1. Introduction
The colostrum and breast milk of mammals contain extremely important nutrients for infants in the first days after delivery. They contain multiple protective peptides and proteins including an iron-binding glycoprotein, lactoferrin (Lf) The concentration of Lf is 5–13 g/L in the colostrum and 1.5–4.5 g/L in milk [
1,
2]. It is plausible that this protein plays an important role during neonatal development as the daily supply of Lf in an infant ranges between 0.4 g and 1.2 g/kg body weight [
3].
Scientists have been developing the idea that Lf is able to modulate the newborn gastrointestinal tract microbiota composition thanks to bactericidal and bacteriostatic properties [
4]. Furthermore, in vitro studies have proved lactoferrin’s antioxidant activity, the potential to inhibit bacterial biofilm formation and the regulation of immune cells’ response to the lipopolysaccharide (LPS), the endotoxin of Gram-negative bacteria [
5].
Lactoferrin is characterized by high stability. The release of iron requires the destabilization of the protein structure, e.g., by lowering the pH. This phenomenon of ferric ion release can occur in the upper gastrointestinal tract of the newborn under the influence of a gastric pH equal to 4.0 [
5,
6]. Thus, both a metal-free form apolactoferrin (apoLf) and an iron-saturated hololactoferrin (holoLf) can exist in the newborn’s gastrointestinal tract at the same time.
It is important to emphasize that every supply of free ferric ions in a gastrointestinal tract may stimulate the overgrowth of those Gram-negative rods that produce siderophores, which are low molecular weight substances (but also proteins) that capture ferric ions and fuel the rapid multiplication of Gram-negative bacteria [
7]. Even a small increase in the
Escherichia coli population in the very low birth weight (VLBW, i.e., below 1500 g) newborn’s gastrointestinal tract can impair the mucosal barrier function and lead to the translocation of bacteria, primarily Gram-negative pathogenic flora, from the intestinal lumen into the blood system, leading to sepsis. In newborns, sepsis is a major cause of morbidity and mortality.
It is possible that different forms of lactoferrin (metal-depleted apolactoferrin, iron-saturated hololactoferrin or manganese-saturated lactoferrin) may be recognized as the key factors affecting the possibility of E. coli translocation from the gut.
Recently, we have observed a growing interest in clinical applications of lactoferrin to improve the intestinal barrier function in preterm infants providing protection against translocation, sepsis and necrotizing enterocolitis.
The first results of the clinical trials conducted about 10 years ago were very promising; it was proved that an oral supplementation of Lf significantly reduced the number of episodes of late-onset sepsis in newborns with a VLBW [
7]. However, in recent publications authors have begun to doubt whether lactoferrin really reduces the episodes of sepsis in newborns. They emphasize the fact that the sample sizes are too small and they conclude that oral supplementation with bovine lactoferrin does not reduce the incidence of sepsis in infants with birth weights lower than 2000 g [
8].
Why are the results of clinical trials so different? As very little is known about how metal saturation of this protein may modify the composition of the newborn’s microbiota, it is probably a great opportunity to return to basic studies on lactoferrin.
Therefore, the main objective of this study was to elucidate how iron-depleted, iron-saturated and manganese-saturated forms of lactoferrin can regulate the populations Gram-negative and Gram-positive bacteria (including Lactobacillus and Bifidobacterium strains) colonizing the newborn’s gastrointestinal tract a few hours after delivery.
We also studied the influence of various lactoferrin forms on the gut microbiota by using prematurely weaned rat pups; namely, levels of potentially pathogenic E. coli as well as probiotic strains of the Lactobacillus genus. The use of an animal model allowed us to observe the effect of lactoferrin forms and saturation levels in a mammalian system similar to those found in human newborns especially those with a VLBW that are separated from their mothers and are at a particularly high risk of developing sepsis.
2. Materials and Methods
2.1. Forms of Lactoferrin Used in Experiments
Bovine lactoferrin was purchased from FrieslandCampina (Amersfoort, The Netherlands) and exhibited iron saturation of 10.2%. The modified forms (iron-depleted (apolactoferrin, apoLf, 1.2 ± 0.2% Fe saturation), iron-saturated (hololactoferrin, holoLf, 71.8 ± 6.5% Fe saturation) and manganese-saturated lactoferrin (MnLf, 1.2 ± 0.2% Fe saturation, 47.1 ± 2.0% Mn
3+ saturation)) were prepared as described before [
9,
10].
In further experiments, all forms of lactoferrin were tested at concentrations 0.6, 5 and 40 mg/mL. High concentrations of lactoferrin have been described as bactericidal/bacteriostatic and have been used in dietary supplements intended for newborns and children [
11].
2.2. Pathogenic Gram-Negative and Gram-Positive Bacterial Strains and Growth Conditions
The experiments involved the Gram-negative reference bacterial species Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603) and the Gram-positive reference strain Staphylococcus aureus (ATCC 25923). Pathogenic Gram-negative species tested included clinical strains of Escherichia coli (CM226), Klebsiella pneumoniae (CM18) and a Gram-positive strain Staphylococcus aureus (CM30). Pathogenic strains were isolated from the blood of very low birth weight neonates with clinical symptoms of sepsis confirmed by blood culture. Neonates were treated in Polish hospitals within The Polish Neonatology Surveillance Network (PNSN). Utilization of the data collected in the PNSN for scientific purposes was approved by the Bioethics Committee of Jagiellonian University Medical College (no. KBET/221/B/2011).
