Copper Adsorption on Lignin for the Removal of Hydrogen Sulfide
Abstract
1. Introduction
2. Materials and Methods
2.1. Copper Adsorption
2.2. H2S Removal Experiments
2.3. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
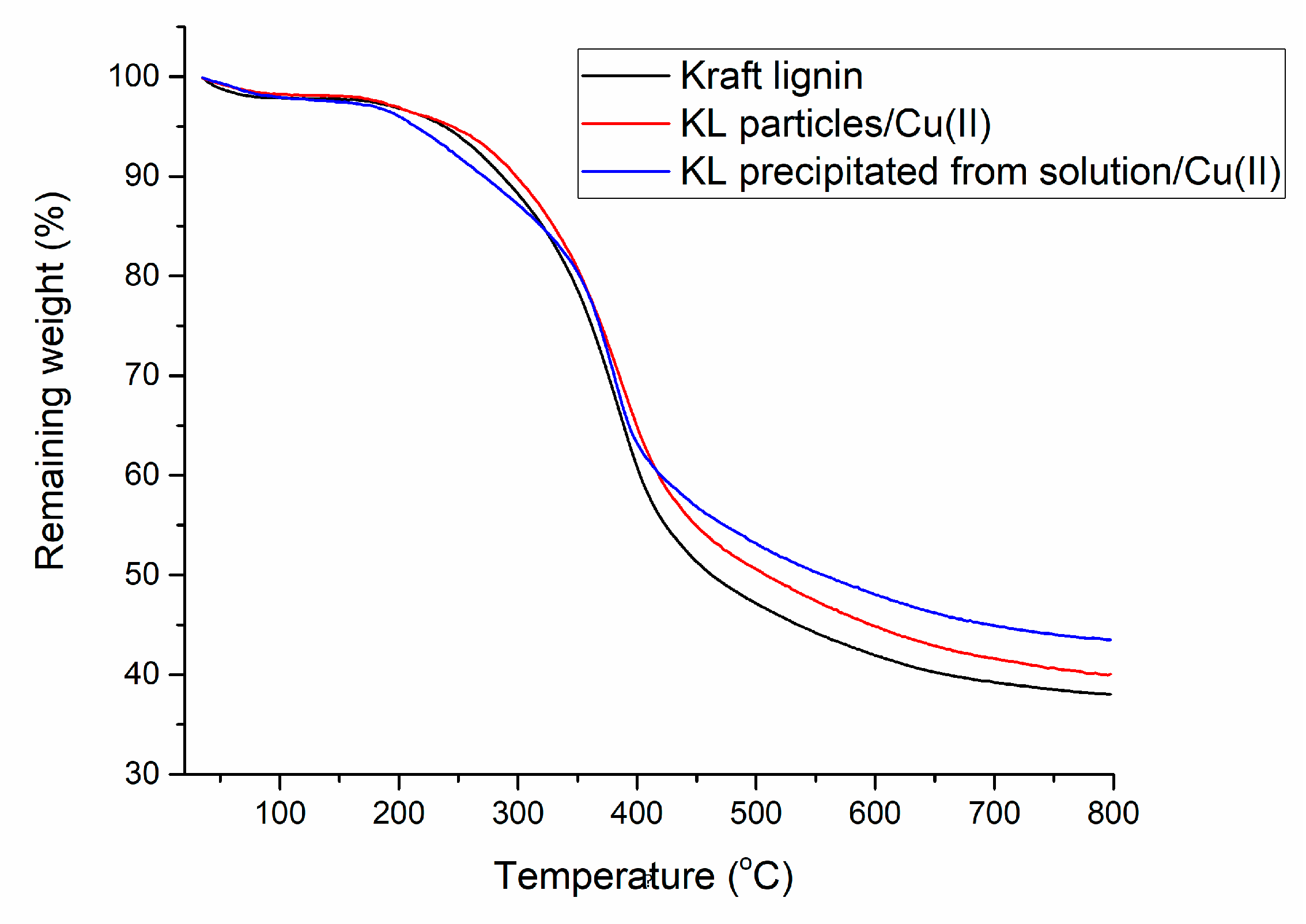
3.1. Copper Adsorption
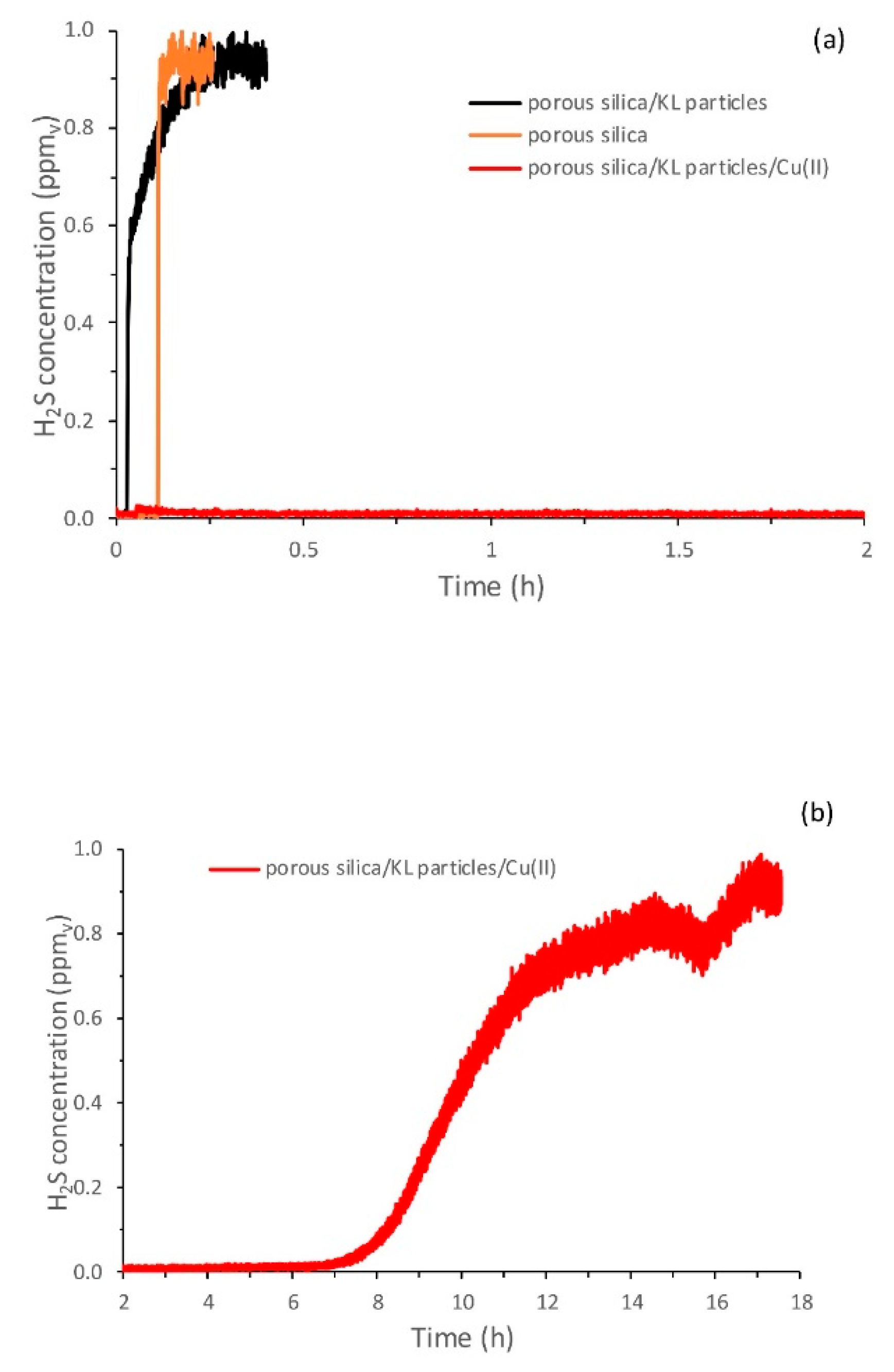
3.2. H2S Removal Experiments
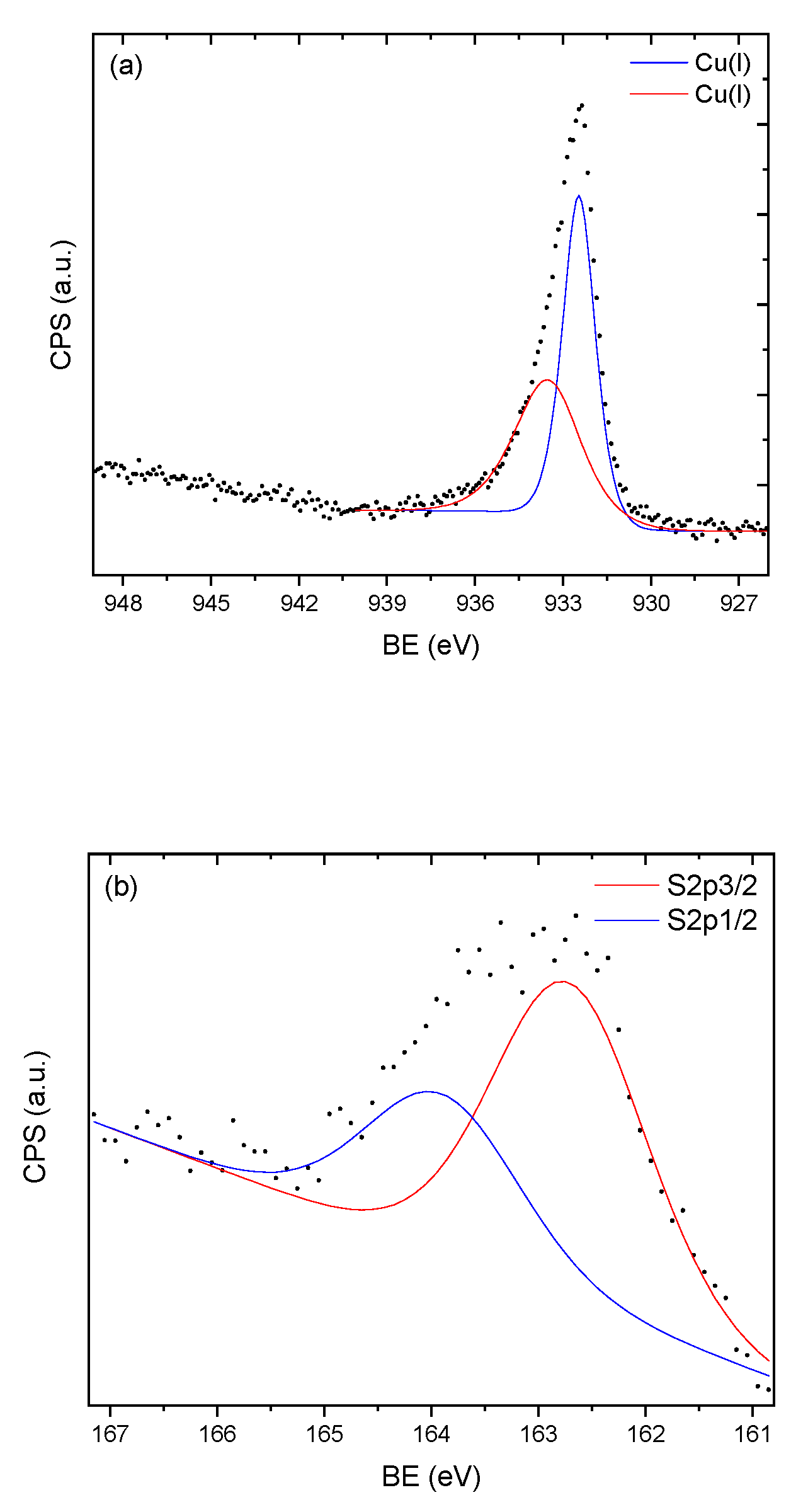
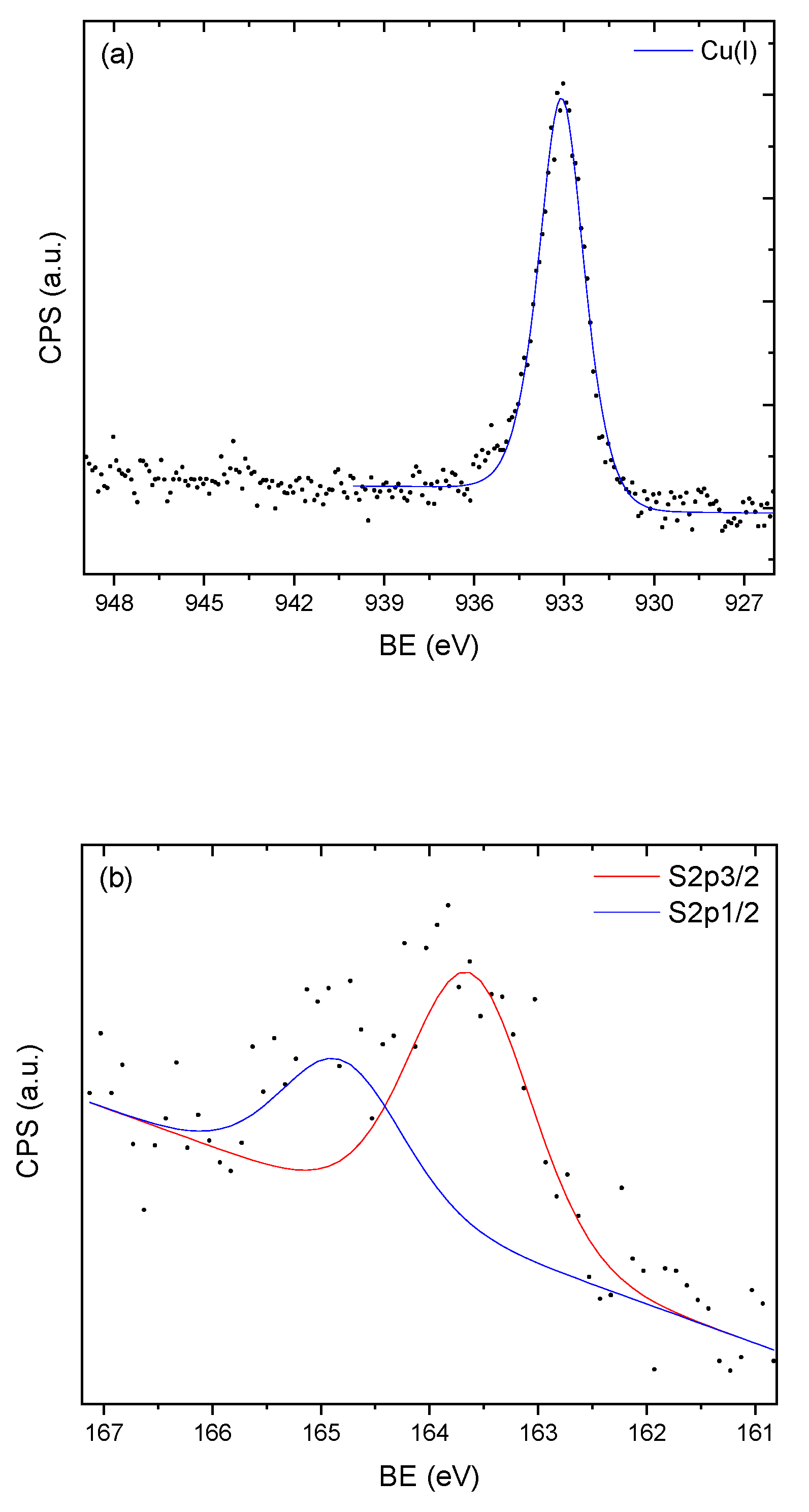
3.3. X-ray Photoelectron Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, M.B.; Strubing, D.; de Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Koch, K.; Kofoed, M.V.W. Stick or leave-Pushing methanogens to biofilm formation for ex situ biomethanation. Bioresour. Technol. 2019, 291, 121784. [Google Scholar] [CrossRef]
- Wall, D.M.; Dumont, M.; Murphy, J.D. Green Gas: Facilitating a Future Green Gas Grid through the Production of Renewable Gas; Murphy, J.D., Ed.; IEA Bioenergy: Paris, France, 2018; ISBN 978-1-910154-37-3. [Google Scholar]
- Bailon, L.; Hinge, J. Biogas Upgrading. Evaluation of Methods for H2S Removal; Danish Technological Institute, 2014. Available online: www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evaluation%20of%20methods%20for%20H2S%20removal.pdf (accessed on 1 September 2020).
- European Chemicals Agency: Homepage. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.029.070. (accessed on 8 June 2020).
