In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements
Abstract
1. Introduction
2. Results
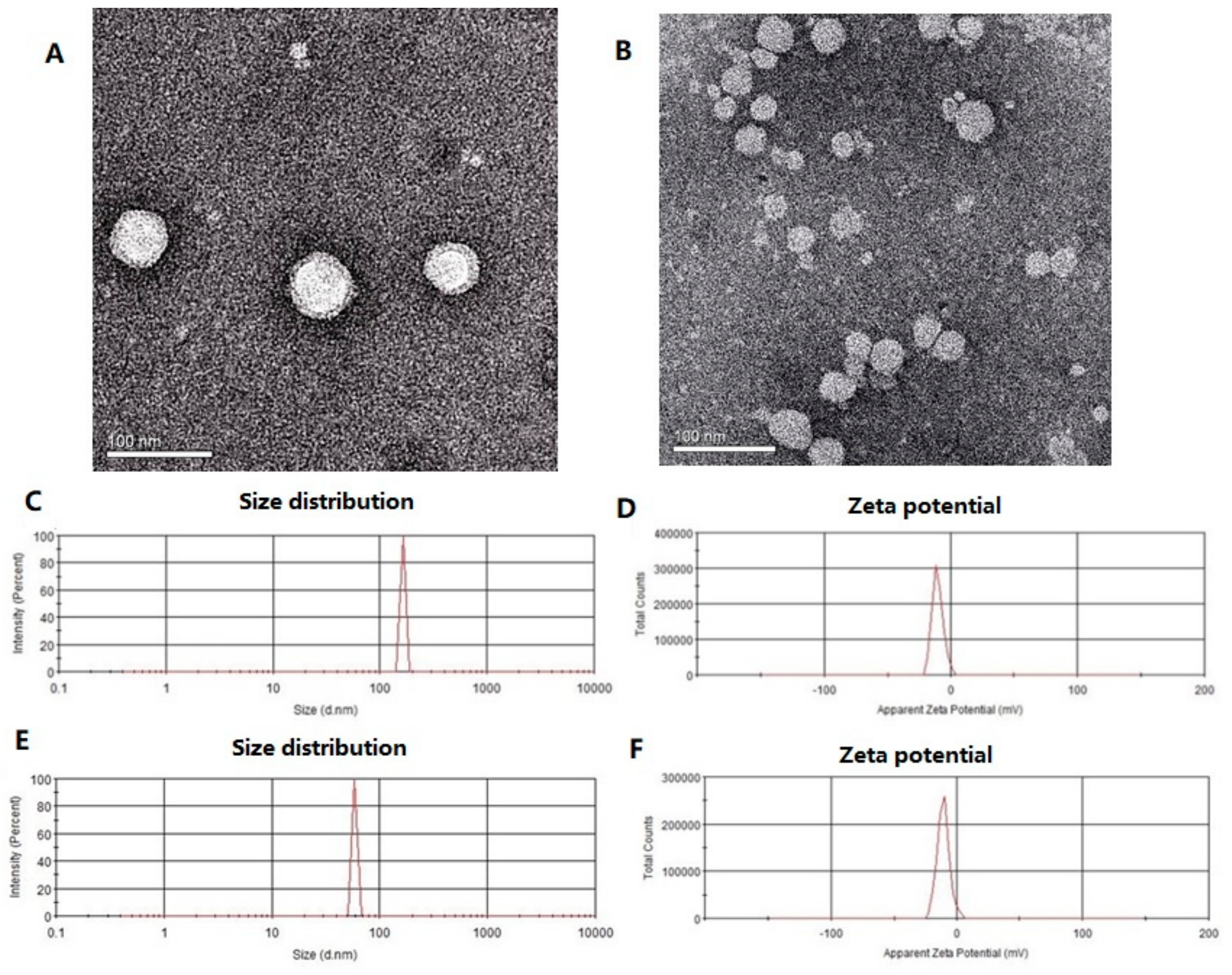
2.1. Physicochemical Characteristics of RSV-SLNs
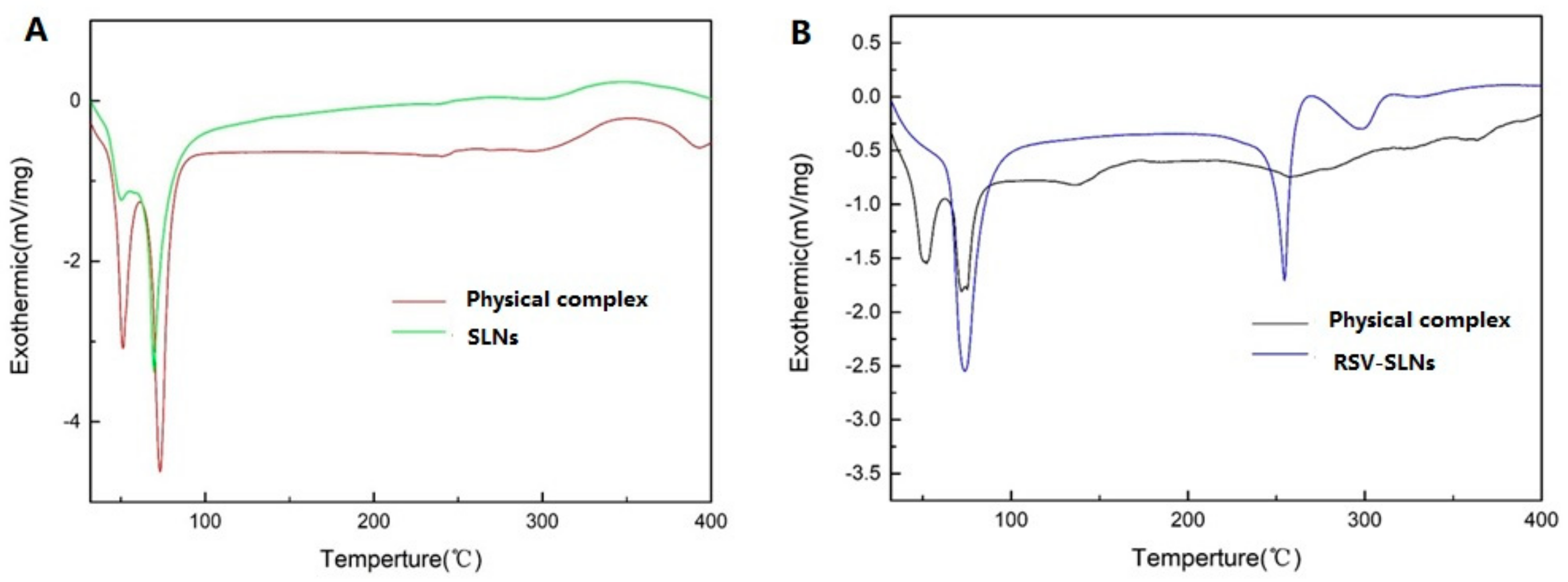
2.2. Differential Scanning Calorimetry (DSC) Measurements
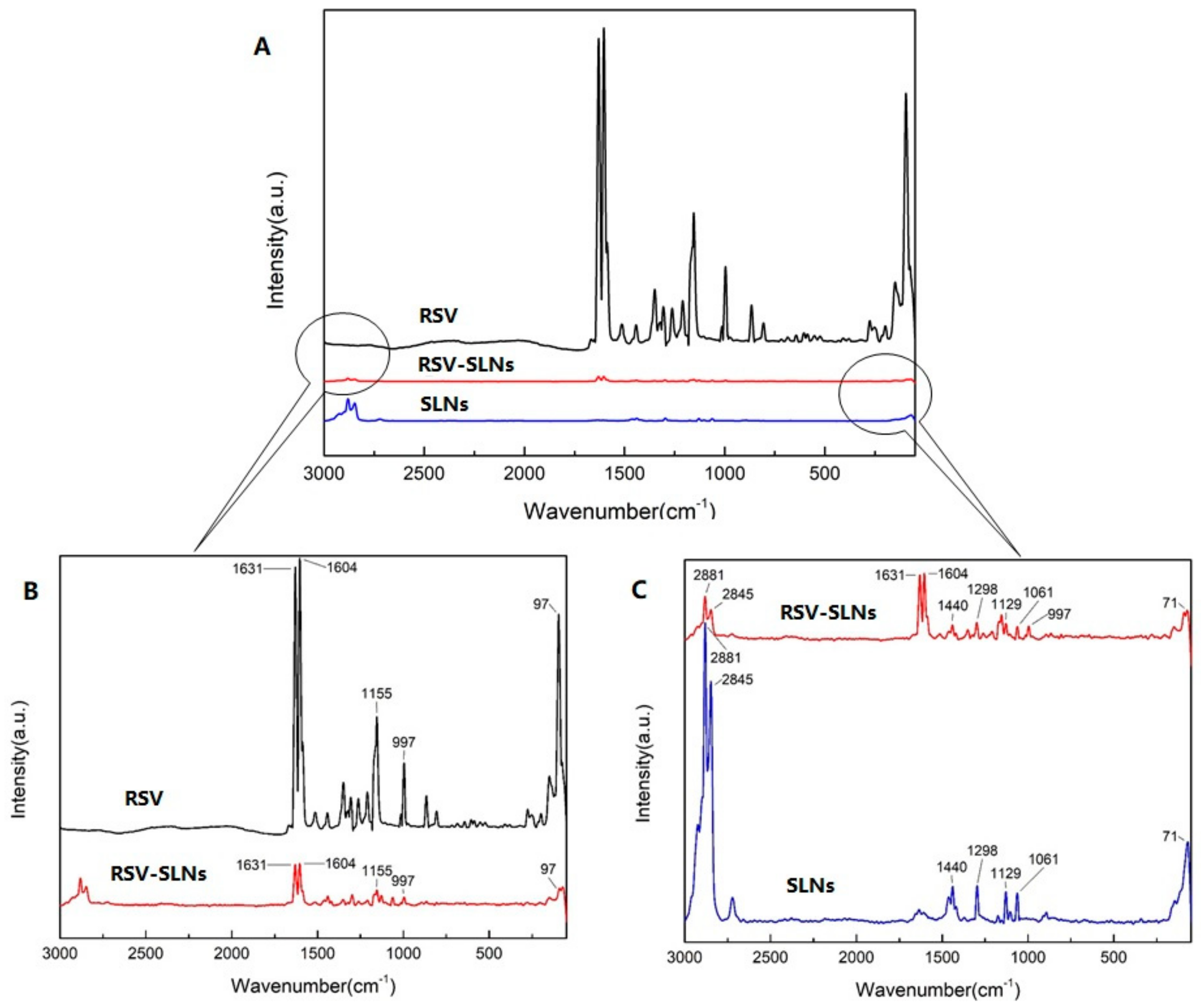
2.3. Raman Spectroscopy
2.4. Effect of RSV-SLNs on Exercise Fatigue Related Tests
2.5. The Biochemical Parameters of Blood Related to Fatigue
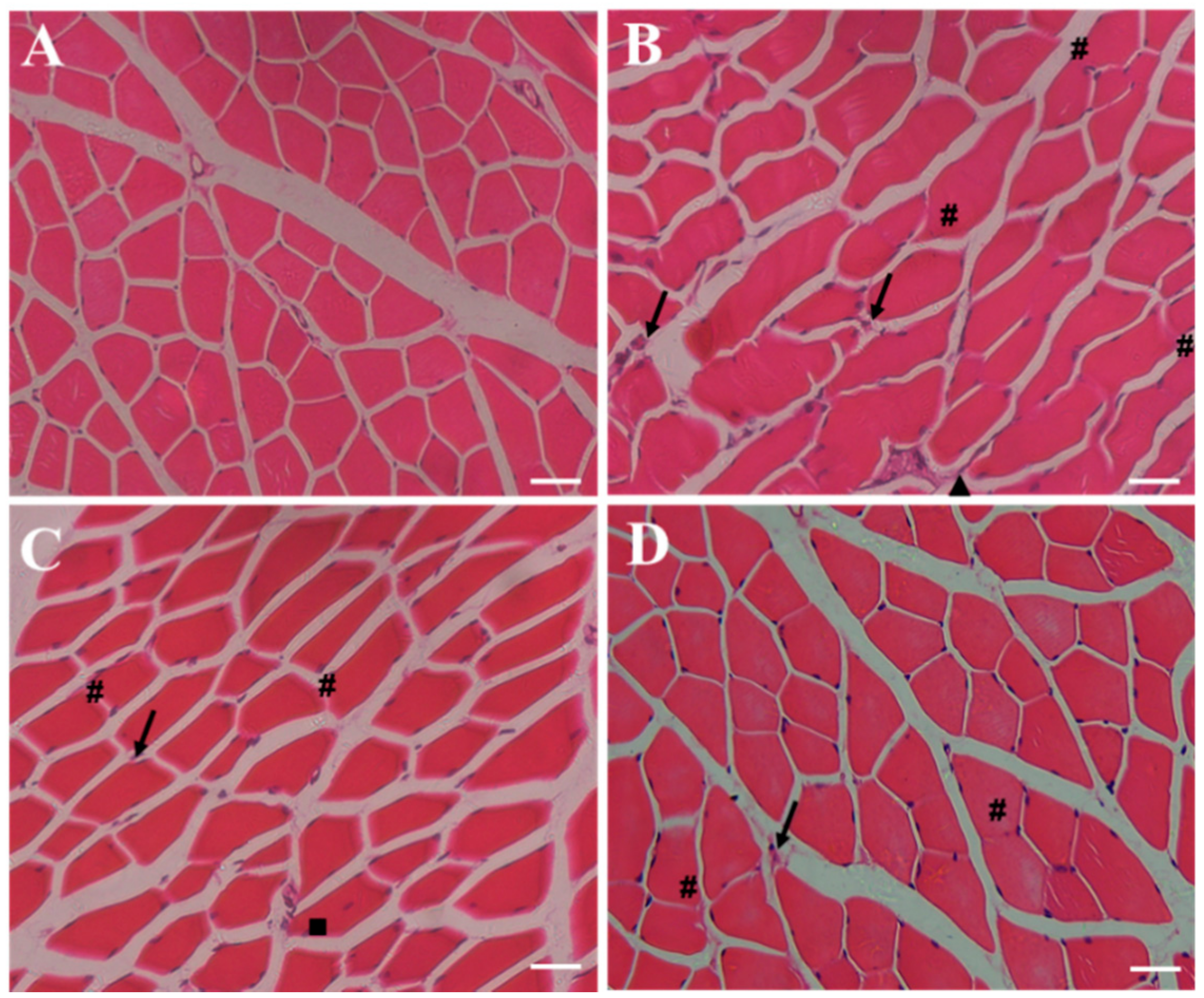
2.6. Effect of RSV-SLNs Supplementation on the Muscular Morphology of Mice that Underwent Excessive Endurance Exercise
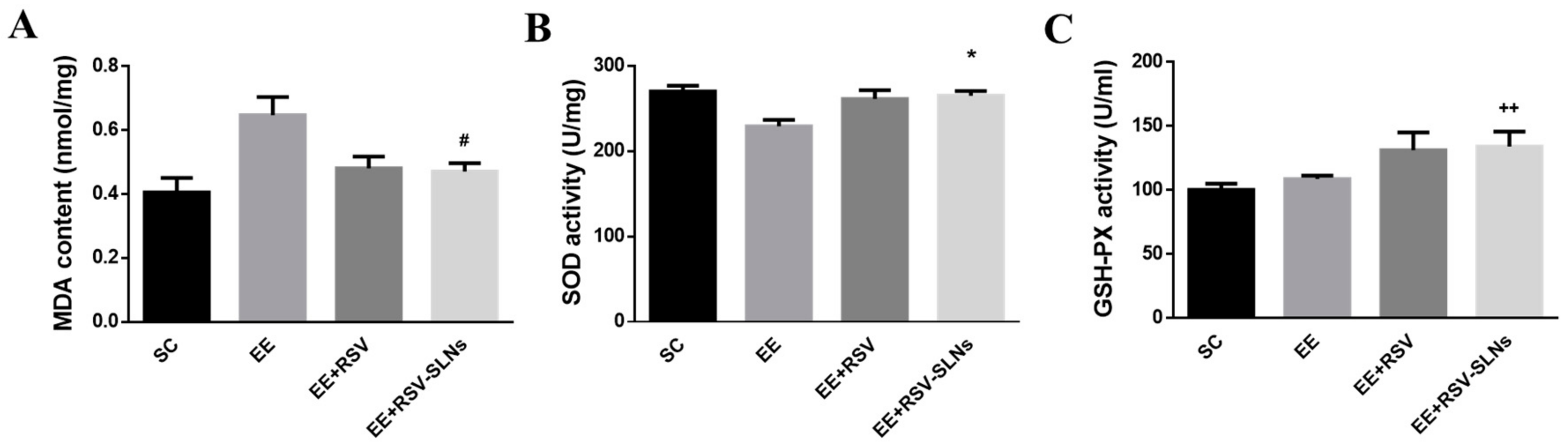
2.7. Antioxidative Effect of RSV-SLNs on Liver
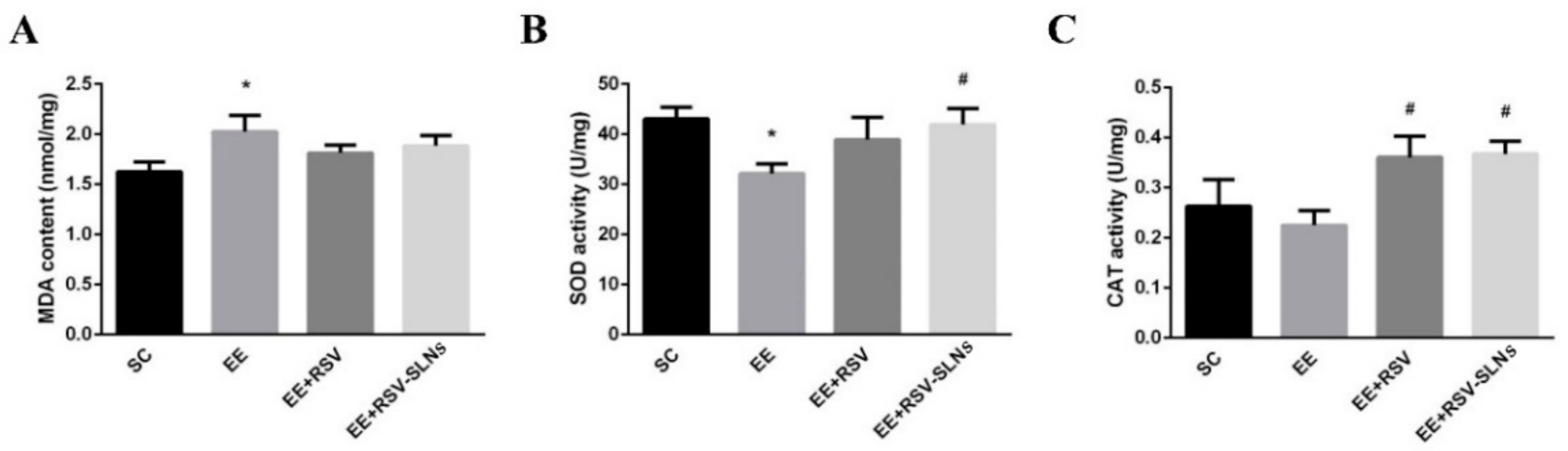
2.8. Effect of RSV-SLNs Supplementation on Lipid Peroxidation and Antioxidant Status in Mice Subjected to Excessive Endurance Exercise
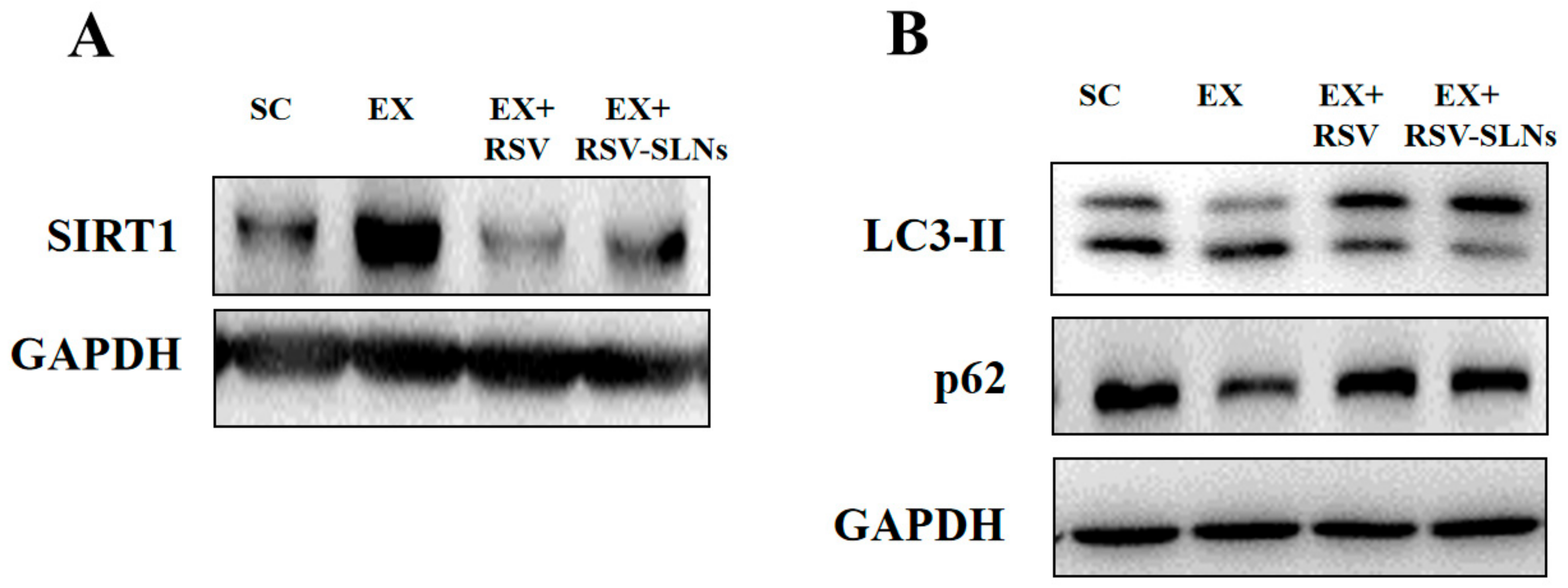
2.9. Effect of Res-SLNs on the Expression of SIRT1, LC3-II and p62 Signaling
3. Discussion
3.1. Characteristics of RSV-SLNs
3.2. The Anti-Fatigue Related Effect of RSV-SLNs