E. coli and K. pneumoniae strains were passaged on MacConkey Agar (Oxoid, UK) and S. aureus on Blood Agar (Oxoid, UK) under aerobic conditions at 37 °C. Before the experiment, all strains were cultured in liquid TSB (tryptic soy broth, Oxoid, UK) overnight.
2.3. Probiotic Bacteria Strains and Growth Conditions
The experiments involved reference probiotic bacterial strains Lactobacillus plantarum (ATCC 14431), Lactobacillus rhamnosus (ATCC 53103), Bifidobacterium breve (ATCC 15700) and Bifidobacterium longum (ATCC 15707).
Investigated probiotic bacteria species included strains isolated from commercially available probiotic formulae (Lactoral® and FFbaby®, Biomed, Poland) L. plantarum (PL02), L. rhamnosus (KL53A), B. breve (PB04) and B. longum (PL03).
Lactobacillus strains were passaged on de Man, Rogosa and Sharpe (MRS) agar (Oxoid, UK) and cultured in MRS broth (Oxoid, UK). Bifidobacterium strains were passaged on trypticase-phytone-yeast agar (TPY, Fluka, Switzerland) and cultured in liquid TPY broth (Fluka, Switzerland). Lactobacillus and Bifidobacterium strains were cultured under anaerobic conditions at 37 °C. Before the experiment, strains were cultured for 48 h in liquid broth.
2.4. The Effect of Various Forms of Lactoferrin on Pathogenic and Probiotic Bacterial Growth
The cultures of Gram-negative rods E. coli and K. pneumoniae were performed in a liquid M9 medium (Sigma-Aldrich, St. Louis, MO, USA), a broth with a defined minimal composition. To observe bacterial growth, it was necessary to add ferric salt 0.2 mM (FeSO4)·7H2O to achieve the final concentration of 20.2 mg/L of Fe (iron and manganese levels were estimated using inductivelycoupled plasma optical emission spectroscopy—ICP-OES). This M9(+Fe) medium constituted the growing medium in the case of the Gram-negative rods. All forms of lactoferrin were then added to the M9(+Fe) broth in order to determine the increase or decrease in the Gram-negative bacterial population numbers. For Staphylococcus aureus, a liquid TSB medium was used as a growing medium.
For probiotics strains Lactobacillus and Bifidobacterium, we chose a liquid MRS and TPY medium, respectively. The contents of the iron and manganese ions were 0.6 mg/L and 20 mg/L for MRS and 4.5 mg/L and <0.5 mg/L for TPY, respectively. In this case, the concentration of iron and manganese ions was too high to observe the effect of lactoferrin metal saturation. It was necessary to reduce these concentrations to the minimum level, thus allowing monitoring of the growth of probiotic bacteria. In order to achieve this, the above mentioned broths were incubated for 2 h with a Chelex® 100 sodium form (Sigma-Aldrich, 50 g/L). An ion-exchange resin was then removed by filtration. That way, we reduced the concentration of Fe ions and Mn ions to a final value of <0.5 mg/L in the MRS and TPY broths. The various lactoferrin forms were added to such media (MRS(−) or TPY(−)), which resulted in final media with various contents of Fe and Mn.
Detailed information on the concentration of metals are presented in
Table 1.
2.5. Bacterial Growth Evaluation
Freshly prepared bacterial suspensions (as described above) at a density of 1 × 108 colony colony-forming unit per mL (CFU/mL) were centrifuged (10 min, 6000× g) and washed twice with sterile phosphate-buffered saline. Dilutions in the appropriate broth were made to adjust the density to 1 × 105 CFU/mL. Finally, 200 μL of this suspension were added to 1.8 mL of the appropriate medium (growing media or growing media with different lactoferrin forms) to obtain a population number of 1 × 104 CFU/mL.
Three independent repetitions of every experiment were performed with three technical replicates in each repetition. We evaluated bacterial growth using two methods:
- (a)
Semi-quantitative assessment. Optical density (OD) monitored every 30 min by measuring the absorbance of 200 μL of the culture media at 620 nm using a Tecan Infinite M200 PRO plate reader.
- (b)
Quantitative assessment. After 24 h (and 48 h for Bifidobacterium) at 37 °C, bacterial cultures were seeded quantitatively on appropriate agar plates.
2.6. Animals
Male albino Wistar rat pups [WistarKrf (Wi) Wu)] with a 20–30 g body weight were used throughout the study. The pups stayed with the mother until 12 days post-birth when they were prematurely weaned. Animals were fed standard laboratory chow and water ad libitum. They were housed in standard cages in temperature-controlled rooms (18–22 °C, 50–60% humidity) under a 12 h light cycle (06:00–18:00) and appropriate environmental enrichment was introduced in the cages. All animal procedures performed conformed with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and approved by the Jagiellonian University Ethical Committee on Animal Experiments (no. 190/2012).
2.7. In Vivo Experimental Design
A total number of 24 male rat pups were used to study the effect of oral administration of various lactoferrin forms on the microbial community in the neonatal gut. The main focus was on population numbers of Escherichia coli and staphylococci as well as the probiotic bacteria of the Lactobacillus and Bifidobacterium genera. Given the typical standard deviations of CFU/g range between 0.5–0.8 log, we calculated that the usage of six animals per group would be enough to observe a significant difference between means of 1.5 log with 80% power (at a significance level of 0.05). Six dams were used and their litters were reduced to four male pups. On day 12 post-birth, rat pups were prematurely weaned and randomly assigned into four cages corresponding to experimental units/groups (every pup from each litter was placed in a different cage). On days 14–16, lactoferrin forms differing in metal saturation (namely metal-depleted apoLf, iron-saturated holoLf and manganese-saturated MnLf; water was used for a control group) were administered orally at a dose of 300 mg/kg/day (every day between 8–9 am in the treatment room). This was to mimic the process of lactoferrin supplementation in humans. On day 17, the animals were euthanized using pentobarbital in the laboratory (100 mg/kg body weight, i.p., standard procedure for euthanasia) and fecal content was extracted and plated quantitatively on selective media. The order in which the animals were treated was random as the procedures were not time-consuming and were performed quickly and efficiently. No adverse events were observed during the study.