- Huertas, J.I.; Giraldo, N.; Izquierdo, S. Removal of H2S and CO2 from Biogas by Amine Absorption. In Mass Transfer in Chemical Engineering Processes; Markos, J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 133–151. ISBN 978-953-307-619-5. [Google Scholar]
- Gosselink, R.J.A.; de Jong, E.; Guran, B.; Abächerli, A. Co-ordination network for lignin—standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- De Jong, E.; Gosselink, R.J.A. Lignocellulose-Based Chemical Products; Elsevier Inc. Academic Press: Cambridge, MA, USA, 2014; pp. 277–313. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon N. Y. 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Khezami, L.; Chetouani, A.; Taouk, B.; Capart, R. Production and characterisation of activated carbon from wood components in powder: Cellulose, lignin, xylan. Powder Technol. 2005, 157, 48–56. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin-from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Biorefining 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E. On the Quantification of Lignin Hydroxyl Groups With 31 P and 13 C NMR Spectroscopy. J. Wood Chem. Technol. 2015, 35, 220–237. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; van Dam, J.E.G.; de Jong, E.; Scott, E.L.; Sanders, J.P.M.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. Holzforschung 2010, 64, 193–200. [Google Scholar] [CrossRef]
- Sumerskii, I.; Korntner, P.; Zinovyev, G.; Rosenau, T.; Potthast, A. Fast track for quantitative isolation of lignosulfonates from spent sulfite liquors. RSC Adv. 2015, 5, 92732–92742. [Google Scholar] [CrossRef]
