Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of NLCs Prepared by Different Methods
2.2. NLC Stability
2.3. TEM Analysis
2.4. HPLC Method Validation
2.5. Characterization and Stability Study of Chili-Extract-Loaded NLCs Incorporated in Gel Formulation
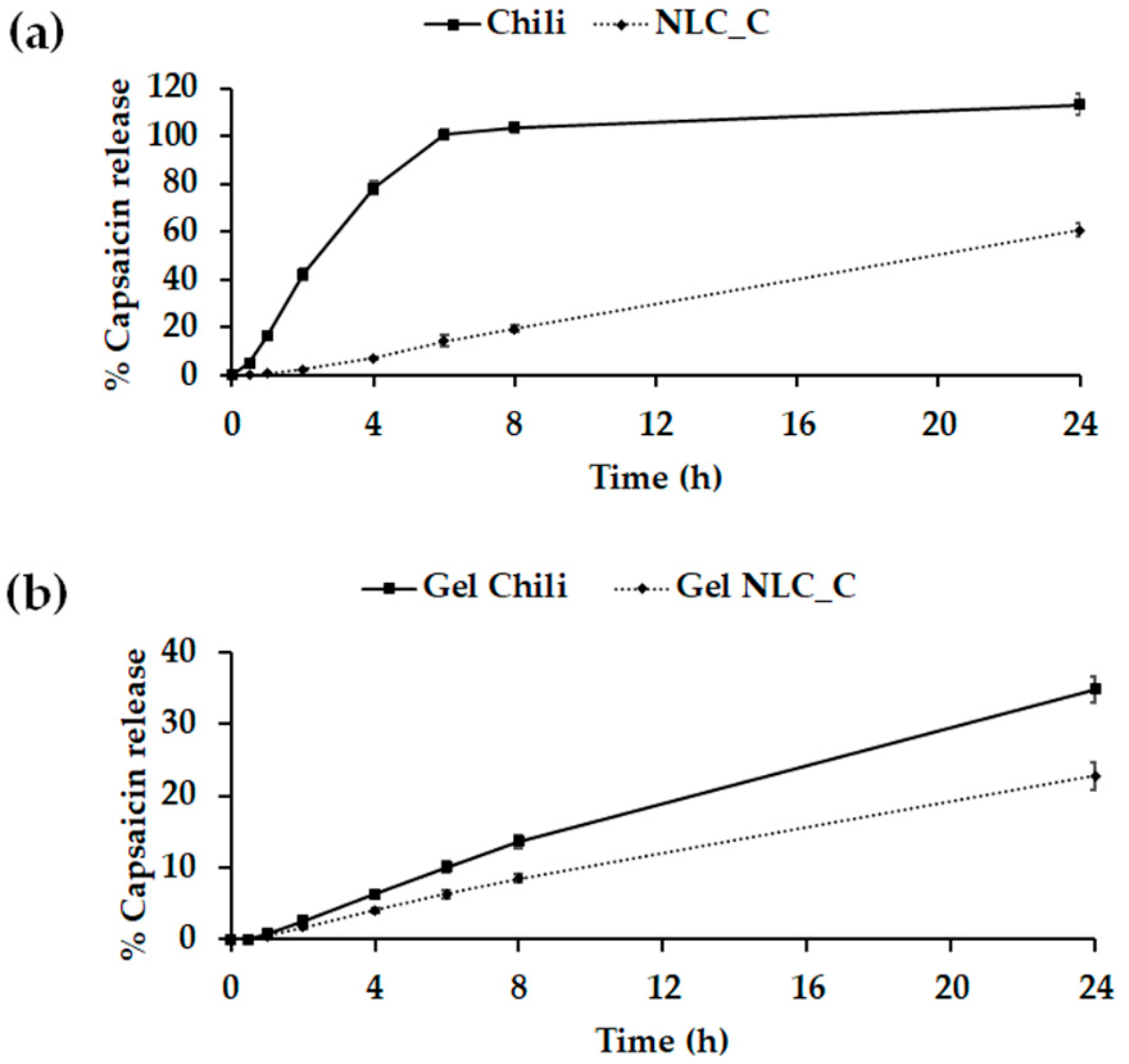
2.6. In Vitro Release Study
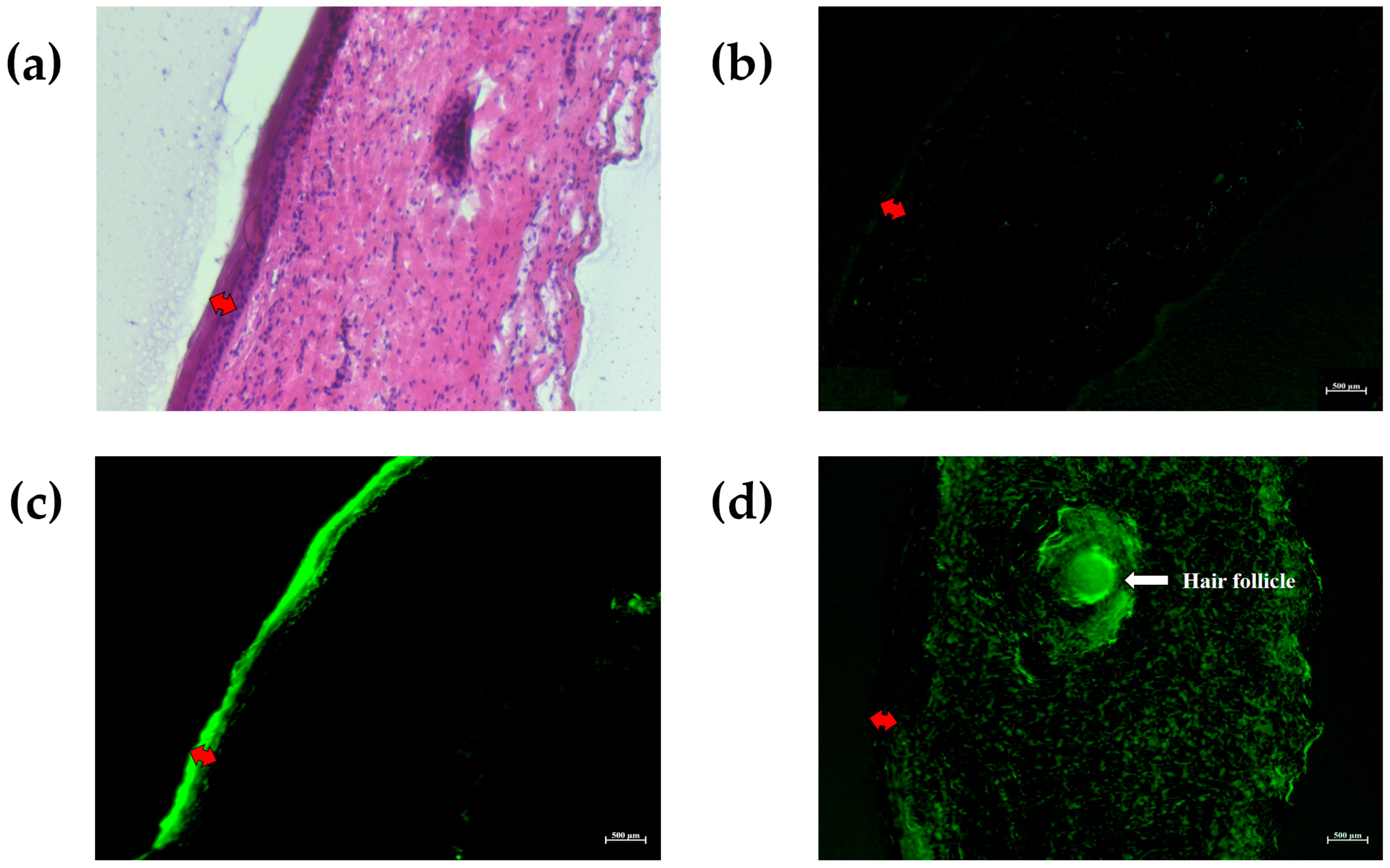
2.7. Study of NLC Distribution through the Skin Layers Using Fluorescence Microscopy
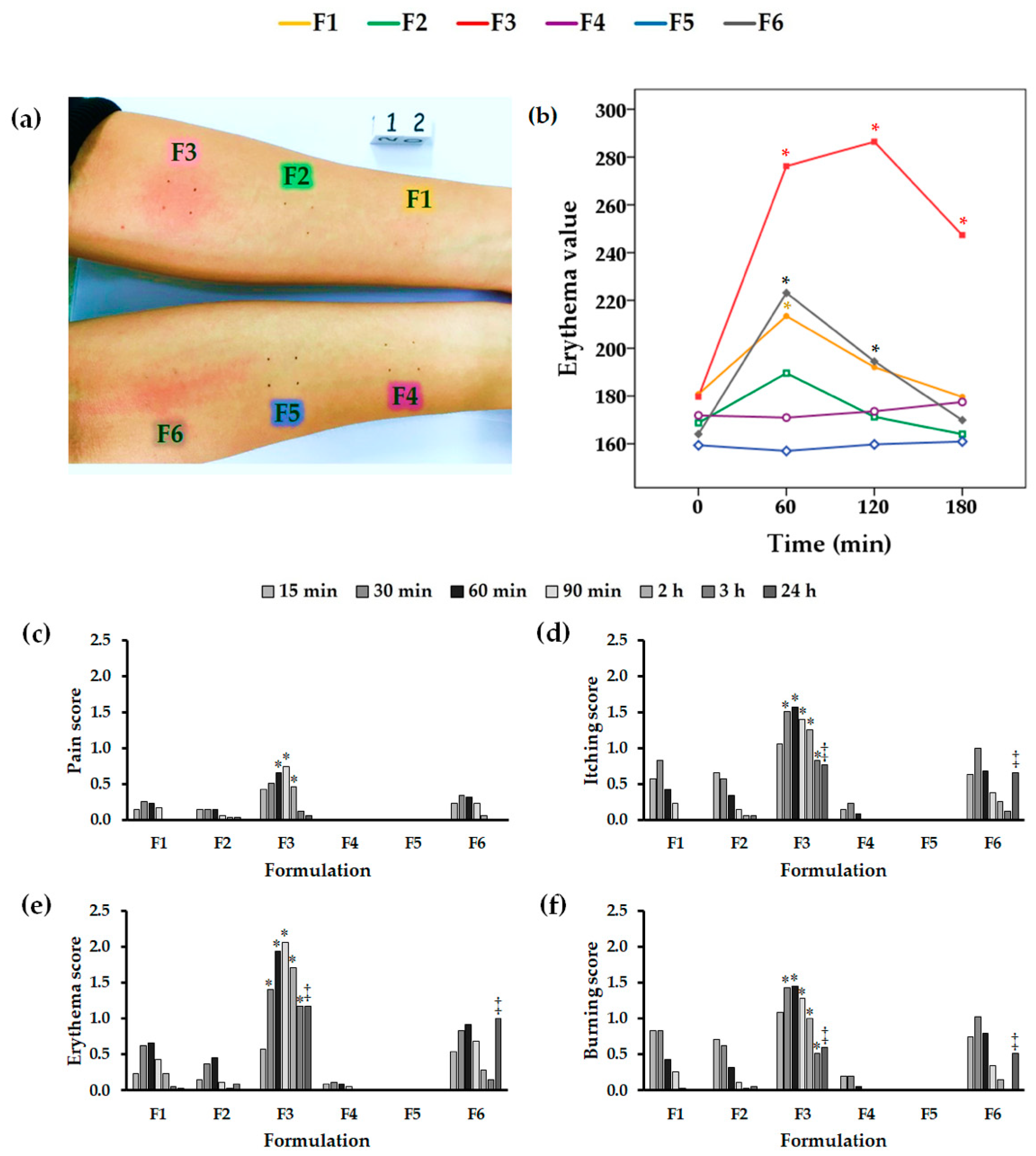
2.8. In Vivo Human Skin Irritation Test in Volunteers
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chili-Extract-Loaded Nanostructured-Lipid-Carrier (NLC) Dispersion
3.3. Preparation of Chili-Extract-Loaded NLCs Incorporated in Gel Formulation
3.4. HPLC Analysis
3.5. Characterization of Chili-Extract-Loaded NLC Dispersion
3.5.1. Particle-Size, Size-Distribution, and Zeta-Potential Analysis
3.5.2. Determination of Entrapment Efficiency (EE)
3.5.3. Transmission Electron Microscopy (TEM) Analysis
3.6. Characterization of Gel Formulation
3.7. Stability Study
3.8. In Vitro Release Study
- Zero order model Q = Q0 + kt;
- First order model Q = Q0 × ekt;
- Higuchi model Q = k × t0.5;
3.9. Study of NLC Distribution through the Skin Layers Using Fluorescence Microscopy
3.10. In Vivo Human Skin Irritation
3.11. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Abdelbary, G.; Haider, M. In vitro characterization and growth inhibition effect of nanostructured lipid carriers for controlled delivery of methotrexate. Pharm. Dev. Technol. 2013, 18, 1159–1168. [Google Scholar] [CrossRef]
- Souto, E.; Wissing, S.; Barbosa, C.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Bhaskar, K.; Mohan, C.K.; Lingam, M.; Mohan, S.J.; Venkateswarlu, V.; Rao, Y.M.; Bhaskar, K.; Anbu, J.; Ravichandran, V. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug Dev. Ind. Pharm. 2009, 35, 98–113. [Google Scholar] [CrossRef]
- Joshi, M.; Patravale, V. Formulation and evaluation of nanostructured lipid carrier (NLC)–based gel of Valdecoxib. Drug Dev. Ind. Pharm. 2006, 32, 911–918. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Jindal, M.; Aggarwal, G.; Jain, S. Development of a novel method for fabrication of solid lipid nanoparticles: Using high shear homogenization and ultrasonication. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 265–274. [Google Scholar]