Experimental units, that is groups given either the vehicle (control group) or lactoferrin form (experimental groups), were arranged as explained below:
Group 1 (n = 6): control—weaned pups administered vehicle (water per os);
Group 2 (n = 6): apoLf—weaned pups administered apolactoferrin;
Group 3 (n = 6): holoLf—weaned pups administered hololactoferrin;
Group 4 (n = 6): MnLf—weaned pups administered manganese-saturated lactoferrin.
2.8. Statistical Analysis
Herein data were presented as mean (growth curves), mean ± SEM (for colony forming unit graphs) or scatter plots (mean and value for every animal in the group for in vivo studies). The test for normality was performed using the Kolmogorov–Smirnov test. Data were analyzed using one-way ANOVA, followed if significant with a post-hoc Tukey test. The statistical analysis was performed in Graphpad Prism v. 5.01 (GraphPad Software, Inc., San Diego, CA, USA). The significance level was set at 0.05.
4. Discussion
Lactoferrin, thanks to its multifunctional activities, has been recognized as one of the most important components of women’s milk. Publications dating from the 1980s to the early years of the 21st century emphasize the strong bacteriostatic and bactericidal activity of lactoferrin, especially the apolactoferrin, versus various microorganisms including
Escherichia coli [
14,
15]. This has prompted the usage of lactoferrin as an innovative nutritional supplement mostly for infant formulas [