3.3. The Molecular Mechanisms of RSV-SLNs
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Resveratrol-Solid Lipid Nanoparticles (RSV-SLNs)
4.4. Morphology Detected by Transmission Electron Micrographs (TEM), Photon Correlation Spectroscopy (PCS) and Zeta Potential Measurement
4.5. Differential Scanning Calorimetry (DSC) Analysis
4.6. Raman Spectra Analysis
4.7. In Vivo Anti-Fatigue Effect of RSV-SLNs
4.7.1. Effect on Running Time to Fatigue
4.7.2. The Biochemical Parameters of Blood and Tissue Sampling
4.7.3. Blood and Tissue Sampling
4.8. Effect of Res-SLNs on the Expression of SIRT1, LC3-II and p62 Signaling
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- You, L.J.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.L.; Lu, R.; Zhou, Y.J.; Ma, R.; Lv, J.Q.; Li, X.L.; Chen, L.J.; Yao, Z. The decapeptide CMS001 enhances swimming endurance in mice. Peptides 2008, 29, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-Rich Extract from Antrodia camphorata Improves Physical Fatigue and Exercise Performance in Mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–8. [Google Scholar]
- Tanaka, M.; Baba, Y.; Kataoka, Y.; Kinbara, N.; Sagesaka, Y.M.; Kakuda, T.; Watanabe, Y. Effects of (-)-epigallocatechin gallate in liver of an animal model of combined (physical and mental) fatigue. Nutrition 2008, 24, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Tur, J.A. Antioxidant Supplementation and Adaptive Response to Training: A Systematic Review. Curr. Pharm. Des. 2019, 25, 1889–1912. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Souza, D.G.; Souza, D.O.; Quincozes-Santos, A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol. Vitro 2014, 28, 479–484. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidantagent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Zhao, H.; Niu, Q.; Li, X.; Liu, T.; Xu, Y.; Han, H.; Wang, W.; Fan, N.; Tian, Q.; Zhang, H.; et al. Long-term resveratrol consumption protects ovariectomized rats chronically treated with D-galactose from developing memory decline without effects on the uterus. Brain Res. 2012, 1467, 67–80. [Google Scholar] [CrossRef]
- MuÈller, R.H.; MaÈder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2000, 47, 165–196. [Google Scholar] [CrossRef]
