Prebiotic Potential and Anti-Inflammatory Activity of Soluble Polysaccharides Obtained from Soybean Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microwave Assisted Enzymatic Extraction
2.3. Characterization of MESP
2.4. Stimulation of Probiotic Growth
2.5. Short Chain Fatty Acid (SCFA) Analysis
2.6. Cytotoxicity Assay
2.7. Measurement of NO and Cytokines
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
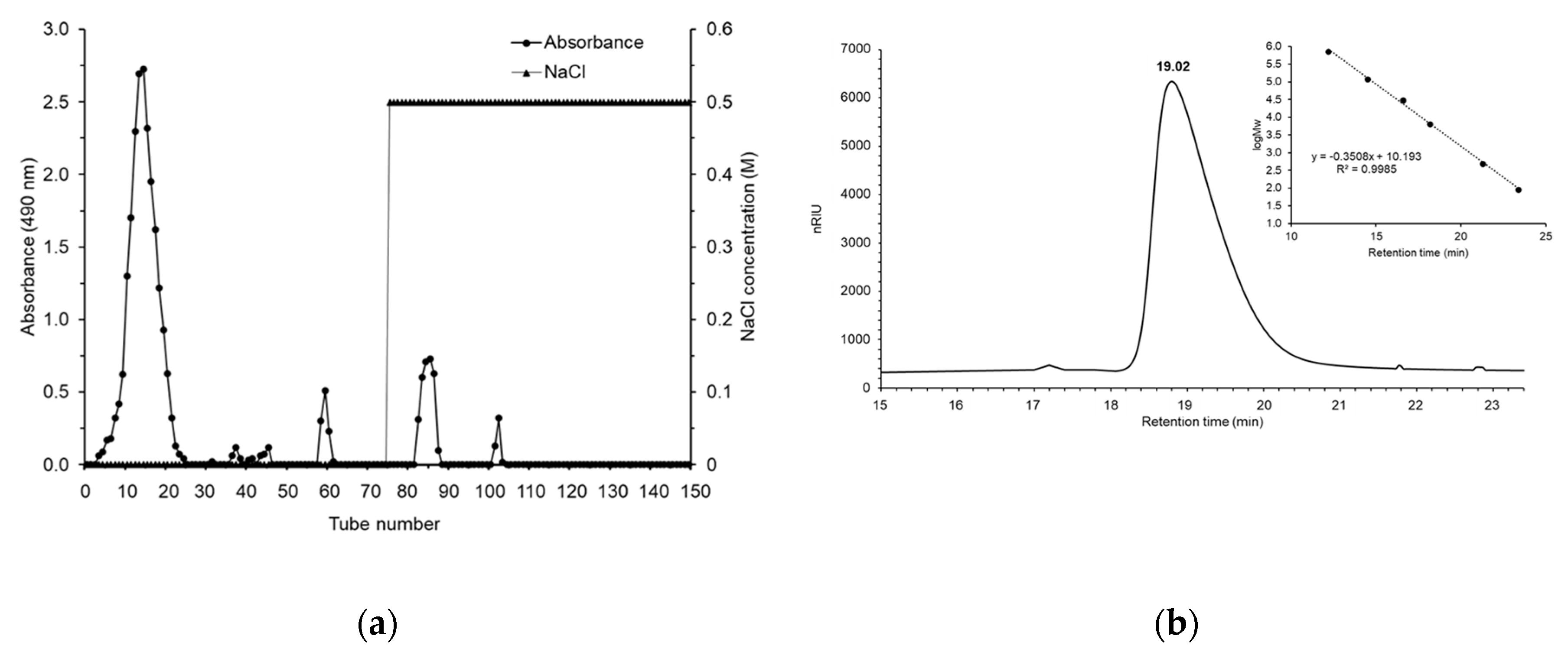
3.1. Polysaccharide Extraction, Isolation, and Purification
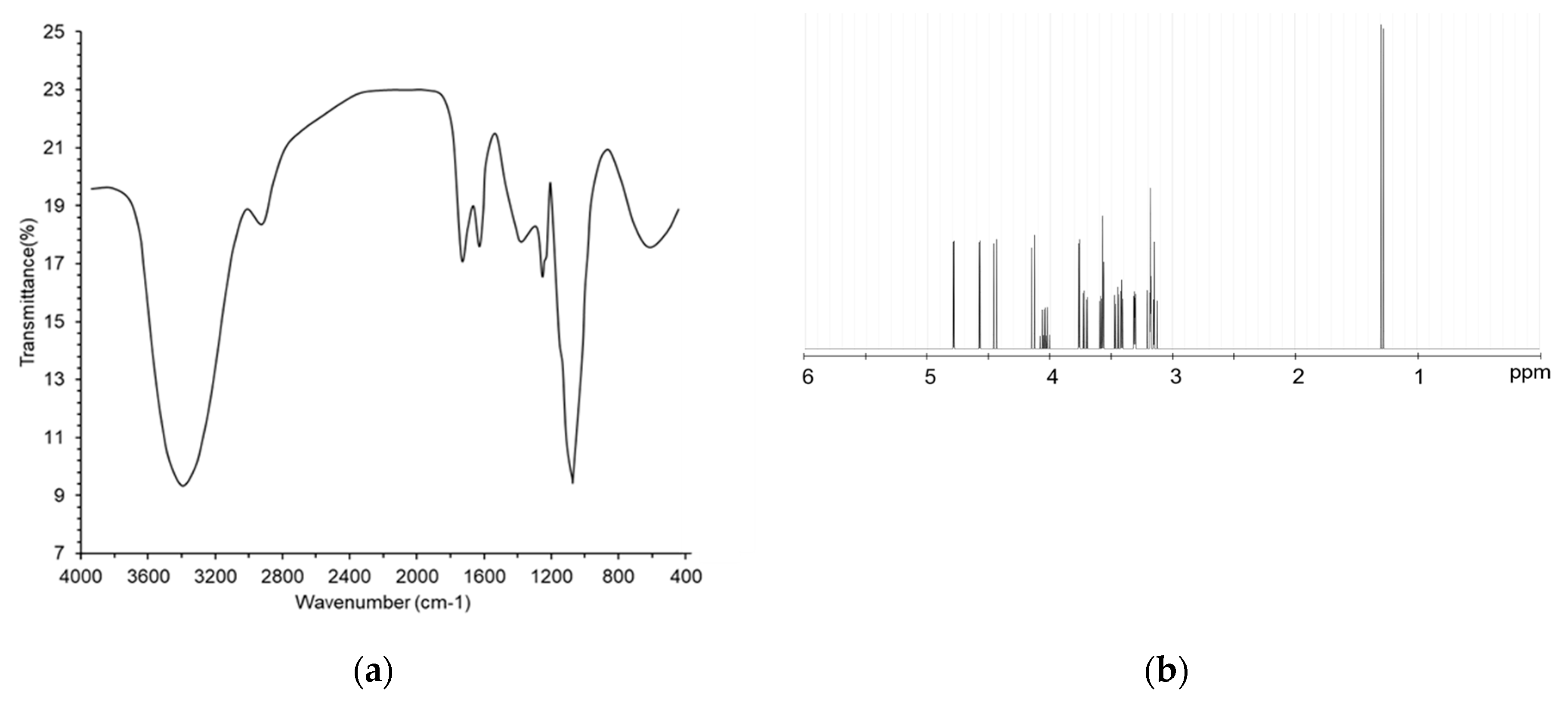
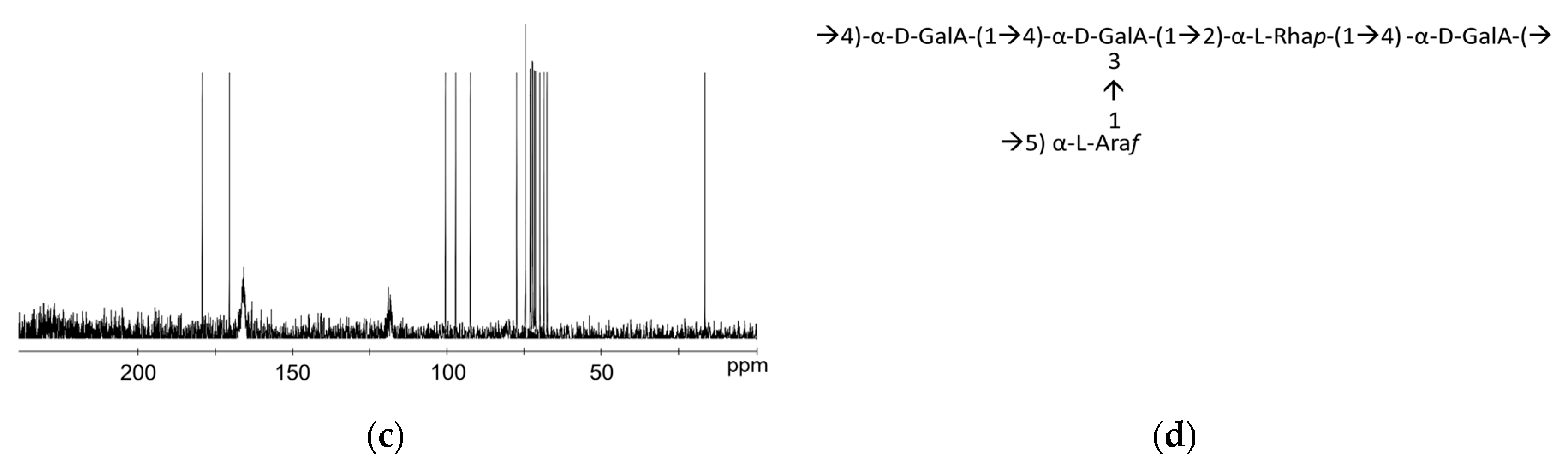
3.2. Structural Characterization
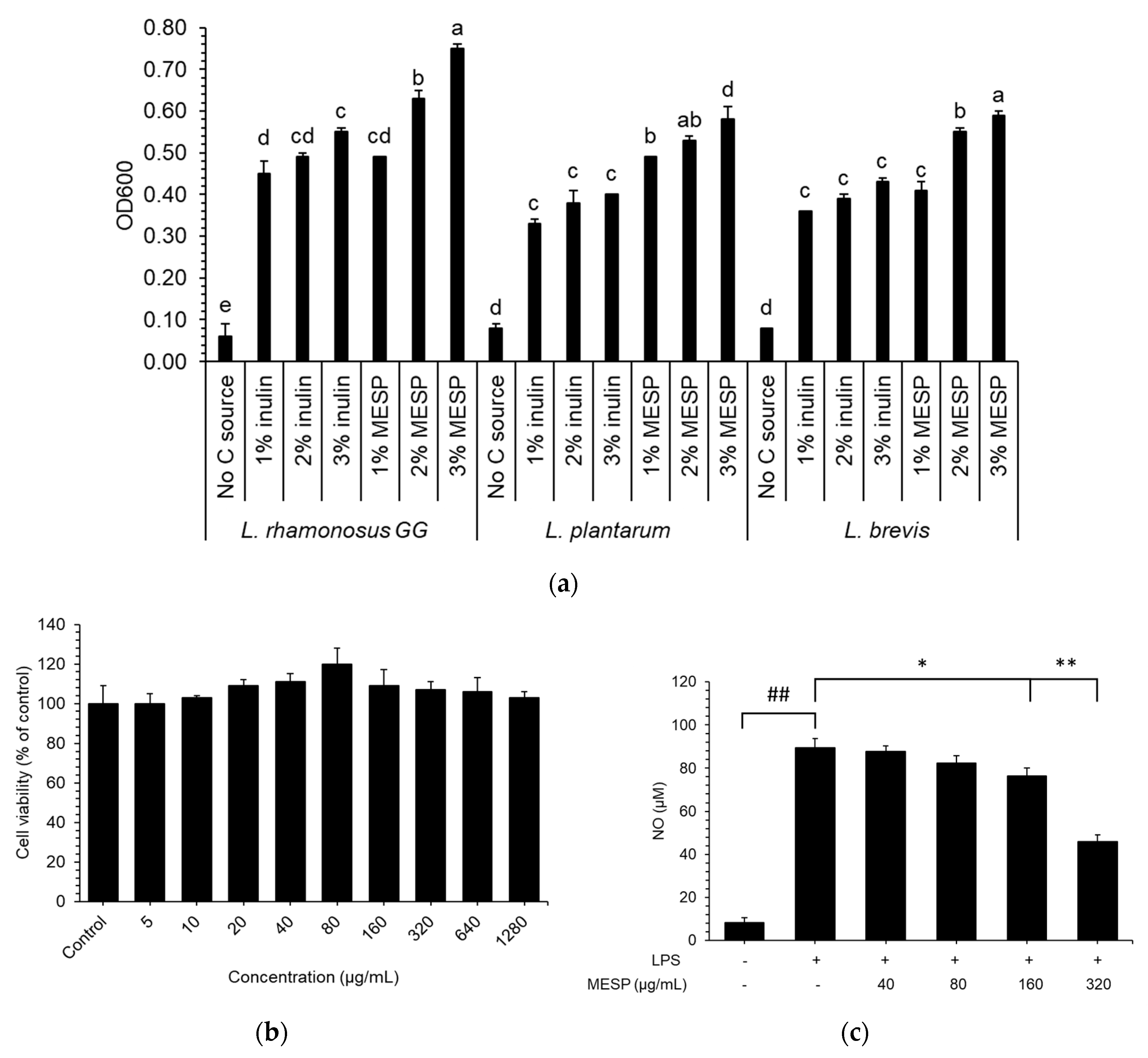
3.3. MESP Stimulated Probiotic Growth
3.4. Effect of MESP on SCFA Production
3.5. Effects of MESP on RAW264.7 Cell Proliferation
3.6. Effects of MESP on NO Production in LPS-Stimulated RAW264.7 Cells
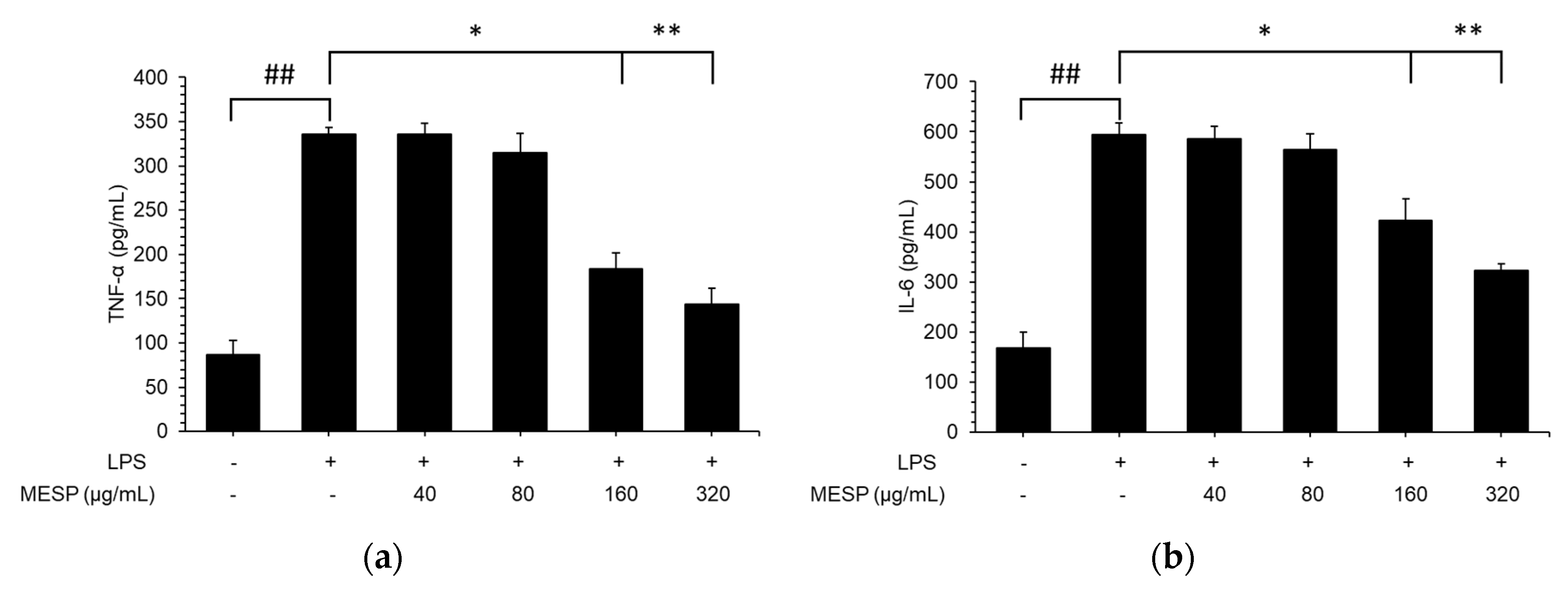
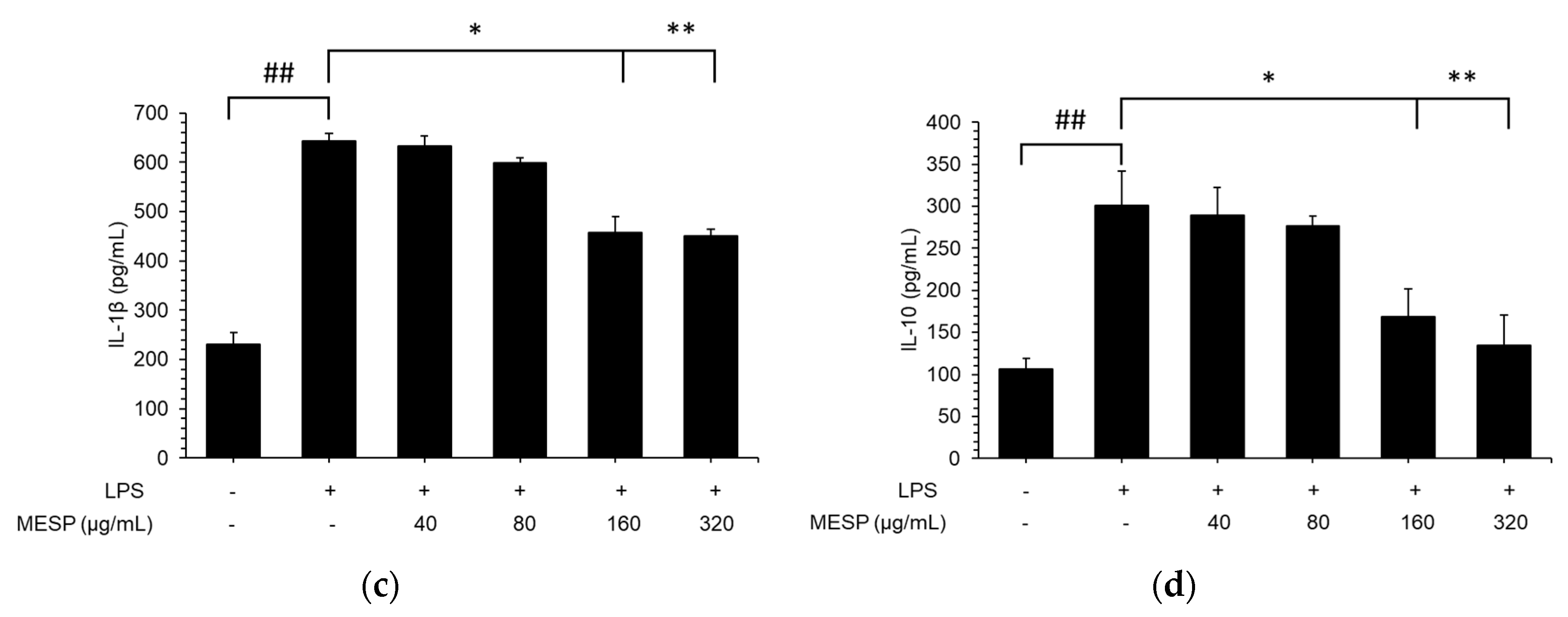
3.7. Effect of MESP on IL-6, TNF-α, and IL-1β Release in LPS-Stimulated RAW264.7 Cells
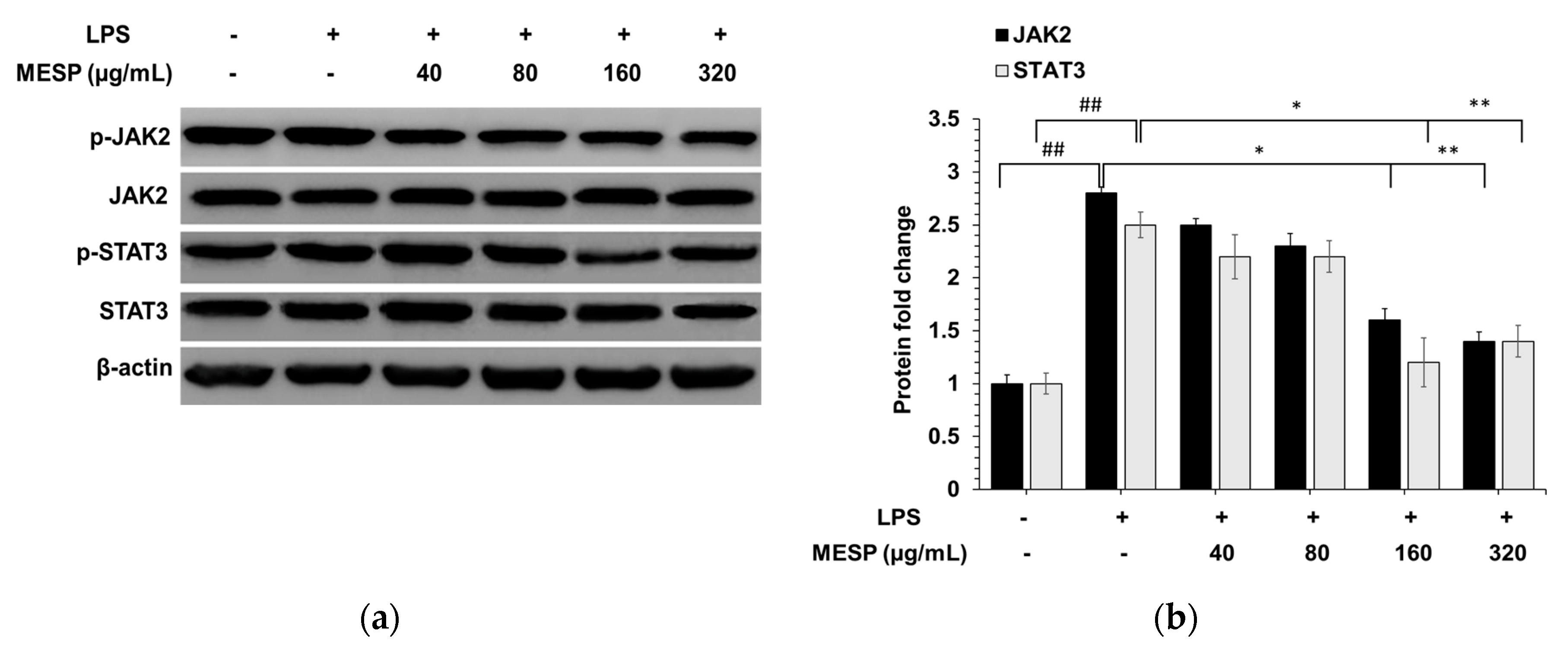
3.8. MESP Blocked the LPS-Triggered Inflammatory Response via the JAK2/STAT3 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamashita, Y.; Sakakibara, H.; Toda, T.; Ashida, H. Insights into the potential benefits of black soybean (Glycine max L.) polyphenols in lifestyle diseases. Food Funct. 2020, 11, 7321–7339. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of by-products from soybean (Glycine max (L.) Merr.) processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Best, T.; Howe, P.; Bryan, J.; Buckley, J.; Scholey, A. Acute effects of a dietary non-starch polysaccharide supplement on cognitive performance in healthy middle-aged adults. Nutr. Neurosci. 2015, 18, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.; Wang, D.; Li, X.; Chen, X.; Chen, X.; Liu, C. Optimization of selenylation conditions for lycium barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef]
- Bai, Z.; Meng, J.; Huang, X.; Wu, G.; Zuo, S.; Nie, S. Comparative study on antidiabetic function of six legume crude polysaccharides. Int. J. Biol. Macromol. 2020, 154, 25–30. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Tafaghodi, M. Environment-friendly green composites based on soluble soybean polysaccharide: A review. Int. J. Biol. Macromol. 2019, 122, 216–223. [Google Scholar] [CrossRef]
- Liu, J.; Wen, X.-Y.; Zhang, X.-Q.; Pu, H.-M.; Kan, J.; Jin, C.-H. Extraction, characterization and in vitro antioxidant activity of polysaccharides from black soybean. Int. J. Biol. Macromol. 2015, 72, 1182–1190. [Google Scholar] [CrossRef]
- Liao, J.; Li, C.; Huang, J.; Liu, W.; Chen, H.; Liao, S.; Chen, H.; Rui, W. Structure characterization of honey-processed Astragalus polysaccharides and its anti-inflammatory activity in vitro. Molecules 2018, 23, 168. [Google Scholar] [CrossRef]
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. Int. J. Biol. Macromol. 2016, 91, 100–105. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Zhang, Y.; Wang, J.; Sun, H.; Wang, J.; Cao, X.; Ye, Y. Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox). Carbohydr. Polym. 