16]. However, the reports published in the last few years present a much less impressive view on the bactericidal effect of lactoferrin. In our study, we examined the bactericidal effect of various forms of lactoferrin (apoLf, nLf, holoLf, MnLf) at concentrations of 0.6, 5 and 40 mg/mL because high concentrations of lactoferrins have been considered safe and used in oral dietary supplements for newborns [
11].
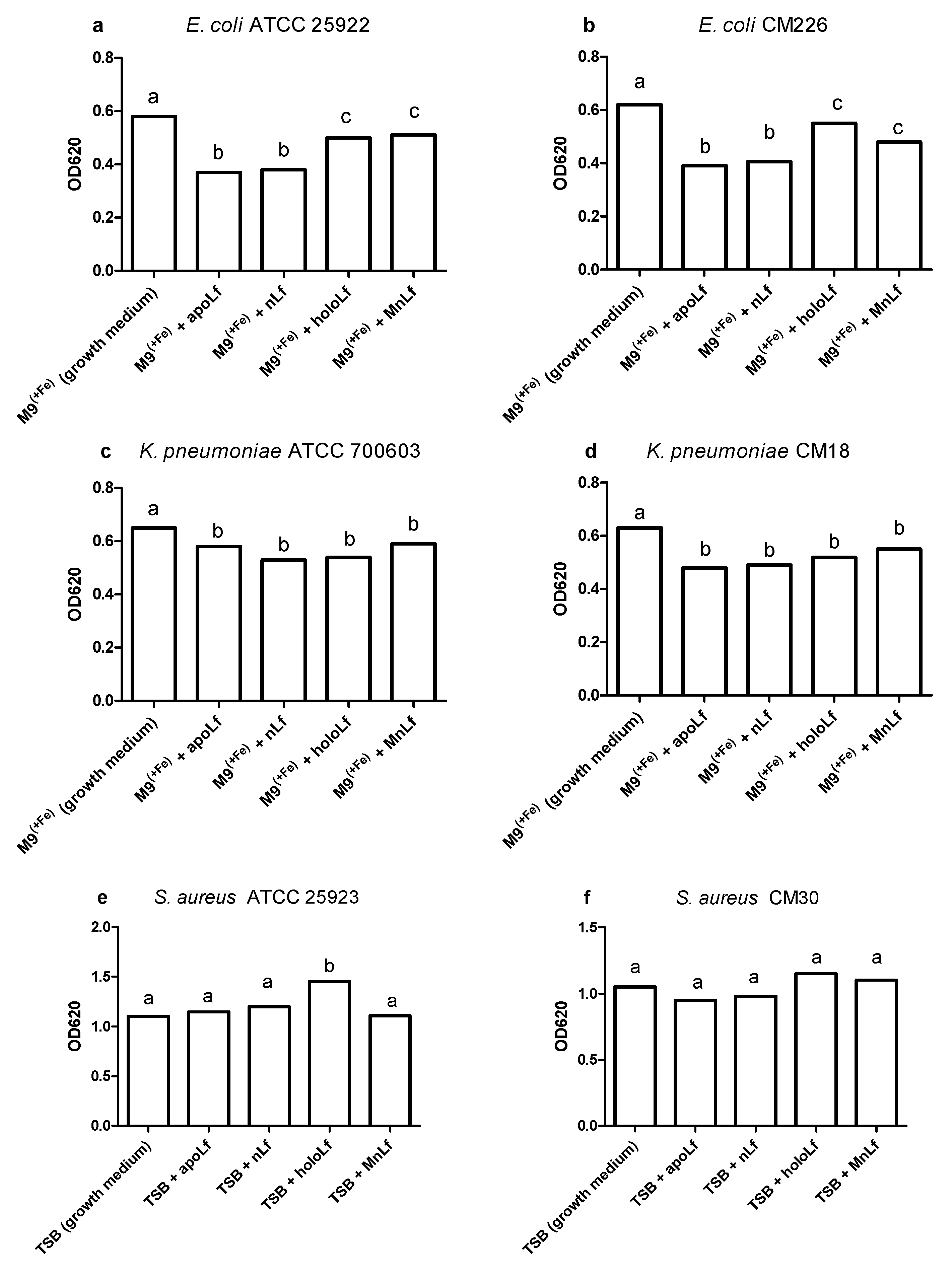
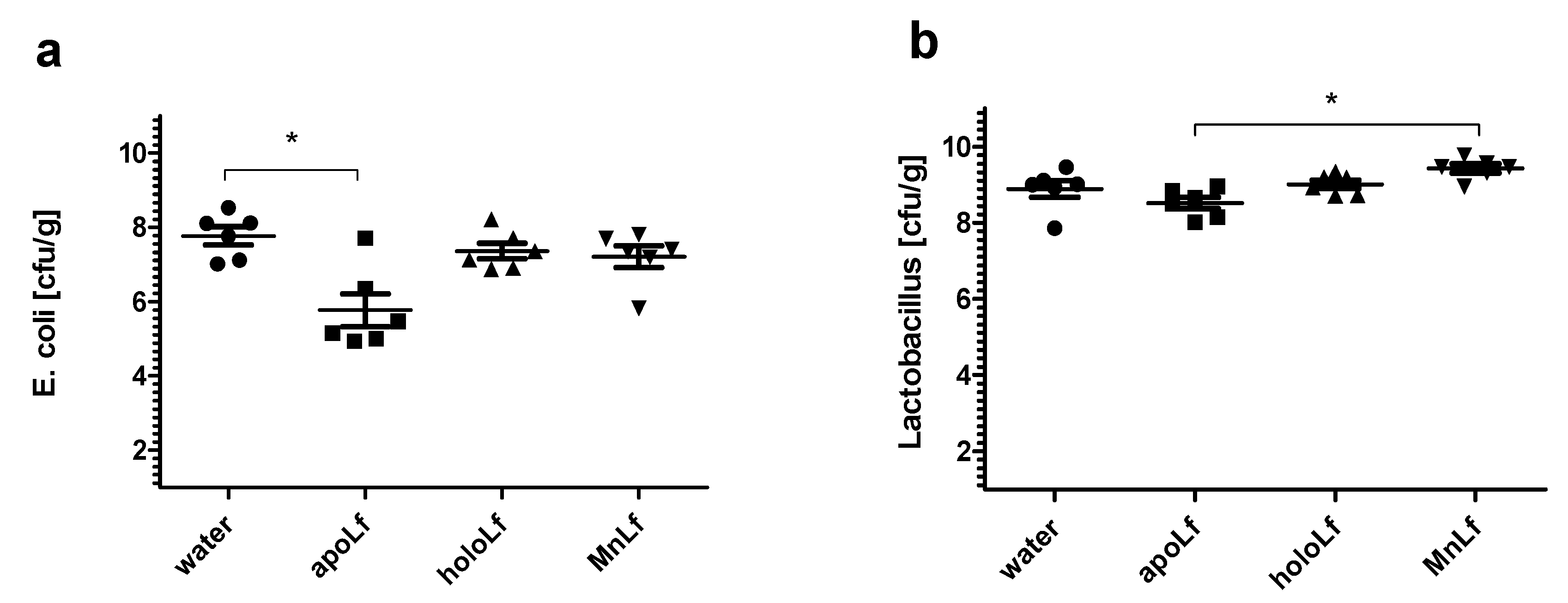
Our studies have shown that lower concentrations of bovine lactoferrin exerted no antibacterial effect against pathogenic bacteria. Using a semi-quantitative method, after 24 h we observed a significantly decreased value of OD for Gram-negative bacteria in both
E. coli and
K. pneumoniae strains under the influence of all forms of lactoferrin (at 40 mg/mL) vs. the control. This may indicate that the lactoferrin was responsible for the bacteriostatic effect for Gram-negative strains (
Figure 1). Moreover, we showed statistical differences in the decrease in the population size between apoLf vs. holoLf in the case of the
E. coli strains (
Figure 2a,b). This might suggest that apoLf could be more potent in limiting the bacterial translocation phenomenon.
On the contrary, this kind of difference between forms was not demonstrated for the
Klebsiella strains. This may indicate that the antibacterial effect of iron-depleted lactoferrin (apoLf) depends on the species of Gram-negative rods and probably
E. coli is a more potent competitor with apoLf for Fe ions of the same pool. It seems plausible that lactoferrin is engaged in a battle for iron acquisition but some pathogens are capable of counteracting such iron-binding proteins by synthesizing siderophores, small high affinity iron-chelating molecules, or through iron acquisition from other sources [
17].
