this Figure are very strong correlations between the fermented food supplementary variable cheese and total lipid content, and between wine/vinegar and TA value, as indeed expected.
A more detailed analysis of these PCA loadings was made with the application of varimax rotation, Kaiser normalization, and a maximal number of 5 PCs considered. For this model, such variable loadings, and the percentage of total variance accounted for by each PC are available in
Table 4. This analysis revealed that cadaverine, tryptamine, 2-phenylethylamine, and tyramine all strongly and positively loaded on PC1, spermidine and histamine strongly and positively loaded on PC3 (along with a more minor contribution from 2-phenylethylamine), and putrescine and spermidine loaded strongly and positively on PC5, albeit also with histamine to a much lesser extent. Interestingly, all aromatic BAs strongly loaded on PC1, as observed above (
Figure 3b), whereas spermidine and its metabolic precursor putrescine both co-loaded onto the same PC (PC5).
The TBARS secondary lipid oxidation index, along with its value normalized to total food lipid content, both loaded strongly and positively on PC2, as might be expected, although histamine also contributed somewhat towards this PC. Moreover, TA and pH values powerfully loaded on PC4 negatively and positively respectively, as would be expected from their anticipated negative correlation in fermented food products (putrescine also made a moderate positive contribution towards this component). Total lipid content was found to load significantly on PCs 1 and 5, positively and negatively so, respectively.
In a related study focused on PCA of both BAs and polyphenolics in Hungarian wines, Cosmos et al. [
26] found that PC scores successfully clustered differential groups of these product classes, and that PC loadings vectors displayed significant patterns of BA and polyphenol levels. However, it should be noted that for this analysis, spermidine, and tyramine strongly loaded on PC1 (positively and negatively, respectively), agmatine and the sum total BA concentration loaded strongly and positively on PC2, spermine and cadaverine both strongly and negatively loaded on PC3, and that histamine loaded strongly and positively on PC4 alone. These associations between the BA analytes tested did not correspond to those found in the present study, although in the above MV analyses we elected not to include the total summed BA concentration value. Furthermore, our study also included the determinations of 2-phenylethylamine and putrescine, and not agmatine, but that reported in [
26] monitored the latter BA but not 2-phenylethylamine and putrescine. However, as noted by the authors of [
26], these PC loadings are only applicable to one region of Hungarian wine production, and their results will not be readily transferable to others, let alone other classes of fermented foods, especially in consideration of the often highly variable methods of fermentation, sources of fermentative micro-organisms, and conditions employed for these purposes. Notwithstanding, these researchers also concluded that in view of the loading patterns of BAs observed, it was unnecessary to measure all BA variables for quality assessments, and that only one per orthogonal PC was sufficient to provide acceptable levels of distinction between different sub-classes of such wines.
From this analysis, the unambiguously strong loadings vectors of the aromatic BAs 2-phenylethylamine and tyramine on PC1 provide evidence that they may indeed arise from the same biological and/or metabolic sources; however, this observation may also be rationalized by the natural production of tyrosine from phenylalanine, i.e., that involving the possible hydroxylation of the latter substrate to the former catalyzed by the enzyme phenylalanine hydroxylase (PAH) potentially available in fermentative lactobacilli employed for the production of fermented food products, followed by enzymatic transformation of the tyrosine product to tyramine by fermentative bacteria. To date, PAH is the only known aromatic amino acid hydroxylase found in bacteria [
40].
The loadings of spermine and spermidine on different orthogonal PCs (PC3 and PC5, respectively) is not simply explicable, although the co-loading of spermidine’s metabolic precursor putrescine on PC5 is consistent with them being featured in the same metabolic pathway. However, the co-loadings of BAs on differential PCs, particularly PC1, may reflect their engenderment from identical or related bacterial sources.
Notably, PC2 was dominated by powerful loading contributions from TBARS level and (TBARS):(total lipid) ratio (both positive), and PC4 by strong loadings from TA and pH values (negative and positive loadings vectors, respectively). These inter-relationships are, of course, expected, and are consistent with the data presented in
Figure 3b. PC5 was retained in the model since it was the only one available which had a strong loading contribution from spermidine.
3.5. PCA of FCLO BAs
The FCLO product considered was primarily investigated separately since only BA contents, and not parameters such as pH and TA were available for it. Moreover, its total lipid content is, of course, not far removed from a value of 100%, and therefore it would be inappropriate to test this index in the above MV analysis models (similarly, total lipid level-normalized TBARS values would also be inappropriate to test in these systems). However, it was possible to explore inter-relationships between FCLO BA concentrations and/or their orthogonality status using a rigorous PCA approach featuring varimax rotation and Kaiser normaliszation in order to maximize success with the assignment of individual BA variables to PCs.