- Yan, G.; Weng, H.; Yang, J.; Bao, W.; Gao, Y.; Yin, Y. Hydrogen sulfide removal by copper sulfate circulation method. Korean J. Chem. Eng. 2016, 33, 2359–2365. [Google Scholar] [CrossRef]
- Nguyen-Thanh, D.; Bandosz, T.J. Activated carbons with metal containing bentonite binders as adsorbents of hydrogen sulfide. Carbon N. Y. 2005, 43, 359–367. [Google Scholar] [CrossRef]
- Montebello, A.M.; Fernández, M.; Almenglo, F.; Ramírez, M.; Cantero, D.; Baeza, M.; Gabriel, D. Simultaneous methylmercaptan and hydrogen sulfide removal in the desulfurization of biogas in aerobic and anoxic biotrickling filters. Chem. Eng. J. 2012, 200, 237–246. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Gantenbein, A.; Schneebeli, J.; Frei, A.; Knorpp, A.J.; Schildhauer, T.J.; Biollaz, S.M.A. Deep removal of sulfur and trace organic compounds from biogas to protect a catalytic methanation reactor. Chem. Eng. J. 2019, 360, 577–590. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Removal of sulfur contaminants from biogas to enable direct catalytic methanation. Biomass Conv. Bioref. 2019. [Google Scholar] [CrossRef]
- Svensson, S. Minimizing the Sulphur Content in Kraft Lignin. Master’s Thesis, Mälardalen University, Västerås, Sweden, 2008. [Google Scholar]
- Feilberg, A.; Liu, D.; Adamsen, A.P.S.; Hansen, M.J.; Jonassen, K.E.N. Odorant Emissions from Intensive Pig Production Measured by Online Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2010, 44, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Merdy, P.; Guillon, E.; Aplincourt, M.; Dumonceau, J.; Vezin, H. Copper