- Chan, O.H.; Stewart, B.H. Physicochemical and drug-delivery considerations for oral drug bioavailability. Drug Discov. Today 1996, 1, 461–473. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; ArabSadeghabadi, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; RezaeiFarimani, A. Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes. Nanoscale Res. Lett. 2019, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol Loaded Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhou, Y.H.; Su, Y.J.; Li, S.; Dong, J.M.; He, Q.; Cao, Y.; Lu, T.F.; Qin, L.L. Resveratrol-loaded solid lipid nanoparticle supplementation ameliorates physical fatigue by improving mitochondrial quality control. Crystals 2019, 9, 559. [Google Scholar] [CrossRef]
- Pratiwi, D.; Fawcett, J.P.; Gordon, K.C.; Rades, T. Quantitative analysis of polymorphic mixtures of ranitidine hydrochloride by Raman spectroscopy and principal components analysis. Eur. J. Pharm. Biopharm. 2002, 54, 337–341. [Google Scholar] [CrossRef]
- Andreev, G.; Schrader, B.; Schulz, H.; Fuchs, R.; Popov, S.; Handjieva, N. Non-destructive NIR-FT-Raman analyses in practice. Part, I. Analyses of plants and historic textiles. Fresen. J. Anal. Chem. 2001, 371, 1009–1017. [Google Scholar]
- Sacheck, J.M.; Blumberg, J.B. Role of vitamin E and oxidative stress in exercise. Nutrition 2001, 17, 809–814. [Google Scholar] [CrossRef]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid. Int. J. Pharm. 2014, 474, 1–2. [Google Scholar] [CrossRef]
- Prakash, R.; Young, T.K. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B 2016, 139, 52–61. [Google Scholar]
- Anne, S.; Keith, C.; Gordon, T.R. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int. J. Pharm. 2006, 314, 56–62. [Google Scholar]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β-and PP2A-independent β-catenin phosphorylation/degradation. Biofactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, O.; Tozan, A.; Omurtag, G.Z.; Cetinel, S.; Contuk, G.; Gedik, N.; Sener, G. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol. Environ. Saf. 2008, 71, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.K.; Hsieh, C.C.; Hsu, J.J.; Yang, Y.K.; Chou, H.N. Preventive effects of Spirulina platensis on skeletal muscle damage under exercise-induced oxidative stress. Eur. J. Appl. Physiol. 