Table 5 lists the BA contents of
n = 10 FCLO product batches. The total concentrations of BAs in these samples was higher than the recommended ”limit” of 200 ppm in only two out of ten batches of the samples tested, albeit marginally so (only 14 and 20% higher). Similarly, bioactive histamine was completely undetectable in this product. As noted in [
6], all BAs monitored were completely undetectable in three other natural, albeit unfermented, CLO products included for comparative purposes. All BAs tested were found to be reasonably soluble in FCLO lipidic matrices, and also in 1/3 (
v/
v) diluted solutions of this product in deuterochloroform (C
2HCl
3), presumably as the uncharged species with their amine functions deprotonated (solubility in these media is expected to increase with increasing amine function substituent chain length and hydrophobicity).
PCA performed on the FCLO BA dataset revealed that cadaverine, putrescine, and tryptamine all loaded strongly and positively on the first of two automatically-selected PCs (PC1), whereas the aromatic BAs 2-phenylethylamine and tyramine loaded strongly and positively on the second (PC2), along with spermidine (
Table 6). These data displayed some consistency with PC loading values obtained on the full fermented food dataset (
Table 4), which had 2-phenylethylamine and tyramine both strongly loading on one PC (PC1). However, such levels will, of course, be critically dependent on the microbial fermentation sources, parameters employed for fermented food production, and production conditions for these processes.
3.6. MV Chemometric Analysis of BA Data Only: Recognition of Fermented Food Class-Distinctive BA Patterns Using CS-Normalization
Additionally, we conducted univariate and MV analyses of datasets which were restricted to the BA profiles only, but also included the
n = 10 FCLO samples reported above. Additionally, these analyses were performed with and without application of constant sum (CS) normalization. The CS-normalized data format was employed in order to facilitate the recognition of fermented food class-specific BA patterns. For the non-CS-normalized format, the total BA content value was also included as an explanatory variable, as indeed it was in [
26].
Firstly, ANOVA performed on the CS-normalized, glog-transformed, and autoscaled dataset found very highly significant, albeit FDR-corrected
p values for three of the sum-proportionate mean BA concentration differences observed between the fermented food classifications explored in this manner. Notably, these differences were observed for cadaverine, 2-phenylethylamine, and tryptamine (
Table 7), and
post-
hoc testing revealed that for cadaverine, the cheese products had significantly greater proportionate levels than three others, and for both 2-phenylethylamine and tryptamine, FCLO had significantly higher ones than all other products examined. These differences in CS-normalized values are readily visualizable in the form of an ANOVA-based heatmap (
Figure 5a), which revealed characteristic BA signatures for three of the fermented food product classifications. Clearly, the cheese, FCLO, and wine/vinegar sampling groups have high proportionate levels of cadaverine, 2-phenylethylamine/tyramine/tryptamine (all aromatic BAs), and metabolic pathway-associated putrescine/spermidine/spermine, respectively. However, when evaluated in this univariate system, ”between-fermented food class” mean differences observed for putrescine, spermine, spermidine, histamine, and tyramine were not found to be statistically significant.
Secondly, both PCA and PLS-DA models were employed, and these approaches were successful in providing evidence for the MV distinctiveness of the FCLO, cheese, and wine/vinegar groups; however, as noted for the analyses conducted on the combined BA/further food quality parameter dataset, unfortunately no distinctions were observed between the fermented fish, fish sauce/paste, and vegetable sauce products (
Figure 5b,c).
For the CS-normalized dataset (without total BA concentrations as an additional variable), PLS-DA variable importance parameter (VIP) values were in the order spermidine (1.48) > putrescine (1.34) > spermine (1.20) > histamine (1.06) > 2-phenylethylamine (0.94), whereas those for the non-CS-normalized dataset were spermidine (1.56) > spermine (1.35) > 2-phenylethylamine (1.32) > putrescine (0.84) (total BA level was a very poor predictor variable for the latter). As expected, there were significant differences between the sequential orders of these values when prior CS-normalization was implemented.