sorption on a straw lignin: Experiments and EPR characterization. J. Colloid Interface Sci. 2002, 245, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Oinonen, P.; Henriksson, G. Characterization of Two Novel Bio-based Materials from Pulping Process Side Streams: Ecohelix and CleanFlow Black Lignin. BioResources 2018, 13, 7606–7627. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.G.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—A biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
- Merdy, P.; Guillon, E.; Aplincourt, M.; Duomonceau, J. Interaction of metallic cations with lignins. Part 1: Stability of iron (III), manganese (II) and copper (II) complexes with phenolic lignin model compounds: Coumaric, ferulic and sinapic acids and coniferyl alcohol. J. Chem. Res. 2000, 2000, 76–77. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Tay, A.S.H.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: Synthesis and adsorption studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef]
- Pajchel, L.; Kolodziejski, W. Solid-state MAS NMR, TEM, and TGA studies of structural hydroxyl groups and water in nanocrystalline apatites prepared by dry milling. J. Nanoparticle Res. 2013, 15, 1868. [Google Scholar] [CrossRef]
- Nikolic, M.; Nguyen, H.D.; Daugaard, A.E.; Löf, D.; Mortensen, K.; Barsberg, S.; Sanadi, A.R. Influence of surface modified nano silica on alkyd binder before and after accelerated weathering. Polym. Degrad. Stab. 2016, 126, 134–143. [Google Scholar] [CrossRef]