2016, 151, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, H.; Furuta, T.; Maeda, H.; Mori, H. Hydrolysis kinetics of okara and characterization of its water-soluble polysaccharides. Biosci. Biotechnol. Biochem. 1996, 60, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Kasai, N.; Murata, A.; Inui, H.; Sakamoto, T.; Kahn, R.I. Enzymatic high digestion of soybean milk residue (okara). J. Agric. Food Chem. 2004, 52, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Suárez, M.J.; Pérez-Cózar, M.L.; Redondo-Cuenca, A. Sequential extraction of polysaccharides from enzymatically hydrolyzed okara byproduct: Physicochemical properties and in vitro fermentability. Food Chem. 2013, 141, 1114–1119. [Google Scholar] [CrossRef]
- Jia, X.; Chen, M.; Wan, J.-B.; Su, H.; He, C. Review on the extraction, characterization and application of soybean polysaccharide. RSC advances 2015, 5, 73525–73534. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of microwave-assisted extraction of polysaccharides from Ulva pertusa and evaluation of their antioxidant activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, X.; Xue, Y.; Hu, J.; Gao, M.-T.; Tsang, Y.F. The influence of soluble polysaccharides derived from rice straw upon cellulase production by Trichoderma reesei. Process Biochem. 2017, 61, 130–136. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, S.; Wang, Z.; Fu, X.; Huang, Q.; Yuan, Y.; Wang, K.; Zhang, B. In vitro fecal fermentation of propionylated high-amylose maize starch and its impact on gut microbiota. Carbohydr. Polym. 