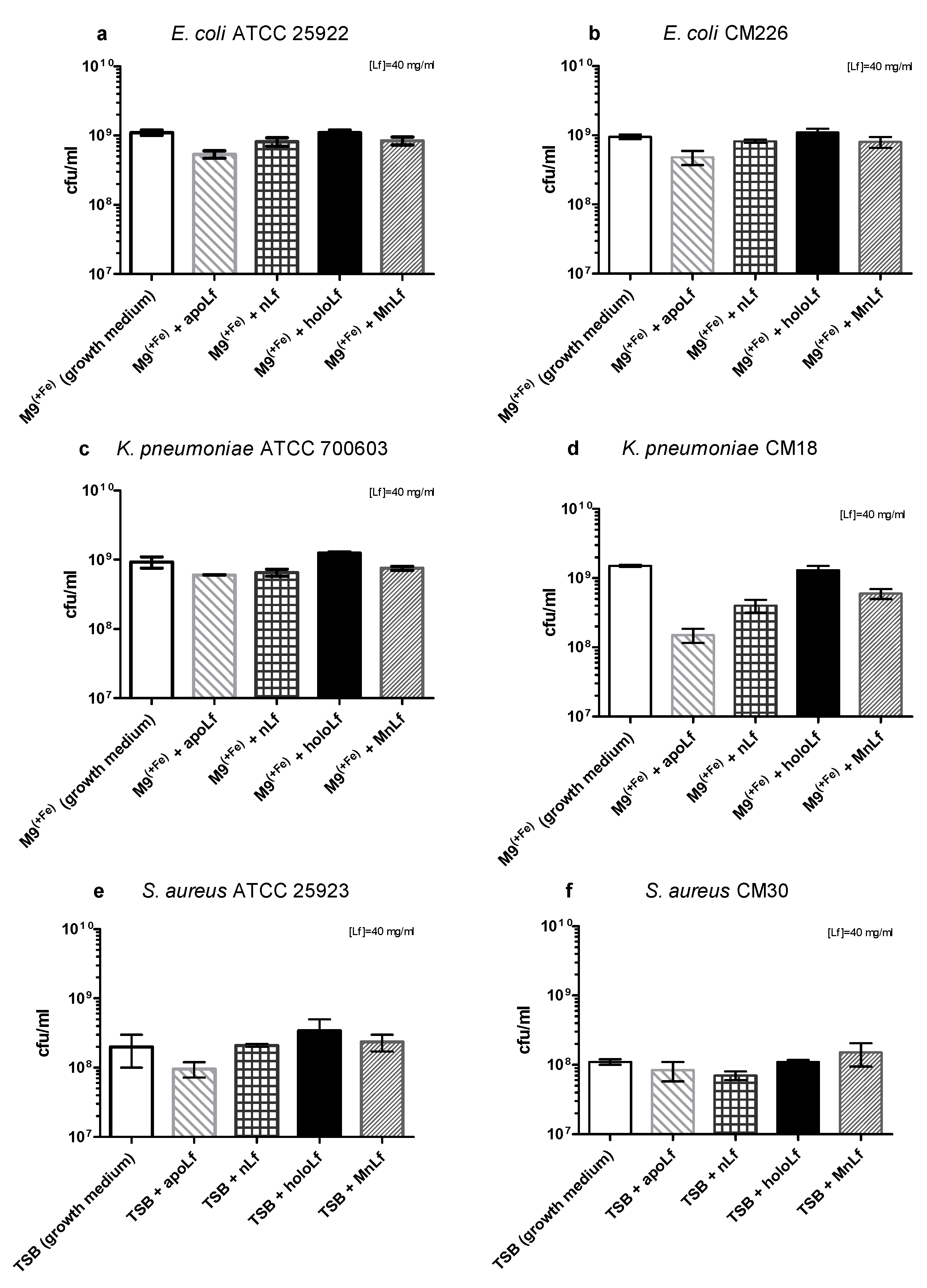
Based on the quantitative (CFU/mL) plating, after 24 h we did not observe any antibacterial effects of any form of lactoferrin on the population of both species of Gram-negative bacteria (
Figure 2a–d).
The differences between the results received using OD and CFU methods are due to the very nature of the optical density measurement. By monitoring optical density, we detected both live and dead bacteria as well as various metabolites such as peptides, DNA or exopolysaccharides (EPS) that comprised the three-dimensional structure of bacterial biofilm. On the other hand, a quantitative culture method allowed for the quantification of only living forms of bacteria. It still constitutes the golden standard for microbiological research.
Interestingly, recently we observed a substantial shift in the literature regarding both the results and methodology of studies of Lf impact on pathogenic species. More recent reports do not show such a strong reduction of population numbers of those bacteria but only minor changes that were observed using semi-quantitative methods. We believe that such fluctuations (not exceeding one order of magnitude) might not be significant enough to affect the microbial ecology of the gut.
Based on these results, we could speculate that—only in case of Gram-negative rods—lactoferrin (at a concentration of 40 mg/mL, regardless of the metal ion saturation) shows more of an anti-biofilm than a bactericidal effect. Such activity has been demonstrated before for lactoferrin. It was shown to inhibit the biofilm formation of
Pseudomonas aeruginosa [
18]. We could theorize that the inhibition of
E. coli and
K. pneumoniae biofilm development by lactoferrin could facilitate the penetration of both antibiotics and reactive oxygen species into the deeper layers of the bacterial biofilm.
Furthermore, based on earlier in vitro studies, we know that lactoferrin can actively block the immunostimulatory action of endotoxin (LPS) from Gram-negative rods, which is of great importance in the case of neonatal bacteremia and endotoxemia originating from infections in the gastrointestinal tract. Perhaps, due to its high affinity for LPS, lactoferrin may also effectively bind to the structure of endotoxin in the cell wall of Gram-negative rods and thus may interfere with the proper production of bacterial biofilms.