- Roonasi, P.; Holmgren, A. A Fourier transform infrared (FTIR) and thermogravimetric analysis (TGA) study of oleate adsorbed on magnetite nano-particle surface. Appl. Surf. Sci. 2009, 255, 5891–5895. [Google Scholar] [CrossRef]
- Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical Composition and Thermal Behavior of Kraft Lignins. Forests 2019, 10, 483. [Google Scholar] [CrossRef]
- Todorciuc, T.; Bulgariu, L.; Popa, V.I. Adsorption of cu(ii) from aqueous solution on wheat straw lignin: Equilibrium and kinetic studies. Cell. Chem. Technol. 2015, 49, 439–447. [Google Scholar]
- Sciban, M.; Klasnja, M. Study of the Adsorption of Copper(II) Ions from Water onto Wood Sawdust, Pulp and Lignin. Adsorpt. Sci. Technol. 2016, 22, 195–206. [Google Scholar] [CrossRef]
- Šćiban, M.B.; Klašnja, M.T.; Antov, M.G. Study of the biosorption of different heavy metal ions onto Kraft lignin. Ecol. Eng. 2011, 37, 2092–2095. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Sun, Y.; Kunc, F.; Balhara, V.; Coleman, B.; Kodra, O.; Raza, M.; Chen, M.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. Quantification of amine functional groups on silica nanoparticles: A multi-method approach. Nanoscale Adv. 2019, 1, 1598–1607. [Google Scholar] [CrossRef]
- Motschi, H. Correlation of EPR-parameters with thermodynamic stability constants for copper(II) complexes Cu(II)-EPR as a probe for the surface complexation at the water/oxide interface. Colloids Surf. 1984, 9, 333–347. [Google Scholar] [CrossRef]