- Anogianaki, A.; Negrev, N.N.; Shaik, Y.B.; Castellani, M.L.; Frydas, S.; Vecchiet, J.; Tete, S.; Salini, V.; Amicis, D.; Lutiis, M.A.; et al. Capsaicin: An irritant anti-inflammatory compound. J. Biol. Regul. Homeost. Agents 2007, 21, 1. [Google Scholar]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The use of nanoencapsulation to decrease human skin irritation caused by capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Escogido, M.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [Green Version]
- Derry, S.; Moore, R.A. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a capsaicin-loaded nanoemulsion for improving skin penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Chantaburanan, T.; Junyaprasert, V.B. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J. Drug Deliv. Sci. Technol. 2017, 39, 300–307. [Google Scholar] [CrossRef]
- Somagoni, J.; Boakye, C.H.; Godugu, C.; Patel, A.R.; Faria, H.A.; Zucolotto, V.; Singh, M. Nanomiemgel-A novel drug delivery system for topical application-in vitro and in vivo evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.R.; Gao, S.Q.; Niu, X.Q.; Li, L.J.; Ying, X.Y.; Hu, Z.J.; Gao, J.Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2017, 12, 3881–3898. [Google Scholar] [CrossRef] [Green Version]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of capsaicin. Colloids Surf. B 2011, 87, 333–339. [Google Scholar] [CrossRef]
- Popescu, M.; Chiutu, L.; Mircioiu, C.; Dima, S. Capsaicin microemulsions: Preparation, characterization and in vitro release study. Farmacia 2014, 62, 58–68. [Google Scholar]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef]
- Sarwa, K.K.; Mazumder, B.; Rudrapal, M.; Verma, V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015, 22, 638–646. [Google Scholar] [CrossRef]
- Agrawal, U.; Gupta, M.; Vyas, S. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Majumdar, A.; Dubey, N.; Malviya, N. Nanostructure lipid carriers: A promising tool for the drug delivery in the treatment of skin cancer. Asian J. Pharm. Clin. Res. 2019, 12, 15–26. [Google Scholar] [CrossRef]