Moreover, for the PLS-DA model adopted without CS-normalization, histamine, spermidine, and spermine contents all loaded significantly on component 1 (loading vector coefficients 0.48, 0.57, and 0.47 respectively); 2-phenylethylamine, cadaverine, tyramine, and total BA levels on component 2 (loadings vector coefficients 0.42, −0.61, −0.57, and −0.61 respectively); 2-phenylethylamine and tryptamine levels on PC3 (loadings vector coefficients 0.50 and 0.57 respectively); and putrescine and spermine on PC4 (loadings vector coefficients 0.75 and −0.73 respectively). For this dataset, a four-component model was found to be most effective (permutation p value 0.0055)
Importantly, it should be noted that one now common issue in chemometrics/metabolomics experiments is the occurrence of a univariately-insignificant variable which remains multivariately- significant. Such observations are readily rationalized, firstly by the complementation (i.e., correlation) between explanatory variables, i.e., separately they do not, but when combined together as a MV composite (e.g., as a sufficiently-loading PC variable), they do serve to explain “between-classification” differences detected; secondly, consistency effects arising from the “masking” of potential univariately-significant differences by high levels of biological source sampling and/or measurement variation may be responsible (such variation may be averaged out via the conversion of datapoints to orthogonal component scores as in the PCA and PLS-DA models applied here); and thirdly, relatively small sample sizes for each classification involved (fermented foods in this case)—unfortunately, strategies applied to correct for FDRs promote the risk of statistical type II errors (i.e., false negatives) [
24].
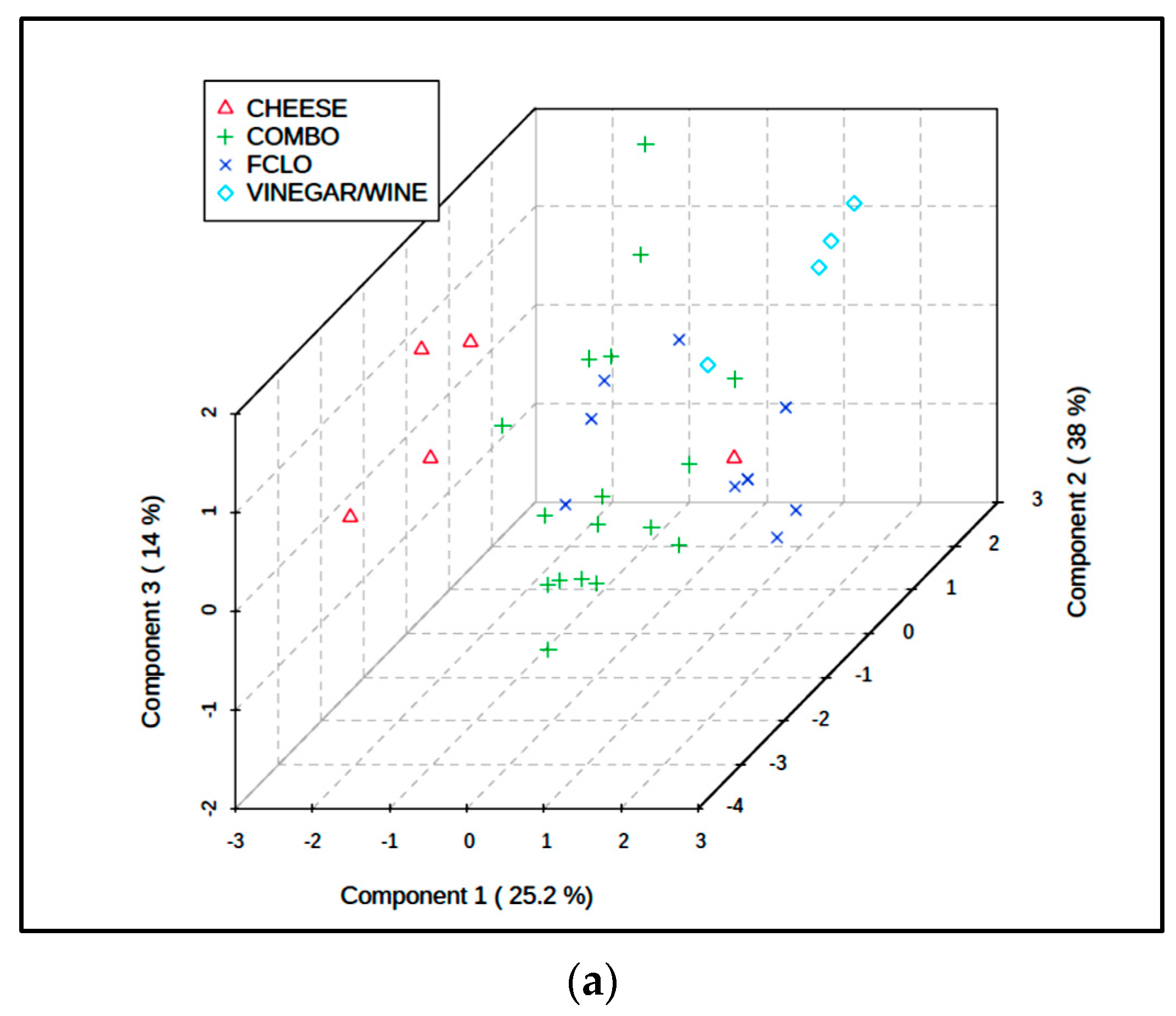
The PLS-DA evaluation was then extended and performed for pairwise comparisons of the differing fermented food classifications (CS-normalized dataset only). Firstly, as expected, Q2 values for the fish vs. fish sauce/paste, fish sauce/paste vs. vegetable sauce, and fish vs. vegetable sauce comparisons were all moderately negative, and p values for associated permutation tests were all >0.10. However, these values for the wine/vinegar vs. FCLO, and FCLO vs. cheese two classification model comparisons revealed that Q2 (permutation p values) indices for these comparisons were 0.71 (0.059) and 0.72 (0.090), but only 0.38 (0.16) for the wine/vinegar vs. cheese one (values were based on models containing two, five, and one components respectively). Hence, these results provide some evidence for the success of this strategy in distinguishing between the FCLO product, and both the cheese and wine/vinegar ones, although permutation test p values obtained for these models were a little higher than the 0.05 significance level, i.e., they were close to statistical significance.