- Cariati, F.; Erre, L.; Micera, G.; Panzanelli, A.; Ciani, G.; Sironi, A. Interaction of metal ions with humic-like models. Part. I. Synthesis, spectroscopic and structural properties of diaquabis(2,6-dihydroxybenzoato) copper(II) and hexaaquaM(II) bis(2,6-dihydroxybenzoate) dihydrate (M = Mn, Fe, Co, Ni, Cu and Zn). Inorganica Chim. Acta 1983, 80, 57–65. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Ravi, K.; Mittermeier, F.; Meier, S.; Riisager, A.; Lidén, G.; Hulteberg, C.P. Oxidative Depolymerization of Kraft Lignin for Microbial Conversion. ACS Sustain. Chem. Eng. 2019, 7, 11640–11652. [Google Scholar] [CrossRef]
- Ghavidel, N.; Fatehi, P. Synergistic effect of lignin incorporation into polystyrene for producing sustainable superadsorbent. RSC Adv. 2019, 9, 17639–17652. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and Characterization of Multifunctional Chitin/Lignin Materials. J. Nanomater. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Ammar, N.S.; Abdel Ghafar, H.H.; Farahat, M. Adsorption of Cd(II), Cu(II) and Pb(II) using recycled waste glass: Equilibrium and kinetic studies. Desalin. Water Treat. 2012, 48, 320–328. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R.; Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chen, C.H.; Cheng, S.; Li, H.Y. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2015, 13, 65–76. [Google Scholar] [CrossRef]