2006, 98, 220. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.; Zafrilla, P.; Almar, M.; Cuevas, M.J.; Lopez, F.J.; Abellan, P.; Gonzalez-Gallego, J. The effects of an antioxidant-supplemented beverage on exercise-induced oxidative stress: Results from a placebo-controlled double-blind study in cyclists. Eur. J. Appl. Physiol. 2005, 95, 543–549. [Google Scholar] [CrossRef]
- Powers, S.K.; Lennon, S.L. Analysis of cellular responses to free radicals: Focus on exercise and skeletal muscle. Proc. Nutr. Soc. 1999, 58, 1025–1033. [Google Scholar] [CrossRef]
- Qin, L.; Wang, W.; You, S.; Dong, J.; Zhou, Y.; Wang, J. In vitro antioxidant activity and in vivo antifatigue effect of layered double hydroxide nanoparticles as delivery vehicles for folic acid. Int. J. Nanomed. 2014, 9, 5701–5710. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, R.; Wang, S.; Li, G.; Sheng, Y.; Rui, H.; Zhang, J.; Xu, J.; Jiang, D. New cofactors and inhibitors for a DNA-cleaving DNAzyme: Superoxide anion and hydrogen peroxide mediated an oxidative cleavage process. Sci. Rep. 2017, 7, 378. [Google Scholar] [CrossRef]