We then elected to statistically combine the fish, fish sauce/paste, and vegetable sauce groups, and repeated the PLS-DA modelling in order to compare the sauce/fish composite, cheese, FCLO, and wine/vinegar groups using the CS-normalized dataset. This analysis exhibited a quite high level of classification success (
Figure 6a); Q
2 for this comparative four-classification analysis was 0.44, and a PLS-DA permutation test confirmed its significance (
p = 0.031). The loadings of each BA variable on PLS-DA components 1 and 2 is shown in
Figure 6b, and this demonstrates three groups of these predictors: the first with highly positive component 1 and highly negative component 2 loadings (all aromatic BAs, i.e., 2-phenylethylamine, tyramine, and tryptamine); the second with low to intermediate positive component 1 but highly positive component 2 loadings (metabolically-related putrescine, spermidine, and spermine, together with histamine); and the third with highly negative loadings on component 1, but negligible loadings on component 2 (cadaverine only). These grouped BA loadings vectors were very consistent with other observations made from the MV analysis of these data as a full six fermented food classification dataset. Specifically, they are completely reflective of the patterns of BA “markers” found in fermented FCLO, wine/vinegar, and cheese products respectively (
Figure 5a).
Finally, RF analysis of this revised dataset showed that this approach had an at least reasonable level of classification success, with all (10/10) FCLO and 88% (15/17) of the fish/sauce combination samples being correctly classified; notwithstanding, only 60 and 50% of the cheese and wine/vinegar fermented food products, respectively, were.
3.7. Scientific Significance and Human Health Implications of Results Acquired
Results acquired from the combined applications of univariate and MV chemometrics techniques in this study clearly demonstrated that the latter strategy was valuable for distinguishing between fermented wine/vinegar products and cheeses, and the discrimination between both of these food classes from either fish, fish sauce/paste, or vegetable sauce products (or a statistical combination of them) was possible on the basis of their BA, total lipid, pH, and TA values; nevertheless, such techniques were not readily able to distinguish between the latter three fermented food classes. However, a rigorously-constrained univariate analysis method selected to overcome complications arising from intra-food classification heteroscedasticities and FDRs was able to successfully distinguish between the vegetable sauce and fish groups through significantly higher and lower levels of spermidine and 2-phenylethylamine, respectively, present in the former class. Moreover, experimental results indicated that cadaverine, tyramine, putrescine, and tryptamine concentrations may all contribute significantly towards food pH values in view of their strong positive correlations with this parameter found, together with corresponding negative ones with TA values (
Figure 3b).