- Bandosz, T.J. On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperatures. J Colloid Interface Sci 2002, 246, 1–20. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; de Arespacochaga, N.; Pérez, L.; Sisani, E. Biogas use in high temperature fuel cells: Enhancement of KOH-KI activated carbon performance toward H2S removal. Int. J. Hydrogen Energy 2017, 42, 10341–10353. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y.; Wan, L. Fabrication of a green porous lignin-based sphere for the removal of lead ions from aqueous media. J. Hazard. Mater. 2015, 285, 77–83. [Google Scholar] [CrossRef]
- Perez-Cantu, L.; Liebner, F.; Smirnova, I. Preparation of aerogels from wheat straw lignin by cross-linking with oligo(alkylene glycol)-α,ω-diglycidyl ethers. Microporous Mesoporous Mater. 2014, 195, 303–310. [Google Scholar] [CrossRef]
- Tian, J.; Ren, S.; Fang, G.; Ma, Y.; Ai, Q. Preparation and Performance of Dimethyl-Acetoxy-(2-Carboxymethyl Ether)-Lignin Ammonium Chloride Amphoteric Surfactant. BioResources 2014, 9, 14. [Google Scholar] [CrossRef][Green Version]
- Ge, Y.; Qin, L.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar] [CrossRef]
- Qin, L.; Ge, Y.; Deng, B.; Li, Z. Poly (ethylene imine) anchored lignin composite for heavy metals capturing in water. J. Taiwan Inst. Chem. Eng. 2017, 71, 84–90. [Google Scholar] [CrossRef]
- Wellinger, A.; Lindberg, A. Biogas Upgrading and Utilization. IEA Bionergy. Task 24. 2000. Available online: http://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/Biogas%20upgrading.pdf (accessed on 1 September 2020).