- Hu, C.; Qian, A.; Wang, Q.; Xu, F.; He, Y.; Xu, J.; Xia, Y.; Xia, Q. Industrialization of lipid nanoparticles: From laboratory-scale to large-scale production line. Eur. J. Pharm. Biopharm. 2016, 109, 206–213. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Dhankhar, P. Homogenization fundamentals. IOSR J. Eng. 2014, 4, 8. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef]
- Makoni, P.A.; Wa, K.K.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef] [Green Version]
- Naik, J.; Lokhande, A.; Mishra, S.; Kulkarni, R. Development of sustained release micro/nanoparticles using different solvent emulsification technique: A review. Int. J. Pharm. Bio Sci. 2012, 3, 573–590. [Google Scholar]
- Kuntsche, J.; Mäder, K. Solid lipid nanoparticles (SLN) for drug delivery. In Handbook of Materials for Nanomedicine; Torchilin, V., Amiji, M.M., Eds.; Pan Stanford Publishing: Singapore, 2010; Volume 1, pp. 383–444. [Google Scholar]
- Gall, V.; Runde, M.; Schuchmann, H.P. Extending applications of high-pressure homogenization by using simultaneous emulsification and mixing (SEM)—An overview. Processes 2016, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Santana, M.H.A.; Souto, E.B. Optimizing SLN and NLC by 22 full factorial design: Effect of homogenization technique. Mater. Sci. Eng. C 2012, 32, 1375–1379. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Li, W. High shear mixers: A review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem. Eng. Process. 2012, 57, 25–41. [Google Scholar] [CrossRef]
- López-García, R.; Ganem-Rondero, A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Occlusive effect and penetration enhancement ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Doktorovova, S.; Zielinska, A.; Silva, A.M. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharm. Dev. Technol. 2019, 24, 1181–1185. [Google Scholar] [CrossRef]
- Maqbool, A.; Mishra, M.K.; Pathak, S.; Kesharwani, A.; Kesharwani, A. Semisolid dosage forms manufacturing: Tools, critical process parameters, strategies, optimization, and recent advances. Ind. Am. J. Pharm. Res. 2017, 7, 882–893. [Google Scholar]
- Shimojo, A.A.; Fernandes, A.R.V.; Ferreira, N.R.; Lopez, E.S.; Santana, M.H.A.; Souto, E.B. Evaluation of the Influence of Process Parameters on the Properties of Resveratrol-Loaded NLC Using 22 Full Factorial Design. Antioxidants 2019, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Üner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006, 61, 375–386. [Google Scholar]