2019, 223, 115069. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Jiang, J.-G.; Li, M.-Q.; Zheng, C.-Y.; Zhu, W. Structural characterization and immunomodulatory activity of novel polysaccharides from Citrus aurantium Linn. variant amara Engl. J Funct Foods 2017, 35, 352–362. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Gan, S.; He, Y.; Chen, Z.; Liu, X.; Fu, C.; Qu, Y.; Zhang, J. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem. Toxicol. 2019, 131, 110539. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, W.; Wang, B.; Hui, A.; Du, B.; Wang, T.; Meng, L.; Bian, H.; Wu, Z. Purification, characterization and anti-fatigue activity of polysaccharide fractions from okra (Abelmoschus esculentus (L.) Moench). Food Funct. 2018, 9, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Al Loman, A.; Ju, L.-K. Enzyme-based processing of soybean carbohydrate: Recent developments and future prospects. Enzyme Microb. Technol. 2017, 106, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duizer, L.; Corredig, M.; Goff, H.D. Addition of soluble soybean polysaccharides to dairy products as a source of dietary fiber. J. Food Sci. 2010, 75, C478–C484. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Kojima, H.; Muramoto, M.; Ota, Y.; Hatanaka, C. Effects of hexametaphosphate on soybean pectic polysaccharide extraction. Biosci. Biotechnol. Biochem. 1996, 60, 2028–2031. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Nan, H.; Liu, Y. Isolation and structural characterisation of okara polysaccharides. Molecules 2012, 17, 753–761. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Xiao, L.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Xing, B. Study on the functional properties and structural characteristics of soybean soluble polysaccharides by mixed bacteria fermentation and microwave treatment. Int. J. Biol. Macromol. 2020, 2020, 561–568. [Google Scholar] [CrossRef]
- Hou, G.; Jin, M.; Ye, Z.; Zhang, X.; Huang, Q.; Ye, M. Ameliorate effects of soybean soluble polysaccharide on adenine-induced chronic renal failure in mice. Int. J. Biol. Macromol. 2020, 149, 158–164. [Google Scholar] [CrossRef]
- Ursekar, B.; Soni, P.; Date, A.A.; Nagarsenker, M. Characterization of soy polysaccharide and its in vitro and in vivo evaluation for application in colon drug delivery. AAPS PharmSciTech 2012, 13, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Giovanni, V.; Meng, X. Effects of soybean oligosaccharides on intestinal microbial communities and immune modulation in mice. Saudi J. Biol. Sci. 2017, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kong, X.; Yang, X.; Yin, Y. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 2012, 90, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.-C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 138–152. [Google Scholar] [CrossRef]
- Li, S.; Gao, A.; Dong, S.; Chen, Y.; Sun, S.; Lei, Z.; Zhang, Z. Purification, antitumor and immunomodulatory activity of polysaccharides from soybean residue fermented with Morchella esculenta. Int. J. Biol. Macromol. 