Additionally, only the apolactoferrin form induced a significant decrease of gut
E. coli population in an in vivo experimental setup using prematurely weaned rat pups. In comparison with the control, only the metal-depleted form of the protein administered orally led to a notable reduction of the population number of
E. coli. This further confirms the hypothesis that apolactoferrin would be the most suitable candidate for oral supplements that could limit the overgrowth of
Enterobacteriaceae, a particularly dangerous group of microorganisms responsible for a lower percentage of late blood infections (30–40%) and are associated with higher mortality [
19,
20].
In the case of
Staphylococcus strains, we did not observe any decrease in the bacterial population numbers using either quantitative plating or semi-quantitative measurements. We may infer that, for
Staphylococcus strains, lactoferrin did not exhibit either antibacterial or anti-biofilm effects. Therefore, it seems that lactoferrin might not be as potent as a supplement in the prevention of early-onset sepsis, which is mostly caused by Gram-positive staphylococci [
21].
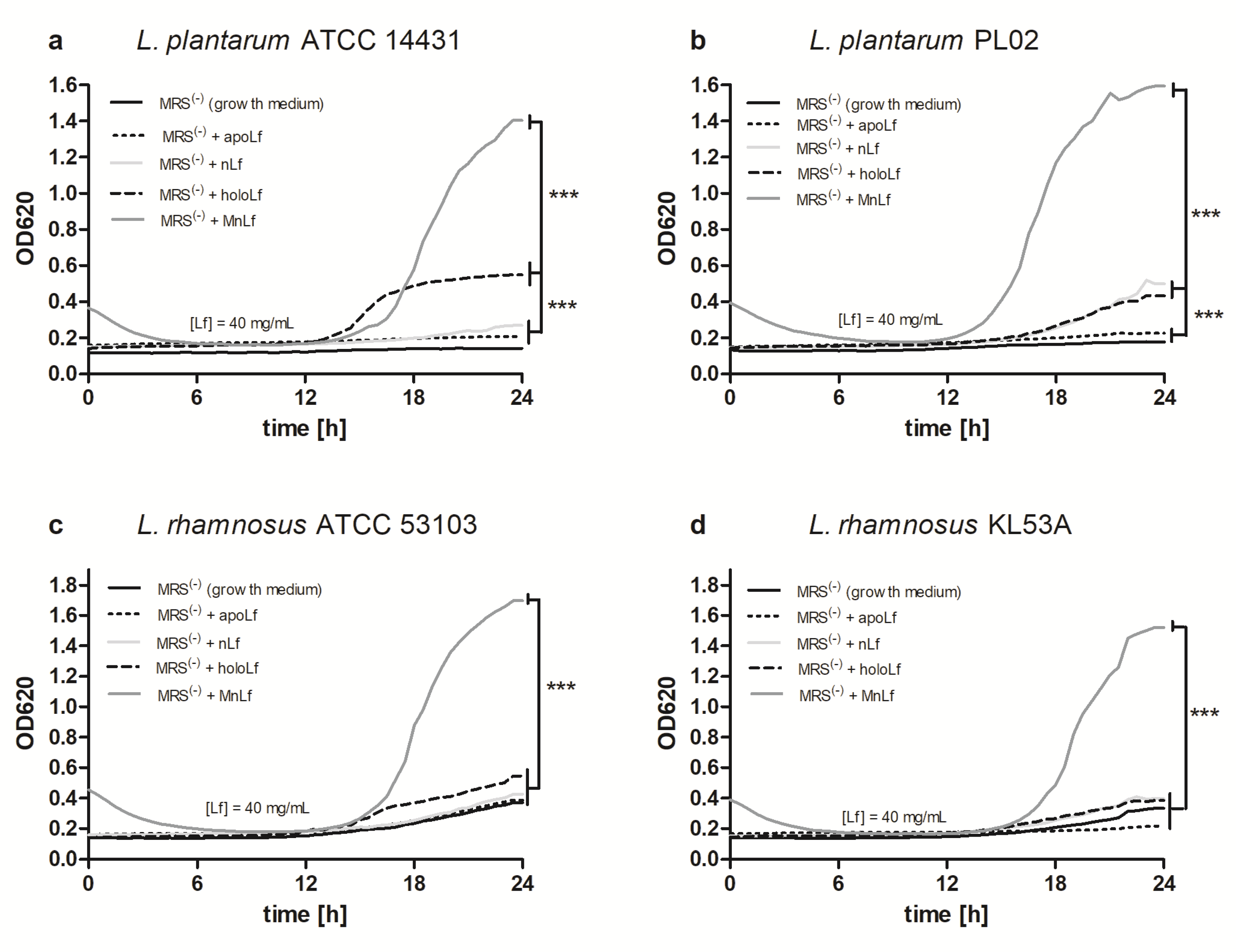
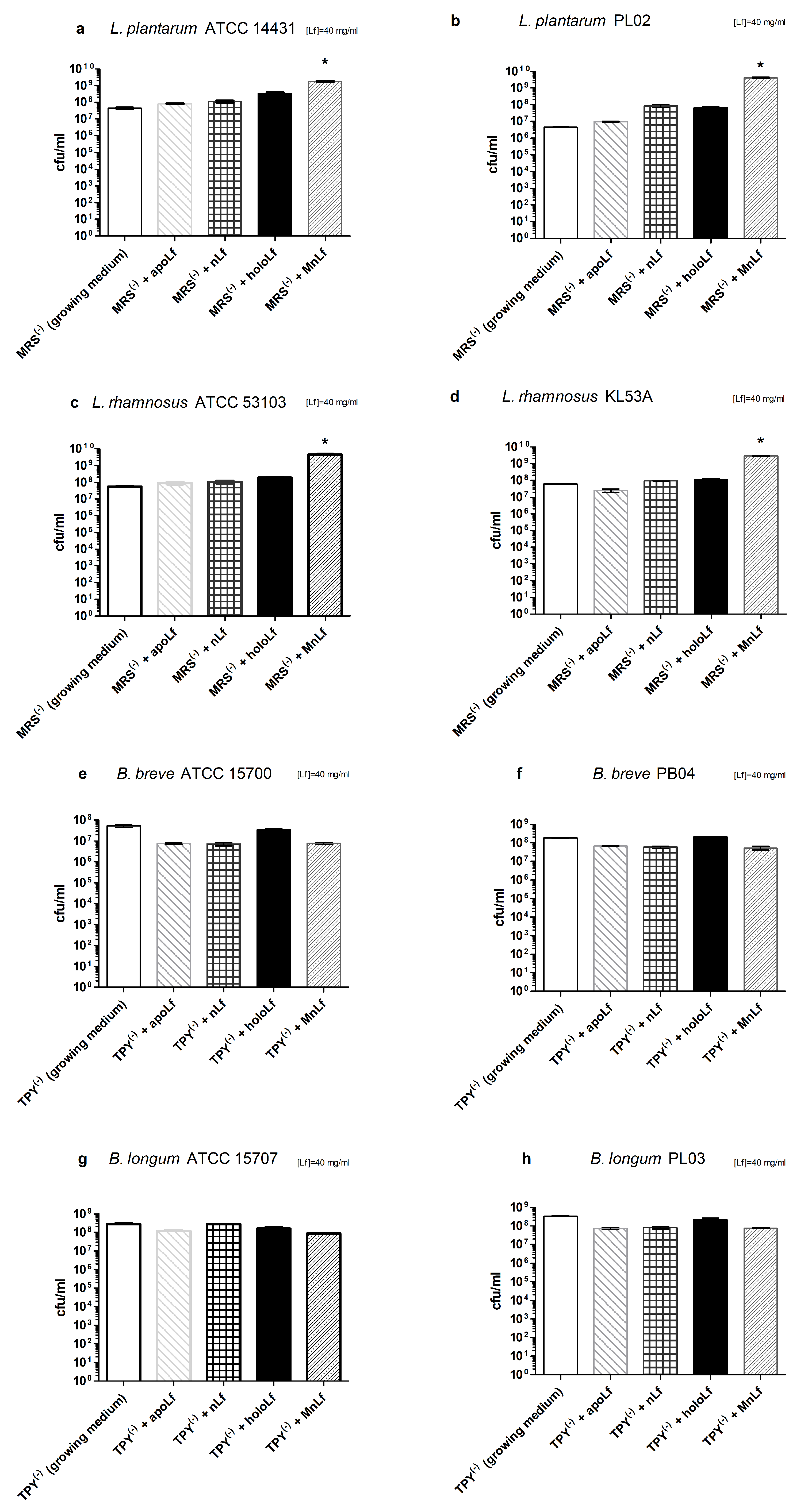
Interestingly, data presented here highlight the substantial role of lactoferrin saturated with manganese in the regulation of the
Lactobacillus strain population. We observed a notable increase in population numbers of all studied strains of
L. plantarum and
L. rhamnosus cultured in the presence of 40 mg/mL MnLf. Additionally, the population numbers for 40 mg/mL MnLf were significantly higher than for the growth medium where we observed an increase ranging from 1.5 to 3 orders of magnitude. In the course of evolution, bacteria of
Lactobacillus genus have become independent of iron supply by replacing iron with manganese in their protein active sites [
2]. In this in vitro study, MnLf was the only source of the element in the culture media and it became a potent supply of the metal for those bacteria, resulting in a significant increase in their population numbers. This phenomenon, however, was not observed for tested
Bifidobacterium strains. Furthermore, it was confirmed in vivo that manganese-saturated lactoferrin could enhance the
Lactobacillus population numbers in the gut as opposed to other tested forms such as apo- and hololactoferrin. It is worth noting that such a potent positive effect on lactobacilli population numbers could translate into a major improvement of the balance in the gut microbial community achieved by concurrently limiting the growth of
Enterobacteriaceae.