- Singh, S.; Singh, M.; Tripathi, C.B.; Arya, M.; Saraf, S.A. Development and evaluation of ultra-small nanostructured lipid carriers: Novel topical delivery system for athlete’s foot. Drug Deliv. Transl. Res. 2016, 6, 38–47. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Müller, R.H.; Junyaprasert, V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—Effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007, 340, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Gardouh, A.R.; Gad, S.; Ghonaim, H.M.; Ghorab, M.M. Design and characterization of glyceryl monostearate solid lipid nanoparticles prepared by high shear homogenization. J. Pharm. Res. Int. 2013, 3, 326–346. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology, Q2 (R1); ICH Secretariat: Geneva, Switzerland, 2005; pp. 1–15. [Google Scholar]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; pp. 12–19. [Google Scholar]
- Islam, M.T.; Rodriguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.K.; Tripathy, S.; Nair, S.K.; Kesharwani, R. Nanostructured lipid carrier (Nlc) a modern approach for topical delivery: A review. World J. Pharm. Pharm. Sci. 2013, 2, 921–938. [Google Scholar]
- Kelchen, M.N.; Brogden, N.K. In vitro skin retention and drug permeation through intact and microneedle pretreated skin after application of propranolol loaded microemulsions. Pharm. Res. 2018, 35, 228. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Ther. 2015, 9, 4209–4218. [Google Scholar] [CrossRef] [Green Version]
- Trombino, S.; Servidio, C.; Laganà, A.S.; Conforti, F.; Marrelli, M.; Cassano, R. Viscosified Solid Lipidic Nanoparticles Based on Naringenin and Linolenic Acid for the Release of Cyclosporine A on the Skin. Molecules 2020, 25, 3535. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Ghaemi, B.; Amani, A. Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: Permeation, antibacterial and safety assessments. Eur. J. Pharm. Sci. 2018, 120, 142–151. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Douglas, K.; Farber, J.; Flynn, S.; Gu, J.; Qin, V.; Shih, J.; Silberg, J.; Wamakima, A.; Cincotta, D.; Tang, J. Zero-Order Controlled Release Kenetics through Polymer Matrices. Available online: https://pdfs.semanticscholar.org/41f6/0e17d70a31503e22d397095bf4d67758a18d.pdf (accessed on 27 October 2020).