2017, 96, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J Funct Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow lupin (Lupinus luteus L.) polysaccharides: Antioxidant, immunomodulatory and prebiotic activities and their structural characterisation. Food Chem. 2018, 267, 319–328. [Google Scholar] [CrossRef]
- Park, S.B.; Song, H.M.; Kim, H.N.; Park, G.H.; Son, H.-J.; Um, Y.; Park, J.; Jeong, J.B. Anti-inflammatory effect of Biji (Soybean curd residue) on LPS-stimulated RAW264. 7 cells. Korean J. Plant Resour. 2018, 31, 117–123. [Google Scholar]
- Lu, Y.; Li, W.; Yang, X. Soluble soybean polysaccharides enhance the protective effects of genistein against hepatic injury in high L-carnitine-fed mice. Food Funct. 2017, 8, 4364–4373. [Google Scholar] [CrossRef]
- Meng, M.; Guo, M.; Feng, C.; Wang, R.; Cheng, D.; Wang, C. Water-soluble polysaccharides from Grifola frondosa fruiting bodies protect against immunosuppression in cyclophosphamide-induced mice via JAK2/STAT3/SOCS signal transduction pathways. Food Funct. 2019, 10, 4998–5007. [Google Scholar] [CrossRef]
- Wang, X.; Chu, Q.; Jiang, X.; Yu, Y.; Wang, L.; Cui, Y.; Lu, J.; Teng, L.; Wang, D. Sarcodon imbricatus polysaccharides improve mouse hematopoietic function after cyclophosphamide-induced damage via G-CSF mediated JAK2/STAT3 pathway. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-L.; Yeh, H.-H.; Lin, C.-C.; Lai, W.-W.; Chang, J.-Y.; Chang, W.-T.; Su, W.-C. Signal transducer and activator of transcription 3 activation up-regulates interleukin-6 autocrine production: A biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Mol. Cancer 2010, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Sample | Molecular Weight (kDa) | Monosaccharide Compositions (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Fuc | Xyl | Man | Gal | Glc | GalA | ||