- Liang, D.; Zhuo, Y.; Guo, Z.; He, L.; Wang, X.; He, Y.; Li, L.; Dai, H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie 2020, 170, 10–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Zhu, W.; Liu, Z.; Liu, H.; Zhou, Y.; Cao, Y.; Liu, C.; Xie, Y. Resveratrol Enhances Autophagic Flux and Promotes Ox-LDL Degradation in HUVECs via Upregulation of SIRT1. Oxid. Med. Cell. Longev. 2016, 2016, 7589813. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Parameter | SC | EE | EE + RSV | EE + RSV-SLNs |
|---|---|---|---|---|
| AST (U/L) | 123.70 ± 6.60 | 178.50 ± 8.30 * | 155.90 ± 9.30 | 133.70 ± 6.60 # |
| ALT (U/L) | 42.90 ± 7.80 | 57.20 ± 2.70 * | 38.90 ± 4.20 | 34.20 ± 1.80 ## |
| ALP (U/L) | 152.50 ± 4.27 | 140.90 ± 5.31 | 121.97 ± 16.20 | 90.75 ± 8.05 * |
| LDH (U/L) | 409.90 ± 78.00 | 445.20 ± 150.40 | 372.60 ± 95.40 | 489.40 ± 43.80 |
| ALB (g/L) | 23.23 ± 0.93 | 24.60 ± 0.16 | 24.53 ± 1.25 | 24.90 ± 0.30 |
| T-BIL (µmol/L) | 3.47 ± 0.58 | 3.23 ± 0.54 | 4.23 ± 1.10 | 4.45 ± 0.65 |