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence microscopy. Cold Spring Harb. Protoc. 2014, 1042–1065. [Google Scholar] [CrossRef] [Green Version]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Korting, M.S. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Stees, M.; Adjei, I.; Labhasetwar, V. A method for quantification of penetration of nanoparticles through skin layers using near-infrared optical imaging. Cosmetics 2015, 2, 225–235. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, F.C.; Depieri, L.V.; Bentley, M. Confocal laser scanning microscopy as a tool for the investigation of skin drug delivery systems and diagnosis of skin disorders. In Confocal Laser Microscopy-Principles and Applications in Medicine, Biology, and the Food Sciences; IntechOpen: London, UK, 2013; pp. 99–140. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirova, D.; Liebsch, M.; Basketter, D.; Spiller, E.; Kejlova, K.; Bendova, H.; Marriott, M.; Kandarova, H. Comparison of human skin irritation and photo-irritation patch test data with cellular in vitro assays and animal in vivo data. In Proceedings of the 6th World Congress on Alternatives & Animal Use in the Lift Sciences, Tokyo, Japan, 21–25 August 2007; pp. 359–365. [Google Scholar]
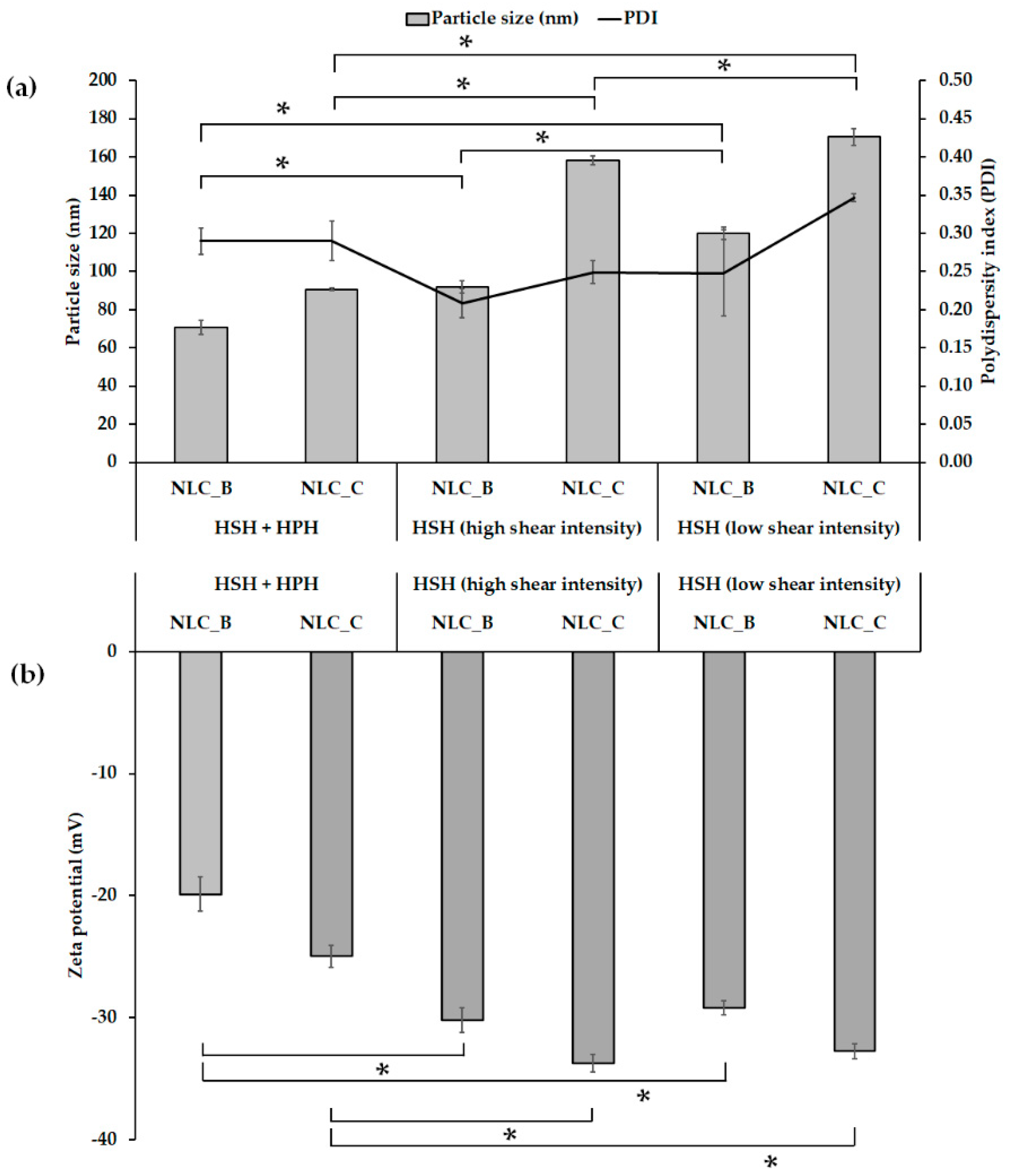
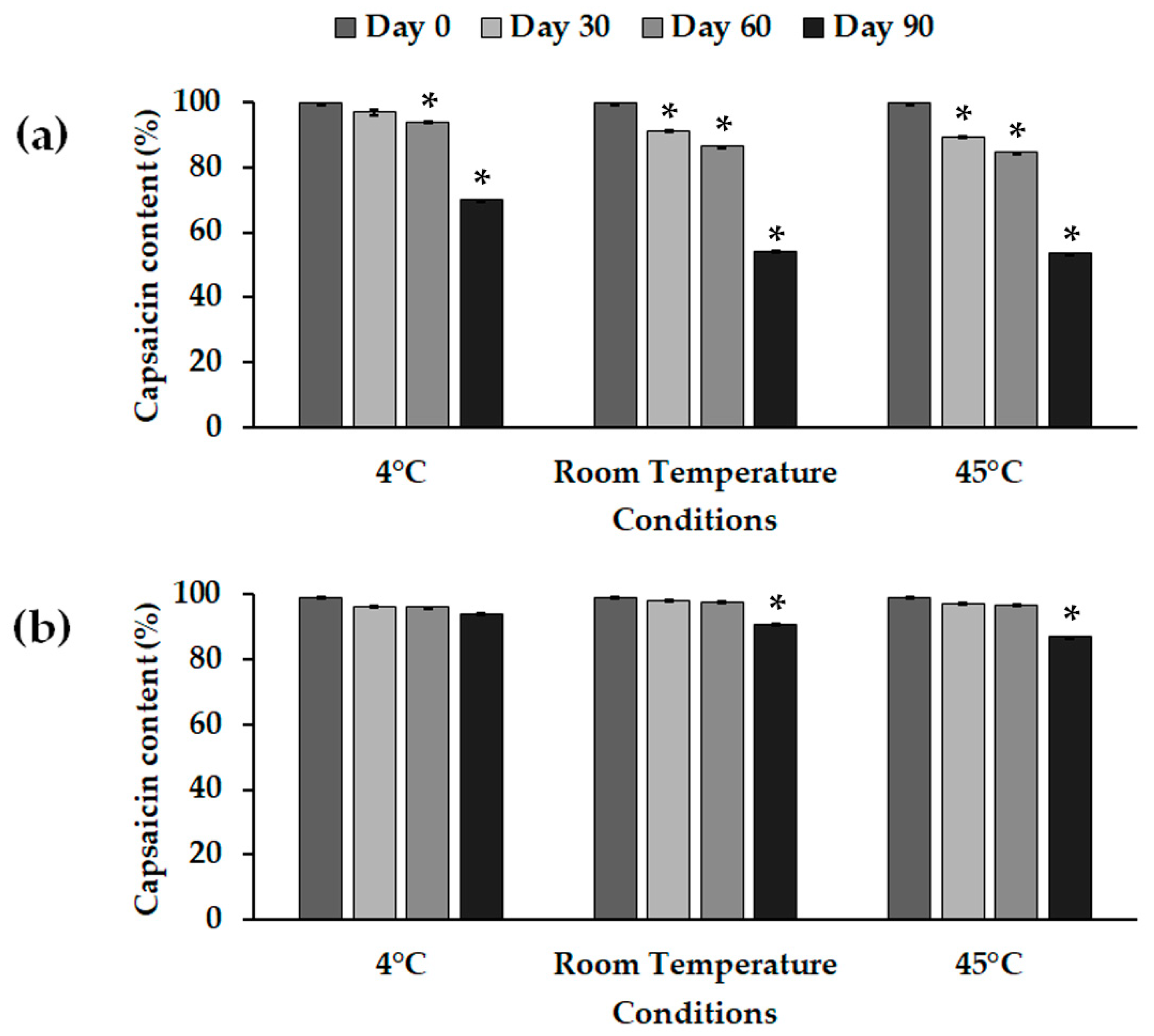
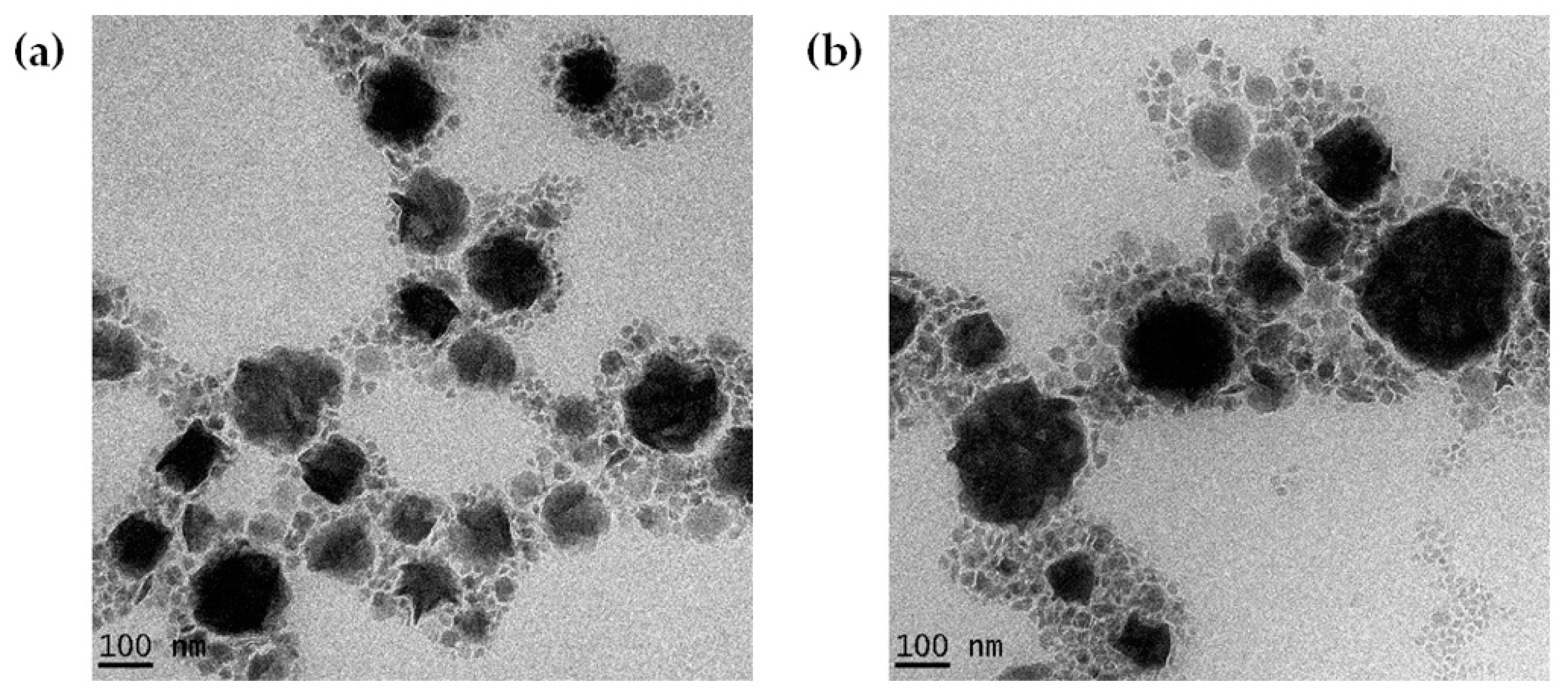
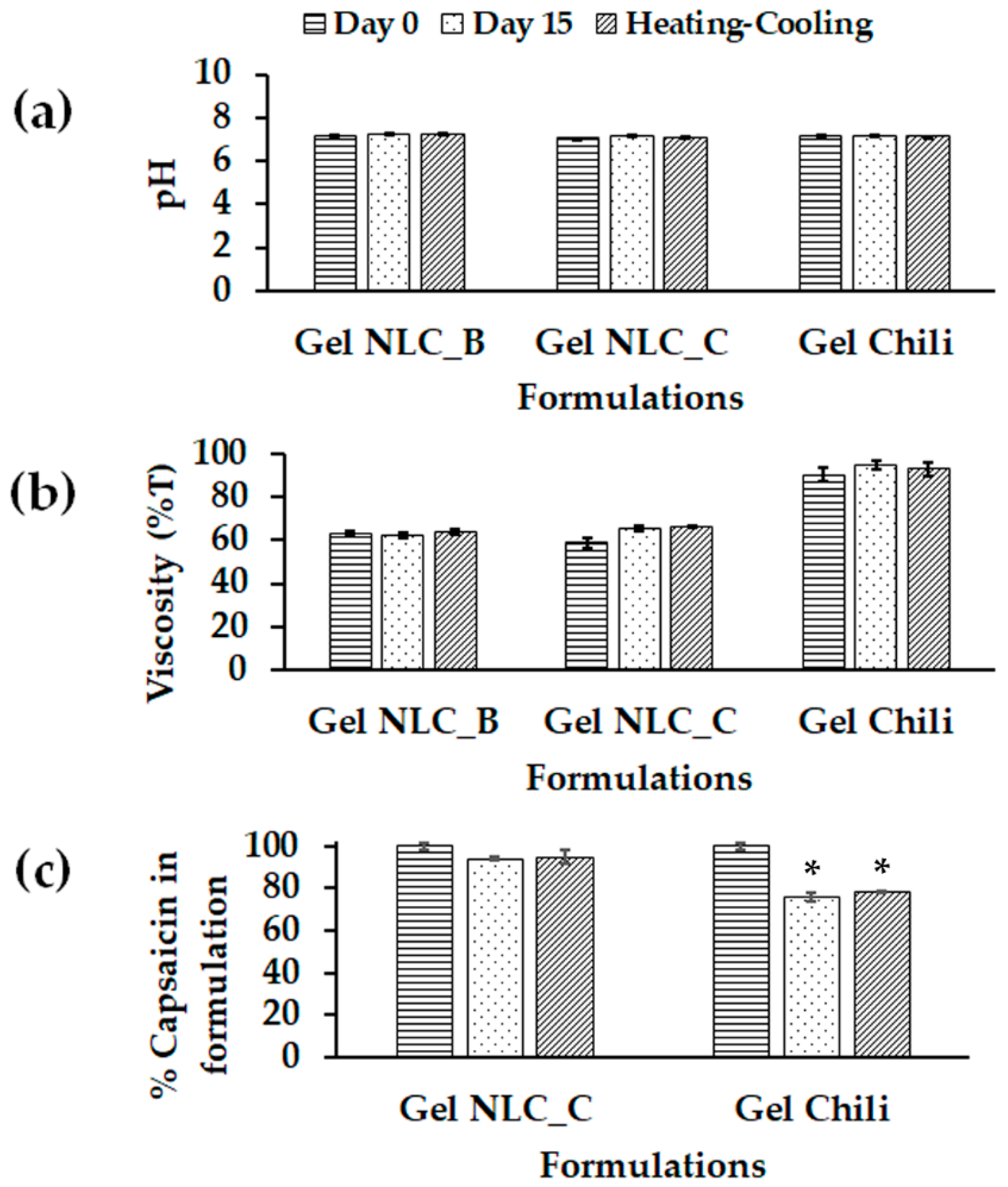
| Characterization | Formulation | Day | HSH + HPH | HSH (High Intensity) | HSH (Low Intensity) |
|---|---|---|---|---|---|
| Size (nm) | NLC_B | 0 | 70.70 ± 3.46 | 91.84 ± 3.15 | 119.89 ± 3.20 |
| 7 | 83.12 ± 6.82 | 110.26 ± 3.26 | 127.75 ± 2.38 | ||
| 14 | 87.53 ± 1.55 | 110.70 ± 3.46 | 126.35 ± 1.64 | ||
| 30 | 95.30 ± 4.25 | 111.75 ± 4.05 | 184.30 ± 2.11 * | ||
| NLC_C | 0 | 90.70 ± 0.66 | 158.30 ± 2.39 | 170.49 ± 4.45 | |
| 7 | 94.81 ± 3.75 | 153.74 ± 3.23 | 174.43 ± 9.55 | ||
| 14 | 98.10 ± 1.24 | 155.45 ± 4.84 | 178.80 ± 5.78 | ||
| 30 | 157.60 ± 2.60 * | 156.30 ± 6.88 | 222.80 ± 2.23 * | ||
| PDI | NLC_B | 0 | 0.29 ± 0.02 | 0.21 ± 0.02 | 0.25 ± 0.06 |
| 7 | 0.31 ± 0.02 | 0.26 ± 0.05 | 0.31 ± 0.13 | ||
| 14 | 0.30 ± 0.05 | 0.25 ± 0.01 | 0.29 ± 0.05 | ||
| 30 | 0.38 ± 0.03 | 0.26 ± 0.02 | 0.58 ± 0.06 * | ||
| NLC_C | 0 | 0.29 ± 0.03 | 0.25 ± 0.01 | 0.35 ± 0.01 | |
| 7 | 0.30 ± 0.10 | 0.26 ± 0.02 | 0.39 ± 0.01 | ||
| 14 | 0.31 ± 0.04 | 0.24 ± 0.02 | 0.36 ± 0.03 | ||
| 30 | 0.61 ± 0.03 * | 0.28 ± 0.01 | 0.54 ± 0.03 * | ||
| ZP (mV) | NLC_B | 0 | −19.88 ± 1.41 | −30.22 ± 1.02 | −29.20 ± 0.61 |