| Soybean residue | 16.41 | 3.46 | 0.91 | 12.89 | ND | 27.56 | 10.23 | 20.3 | |
| MESP | 3.4 | 27.4 | 11.41 | 1.32 | 5.71 | ND | 2.53 | 1.53 | 18.1 |
| Residues | Chemical Shift Assignment of MESP | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| A | →4)-α-D-GalA-(1→ | H | 4.58 | 3.18 | 3.58 | 3.42 | 3.76 | |
| C | 94.3 | 74.0 | 74.9 | 73.2 | 76.4 | 172.2 | ||
| B | →2)-α-L-Rhap-(1→ | H | 4.53 | 3.57 | 3.46 | 3.15 | 4.03 | 1.21 |
| C | 99.0 | 79.2 | 69.4 | 71.7 | 70.4 | 18.3 | ||
| C | α-D-GalA-(1→ | H | 4.45 | 3.18 | 3.33 | 3.71 | 4.14 | |
| C | 98.4 | 74.1 | 74.2 | 73.5 | 74.6 | 181.1 | ||
| D | →5) α-L-Araf (1→ | H | 4.88 | 3.56 | 3.52 | 3.37 | 3.95 | |
| C | 98.59 | 72.2 | 69.6 | 71.23 | 61.01 | 15.9 | ||
| SCFAs (mM) | Sample | Fermentation Time (h) | ||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | 48 | ||
| Acetic acid | Control | 3.89 ± 0.48 H 1 | 4.29 ± 0.69 cH | 16.48 ± 0.71 bF | 15.39 ± 0.67 cF | 15.48 ± 0.99 cF |
| 1% inulin | 9.48 ± 0.49 bG | 21.86 ± 0.89 bE | 29.82 ± 0.08 bC | 24.36 ± 0.53 bD | ||
| 1% MESP | 24.18 ± 0.36 aD | 51.84 ± 0.05 aB | 69.48 ± 0.74 aA | 69.14 ± 0.62 aA | ||
| Butyric acid | Control | 2.18 ± 0.31 E | 2.95 ± 0.12 bE | 4.85 ± 0.49 bD | 8.62 ± 0.18 bB | 6.21 ± 0.83 cC |
| 1% inulin | 4.31 ± 0.81 aD | 6.14 ± 0.48 aC | 9.89 ± 0.84 bB | 9.18 ± 0.23 bB | ||