5. Conclusions
In conclusion, our data suggest the hypothesis that lactoferrin might contribute to the regulation of the homeostasis in the gut in various ways:
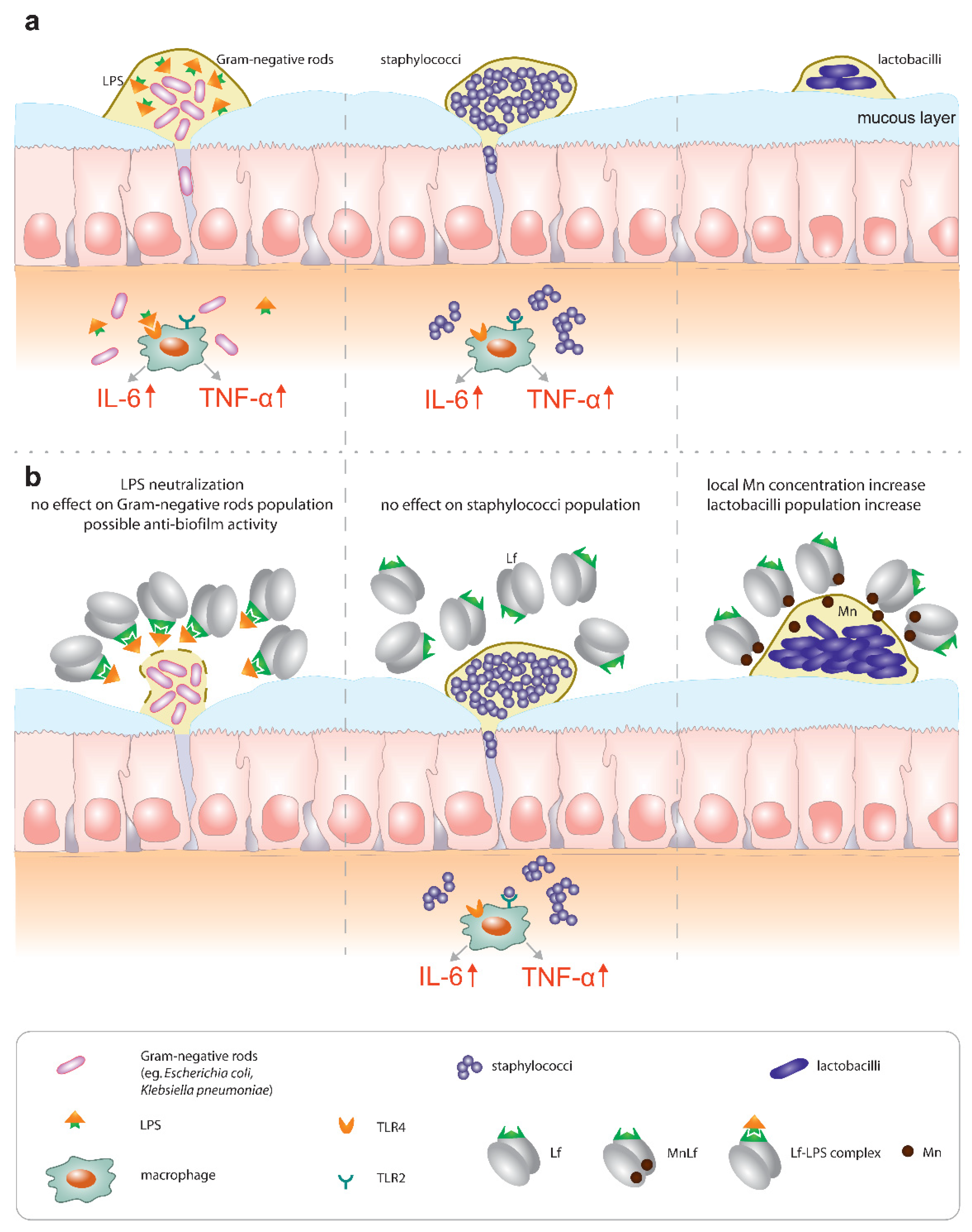
Primarily, lactoferrin (particularly apolactoferrin) could provide mild bacteriostatic or potentially anti-biofilm forming activity towards Gram-negative rods, which was confirmed by in vivo studies. Additionally, our previous studies confirmed that Lf can counteract the proinflammatory effect of endotoxins by sequestrating them into an Lf-LPS complex and reducing the local inflammatory response in the gut [
5] (
Figure 7, left).
On the contrary, our results did not confirm any antimicrobial effect of Lf against Staphylococcus strains (
Figure 7, middle). We infer that the major activity of lactoferrin does not lie in the regulation of population numbers of Gram-positive pathogenic species. We suspect that lactoferrin would not be an effective agent in the prevention of early-onset sepsis commonly caused by staphylococci.
Finally, a very promising activity could be seen for manganese-saturated lactoferrin as it was found to possess the ability to enhance
Lactobacillus population numbers (
Figure 7, right), which was also confirmed using the animal model.
Lactoferrin has long been implicated as a potent bioactive ingredient of dietary supplements such as infant formulae. However, little attention has been paid to both the metal saturation of lactoferrin as well as the necessity to provide a continuous supply of this protein in the neonatal gut. For instance, the endotoxin-neutralizing properties of lactoferrin require the constant presence of the protein in the gut lumen, which can only be achieved by breastfeeding on demand or oral supplementation with proper formulas. Lactoferrin seems to be relevant for the prevention of late-onset sepsis (of Gram-negative origin, associated with higher mortality) because it is capable of hindering the impact of Enterobacteriaceae, both directly (by limiting their growth and possibly biofilm formation) and through LPS neutralization. On the other hand, lactoferrin does not seem to counteract Gram-positive bacteria (the most common pathogens causing late-onset sepsis), which underlines the importance of proper sepsis diagnostics. Lastly, manganese-saturated lactoferrin introduces an interesting opportunity to tip the balance of the microbial community in favor of Lactobacillus strains that exert antibacterial properties against pathogenic species and effectively compete with them for receptor sites on the surface of intestinal epithelial cells.
Our findings confirmed in both in vitro and in vivo studies seem to correspond with the latest clinical trial results that no longer confirm the previously established effectiveness of oral lactoferrin supplementation for sepsis prevention. We suggest that the concept of omnipotent lactoferrin should be revised. Further studies are required to either confirm or deny its properties and verify whether its activity is dependent upon metal saturation. Our next studies will focus on evaluating the role of various lactoferrin forms in supplementation in the rat model of bacterial translocation.