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Majeski, M.W.; Bolotin, I.L.; Hanley, L. Cluster beam deposition of Cu2-XS nanoparticles into organic thin films. ACS Appl. Mater. Interfaces 2014, 6, 12901–12908. [Google Scholar] [CrossRef]
- Silvester, E.J.; Grieser, F.; Sexton, B.A.; Healy, T.W. Spectroscopic studies on copper sulfide sols. Langmuir 1991, 7, 2917–2922. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Hart, B.R.; Polack, R.; Kobe, B.A.; Smart, R.S.C. Analysis of mineral surface chemistry in flotation separation using imaging XPS. Miner. Eng. 2007, 20, 152–162. [Google Scholar] [CrossRef]
- Kundu, M.; Hasegawa, T.; Terabe, K.; Yamamoto, K.; Aono, M. Structural studies of copper sulfide films: Effect of ambient atmosphere. Sci. Technol. Adv. Mater. 2008, 9, 35011. [Google Scholar] [CrossRef]
- Mott, D.; Yin, J.; Engelhard, M.; Loukrakpam, R.; Chang, P.; Miller, G.; Bae, I.-T.; Chandra Das, N.; Wang, C.; Luo, J.; et al. From Ultrafine Thiolate-Capped Copper Nanoclusters toward Copper Sulfide Nanodiscs: A Thermally Activated Evolution Route. Chem. Mater. 2010, 22, 261–271. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P. Handbook of X-ray Photoelectron Spectroscopy; Chastain, K.B.J., Ed.; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Guillon, E.; Merdy, P.; Aplincourt, M.; Dumonceau, J.; Vezin, H. Structural Characterization and Iron(III) Binding Ability of Dimeric and Polymeric Lignin Models. J. Colloid Interface Sci. 2001, 239, 39–48. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample | H2S Adsorption Capacity (mg/g) | |
|---|---|---|
| Low Relative Humidity (14% RH) | High Relative Humidity (90–100% RH) | |
| Porous silica/Cu(II) | 0.62 ± 0.02 | 0.38 ± 0.05 |
| KL particles/Cu(II) | 0.90 ± 0.39 | 0.52 ± 0.11 |
| KL precipitated from solution/Cu(II) | 2.05 ± 0.40 | 1.80 ± 0.60 |
| Sample | Cu 2p3/2 | S 2p3/2 | Cu (LMM *) |
|---|---|---|---|
| Porous silica/Cu(II) | 932.4, 933.5 | 162.7 | 568.4, 571.7 |
| Porous silica/Cu(II) (adsorption at 90–100% RH) | 932.4, 933.5 | 162.9 | 568.9, 572.3 |
| Porous silica/KL precipitated from solution/Cu(II) | 933.1 | 163.8 | 571.6 |
| Porous silica/KL precipitated from solution/Cu(II) (adsorption at 90–100% RH) | 933.6 | 164.0 | 571.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, M.; Cáceres Najarro, M.; Johannsen, I.; Iruthayaraj, J.; Ceccato, M.; Feilberg, A. Copper Adsorption on Lignin for the Removal of Hydrogen Sulfide. Molecules 2020, 25, 5577. https://doi.org/10.3390/molecules25235577
Nikolic M, Cáceres Najarro M, Johannsen I, Iruthayaraj J, Ceccato M, Feilberg A. Copper Adsorption on Lignin for the Removal of Hydrogen Sulfide. Molecules. 2020; 25(23):5577. https://doi.org/10.3390/molecules25235577
Chicago/Turabian StyleNikolic, Miroslav, Marleny Cáceres Najarro, Ib Johannsen, Joseph Iruthayaraj, Marcel Ceccato, and Anders Feilberg. 2020. "Copper Adsorption on Lignin for the Removal of Hydrogen Sulfide" Molecules 25, no. 23: 5577. https://doi.org/10.3390/molecules25235577
APA StyleNikolic, M., Cáceres Najarro, M., Johannsen, I., Iruthayaraj, J., Ceccato, M., & Feilberg, A. (2020). Copper Adsorption on Lignin for the Removal of Hydrogen Sulfide. Molecules, 25(23), 5577. https://doi.org/10.3390/molecules25235577