| 7 | −16.08 ± 1.83 | −31.30 ± 4.36 | −27.20 ± 0.61 | ||
| 14 | −19.00 ± 0.66 | −25.38 ± 0.83 | −26.20 ± 0.72 | ||
| 30 | −18.78 ± 0.56 | −29.39 ± 2.75 | −14.35 ± 1.45 * | ||
| NLC_C | 0 | −24.97 ± 0.91 | −33.74 ± 0.71 | −32.76 ± 0.60 | |
| 7 | −27.64 ± 1.11 | −38.10 ± 0.43 | −30.76 ± 0.70 | ||
| 14 | −26.29 ± 0.48 | −35.66 ± 0.98 | −24.55 ± 0.53 | ||
| 30 | −17.29 ± 0.44 * | −39.27 ± 0.28 | −14.33 ± 0.74 * |
| Day | Characterization | 4 °C | Room Temperature | 45 °C |
|---|---|---|---|---|
| 0 | Size (nm) | 180.20 ± 1.33 | ||
| PDI | 0.22 ± 0.02 | |||
| ZP (mV) | −35.84 ± 0.78 | |||
| 30 | Size (nm) | 187.22 ± 9.65 | 181.20 ± 5.27 | 155.32 ± 2.27 * |
| PDI | 0.31 ± 0.03 | 0.25 ± 0.03 | 0.22 ± 0.02 | |
| ZP (mV) | −34.72 ± 1.53 | −34.46 ± 0.78 | −34.16 ± 1.11 | |
| 60 | Size (nm) | 198.30 ± 24.43 | 179.60 ± 3.55 | 160.84 ± 5.62 * |
| PDI | 0.39 ± 0.08 | 0.29 ± 0.04 | 0.26 ± 0.04 | |
| ZP (mV) | −40.16 ± 0.44 | −41.34 ± 0.39 | −45.74 ± 1.72 | |
| 90 | Size (nm) | 196.58 ± 17.46 | 180.72 ± 5.36 | 205.98 ± 3.10 * |
| PDI | 0.49 ± 0.05 | 0.30 ± 0.04 | 0.30 ± 0.02 | |
| ZP (mV) | −41.50 ± 1.93 | −45.56 ± 1.52 | −43.34 ± 1.24 | |
| Formulation | Zero-Order | First-Order | Higuchi |
|---|---|---|---|
| Chili extract | 0.5883 | 0.3872 | 0.8007 |