Moreover, BA-targeted univariate and multivariate analyses of CS-normalized data was found to be valuable for providing useful discriminatory information, which highlighted the characteristic patterns of BA biomolecules, which may be valuable for further investigations of the particular nature and/or geographic origins of fermented foods, and the mechanisms involved in their formation. Indeed, the present study found that such patterns comprised cadaverine only for cheese samples, three aromatic BAs (2-phenylethylamine, tyramine, and tryptamine), for FCLOs (sourced from fermented cod livers), and those from the sequential metabolic pathway which transforms the amino acid substrates ornithine or arginine to spermine (i.e., putrescine, spermidine, and spermine itself) for wine/vinegar products. Such idiosyncratic, fermented food product-dependent signatures for CS-normalized fermented food BA concentrations may serve to provide valuable information regarding the fermentative bacterial sources, routes involved in fermentation, and product manufacturing conditions employed for them.
For the putrescine → spermidine → spermine metabolic pathway, which was identified as representing a wine/vinegar-specific one from analysis of the CS-normalized dataset, and which accounted for >70% of total BAs in this fermented food class (
Table 2), both positive or negative correlations could arise between a BA catabolite and its immediate upstream precursor, but not necessarily between the terminal spermine metabolite and that upstream of its spermidine substrate (i.e., putrescine).
With regard to toxic concentrations and health risk recommendations available in [
25], it should be noted that all mean histamine levels determined in the fermented food samples tested here lie markedly below the recommended 100 ppm limit for it (with no single product exceeding this value—the highest level observed was 57 ppm in one of the fish sauce products assessed). Furthermore, with the exception of the cheese products evaluated, the mean total BA values all food groups were <200 ppm, the wine/vinegar classification substantially so (
Table 2). However, although three of the cheese products tested had total BA contents of <200 ppm, two of them had levels ranging from 600–800 ppm, and therefore their dietary consumption may present a health risk for susceptible individuals.
Mean BA concentrations for the FCLO product examined ranged from 0 (histamine) to only 34 ppm (2-phenylethylamine), with the highest levels observed for the most predominant species, 2-phenylethylamine and tyramine, being 103 and 88 ppm. Since the United States of America’s recommended dietary intake of health-friendly, highly unsaturated omega-3 (O-3) fatty acids (FAs) is a maximum of 1.0 g/day [
41], and the oil explored here contains a mean of 29% (
w/
w) total O-3 FAs (predominantly the sum of eicosapentaenoic and docosahexaenoic acids) [
6], then daily consumption of 100/29% × 1.0 g = 3.45 g of this FCLO product would provide estimated absolute maximal daily intake levels of 3.45 × 103 µg = 355 µg, and 3.45 × 88 µg = 304 µg of 2-phenylethylamine and tyramine, respectively. Based on the 10 samples of this product analyzed, estimated mean daily intakes of these BAs will be 111 and 95 µg only. Therefore, it appears that daily consumption of this product at the recommended U.S.-recommended dosage levels will certainly not provide any health risks to consumers, even if they are susceptible to the adverse effects experienced by their excessive intake (e.g., migraines induced by 2-phenylethylamine).
As noted above, one potentially important health benefit offered by the ingestion of dietary BAs is their novel antioxidant properties, both for the prevention of food spoilage during storage or transport episodes, but also in vivo following their ingestion. Indeed, our laboratory recently explored the powerful antioxidant capacities of BA-containing natural FCLO products, and their resistivities to thermally-mediated oxidative damage to unsaturated FAs therein, particularly O-3 PUFAs [
6]. These marine oil products, which arise from the pre-fermentation of cod livers (
Section 2), were indeed found to display a very high level of antioxidant activity, and PUFAs therein were also more resistant towards thermally-mediated peroxidation than other natural cod liver oil products evaluated. Resonances assignable to aromatic BAs, specifically those arising from 2-phenylethylamine and tyramine, were directly observable in the
1H NMR profiles of ca. 1/3 (
v/
v) diluted solutions of these products with C
2HCl
3. Additionally, corresponding spectra acquired on both
2H
2O and C
2H
3O
2H extracts of these oils confirmed the presence of both these BAs, together with a series of others, both aromatic and aliphatic. In the present study, mean concentrations of 2-phenylethylamine and tyramine detectable in these products were found to be 34 and 24 ppm, respectively. Antioxidant actions of the phenolic BA antioxidant tyramine found in this FCLO product may be explicable by its chain-breaking antioxidant effects, and this may offer contributions towards the potent resistance of PUFAs, particularly O-3 FAs, present therein. However, in view of the absence of a phenolic function in 2-phenylethylamine, its antioxidant potential is likely to involve an alternative radical-scavenging mechanism, presumably that involving O
2-consuming carbon-centered pentadienyl radical species, as found in [
22,
23].