| TP (g/L) | 51.23± 2.16 | 56.73 ± 2.89 | 60.83 ± 6.45 | 66.70 ± 8.40 |
| BUN (mmol/L) | 12.33 ± 0.72 | 13.60 ± 0.78 | 10.80 ± 0.56 # | 9.41± 0.72 ## |
| CRE (µmol/L) | 7.19 ± 1.13 | 6.58 ± 0.54 | 6.13 ± 0.63 | 4.80 ± 0.89 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Lu, T.; Qin, Y.; He, Y.; Cui, N.; Du, A.; Sun, J. In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements. Molecules 2020, 25, 5302. https://doi.org/10.3390/molecules25225302
Qin L, Lu T, Qin Y, He Y, Cui N, Du A, Sun J. In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements. Molecules. 2020; 25(22):5302. https://doi.org/10.3390/molecules25225302
Chicago/Turabian StyleQin, Lili, Tianfeng Lu, Yao Qin, Yiwei He, Ningxin Cui, Ai Du, and Jingyu Sun. 2020. "In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements" Molecules 25, no. 22: 5302. https://doi.org/10.3390/molecules25225302
APA StyleQin, L., Lu, T., Qin, Y., He, Y., Cui, N., Du, A., & Sun, J. (2020). In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements. Molecules, 25(22), 5302. https://doi.org/10.3390/molecules25225302