| NLC_C | 0.9988 | 0.5554 | 0.9334 |
| Gel Chili | 0.9940 | 0.6829 | 0.9714 |
| Gel NLC_C | 0.9957 | 0.6656 | 0.9694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment. Molecules 2020, 25, 5575. https://doi.org/10.3390/molecules25235575
Anantaworasakul P, Anuchapreeda S, Yotsawimonwat S, Naksuriya O, Lekawanvijit S, Tovanabutra N, Anantaworasakul P, Wattanasri W, Buranapreecha N, Ampasavate C. Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment. Molecules. 2020; 25(23):5575. https://doi.org/10.3390/molecules25235575
Chicago/Turabian StyleAnantaworasakul, Phunsuk, Songyot Anuchapreeda, Songwut Yotsawimonwat, Ornchuma Naksuriya, Suree Lekawanvijit, Napatra Tovanabutra, Pimporn Anantaworasakul, Wajee Wattanasri, Narinthorn Buranapreecha, and Chadarat Ampasavate. 2020. "Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment" Molecules 25, no. 23: 5575. https://doi.org/10.3390/molecules25235575
APA StyleAnantaworasakul, P., Anuchapreeda, S., Yotsawimonwat, S., Naksuriya, O., Lekawanvijit, S., Tovanabutra, N., Anantaworasakul, P., Wattanasri, W., Buranapreecha, N., & Ampasavate, C. (2020). Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment. Molecules, 25(23), 5575. https://doi.org/10.3390/molecules25235575