| 1% MESP | 4.18 ± 0.69 aD | 6.83 ± 0.41 aC | 13.21 ± 0.55 aA | 13.23 ± 0.81 aA | ||
| Propionic acid | Control | 1.22 ± 0.23 D | 1.33 ± 0.04 bD | 2.89 ± 0.63 bD | 4.26 ± 0.47 bC | 4.11 ± 0.84 bC |
| 1% inulin | 4.15 ± 0.28 aC | 6.81 ± 0.16 aB | 11.51 ± 0.04 aA | 11.81 ± 0.41 aA | ||
| 1% MESP | 4.92 ± 0.85 aC | 7.21 ± 0.74 aB | 12.21 ± 0.56 aA | 12.18 ± 0.07 aA | ||
| Total SCFAs | Control | 9.12 ± 0.11 G | 9.34 ± 0.75 cG | 22.14 ± 0.44 cE | 34.95 ± 0.12 bD | 32.43 ± 0.64 cD |
| 1% inulin | 11.15 ±0.07 bF | 33.81 ± 0.49 bD | 45.12 ± 0.17 bC | 44.26 ± 0.16 bC | ||
| 1% MESP | 29.81 ± 0.09 aE | 63.31 ± 0.18 aB | 83.49 ± 0.78 aA | 82.46 ± 0.36 aA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, B.; Pham, T.N.A.; Yang, S.H. Prebiotic Potential and Anti-Inflammatory Activity of Soluble Polysaccharides Obtained from Soybean Residue. Foods 2020, 9, 1808. https://doi.org/10.3390/foods9121808
Le B, Pham TNA, Yang SH. Prebiotic Potential and Anti-Inflammatory Activity of Soluble Polysaccharides Obtained from Soybean Residue. Foods. 2020; 9(12):1808. https://doi.org/10.3390/foods9121808
Chicago/Turabian StyleLe, Bao, Thi Ngoc Anh Pham, and Seung Hwan Yang. 2020. "Prebiotic Potential and Anti-Inflammatory Activity of Soluble Polysaccharides Obtained from Soybean Residue" Foods 9, no. 12: 1808. https://doi.org/10.3390/foods9121808
APA StyleLe, B., Pham, T. N. A., & Yang, S. H. (2020). Prebiotic Potential and Anti-Inflammatory Activity of Soluble Polysaccharides Obtained from Soybean Residue. Foods, 9(12), 1808. https://doi.org/10.3390/foods9121808