The TBARS method employed here to determine the lipid peroxidation status of fermented foods, which involved an extended low temperature equilibration process [
28], successfully avoids the artefactual generation of TBA-reactive aldehydes, including malondialdehyde (MDA), during commonly-employed alternative protocols for this assay system, which generally involve a short (ca. 10–15 min) heating stage at 95–98 °C in order to develop the monitored pink/red chromophore rapidly. However, from an analysis of TBARS and (TBARS):(total lipid) ratios determined on the preliminary FCLO-excluded fermented food samples, there appears to be only little evidence for the ability of BAs to offer any protection against lipid peroxidation in such products. Although
Table 3 shows that the above ratio is significantly greater in the wine/vinegar group, this observation is perceived to be derived from their very low lipid contents, and the presence of a range of non-MDA TBA-reactive aldehydes present therein, including acetaldehyde and acrolein, for example, although these are also lipid oxidation products. Moreover, despite taking steps to avoid the artefact-generating heating stage of this assay, this test still remains poorly specific in view of the reactions of a variety of non-aldehydic substrates to react with it to form interfering chromophores, which also absorb at a monitoring wavelength of 532 nm. Nevertheless, TBARS level appeared to be positively correlated with fermented food spermidine concentration (
Figure 2), and both this lipid peroxidation index and its lipid-normalized value appeared to be positively correlated with fermented food histamine content (loading on PC2,
Table 4). However, in view of the many complications associated with this TBARS lipid peroxidation index, which offers only a very limited and still often erroneous viewpoint on the highly complex lipid peroxidation process [
42], such observations cannot be rationally considered at this stage. As expected, the lipid-normalized TBARS value was negatively correlated with total lipid content (
Figure 3b). The latter variable also appeared to be negatively correlated with histamine and putrescine levels (loading on PC5,
Table 4). Unfortunately, results from unspecific TBARS assays are still widely employed as important quality indices throughout the food industry.
One quite surprising observation made in the current study was the detection of lipids, albeit at low levels, in wine and vinegar samples. Notwithstanding, as noted above, FAs have been detected in Zhenjiang aromatic vinegar products at similar contents to those found here [
32]. Furthermore, Yunoki et al. [
43] explored the FA constituents of some commercially-available red wine products, and found that lipid constituent concentrations varied from 27 to 96 mg/100 mL for
n = 6 domestic (Japanese) wines, and 31 to 56 mg/100 mL for
n = 6 foreign products, and that a total of 12 different FAs were detectable, mainly saturated ones. Although the extraction method described in the latter report was a 2:1 chloroform:methanol (Folch) one that targets non-polar triacylglycerols (TAGs) and more polar phospholipids, it is likely that the FAs detectable in the wine/vinegar products explored here, and also those present in Zhenjiang aromatic vinegars [
32], are present as free non-glycerol-esterified species and their corresponding anions, and this would account for their higher levels detectable in these studies than those reported in [
43]. Indeed, fermentation processes readily induce the hydrolysis of TAGs to free FAs, together with mono- and diacylglycerol adducts, and free glycerol [
44]; such FAs will be expected to contribute towards the food pH values determined here. Similarly, Phan et al. [
45] found a broad spectrum of lipidic species, specifically TAGs, polar lipids, free FAs, sterols, and cholesterol esters present in pinot noir wines.
The official AOAC gravimetric method for lipid determination employed in the current study involves an acid hydrolysis step involving HCl in any case, followed by extraction with mixed ethers, i.e., both diethyl and petroleum ethers. Hence, the HCl added will be sufficient to hydrolyze any residual TAGs present to free FAs and glycerol, and also fully protonate the former so that they are extractable as such into ether solvents. Indeed, it has been demonstrated that such free FAs are readily soluble and extractable into these ether solvent systems [
46,
47]. Hence, the passage of lipidic species from grapes and/or micro-organisms to finalized bottled wine and vinegar products has been confirmed in further investigations.
Interestingly,
1H NMR analysis of
2H
2O extracts of the FCLO product investigated found proportionately high concentrations of free FAs and free glycerol therein (data not shown). These FAs were mainly present as PUFAs, as would be expected from the overall lipid composition of this product which contains high levels of omega-3 FAs as TAG species prior to fermentation induction. This observation is fully consistent with the ability of lactobacilli-mediated fermentation processes to partially hydrolyze TAGs in such a product. High levels of the short-chain organic acids propionic and acetic acids (as their propionate and acetate anions in neutral solution media), both lactobacilli fermentation catabolites, the former arising from the metabolic reduction of lactate [
48], were also detectable in these extracts. These results will be reported in detail elsewhere.