Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Feed, and NAFLD Induction
2.2. Sample Collection and Preparation
2.3. Serum Biochemistry, Insulin and Insulin Sensitivity
2.4. Liver Histology
2.5. Quantitative PCR
2.6. Lipid Analyses
2.7. Statistical Analyses
3. Results
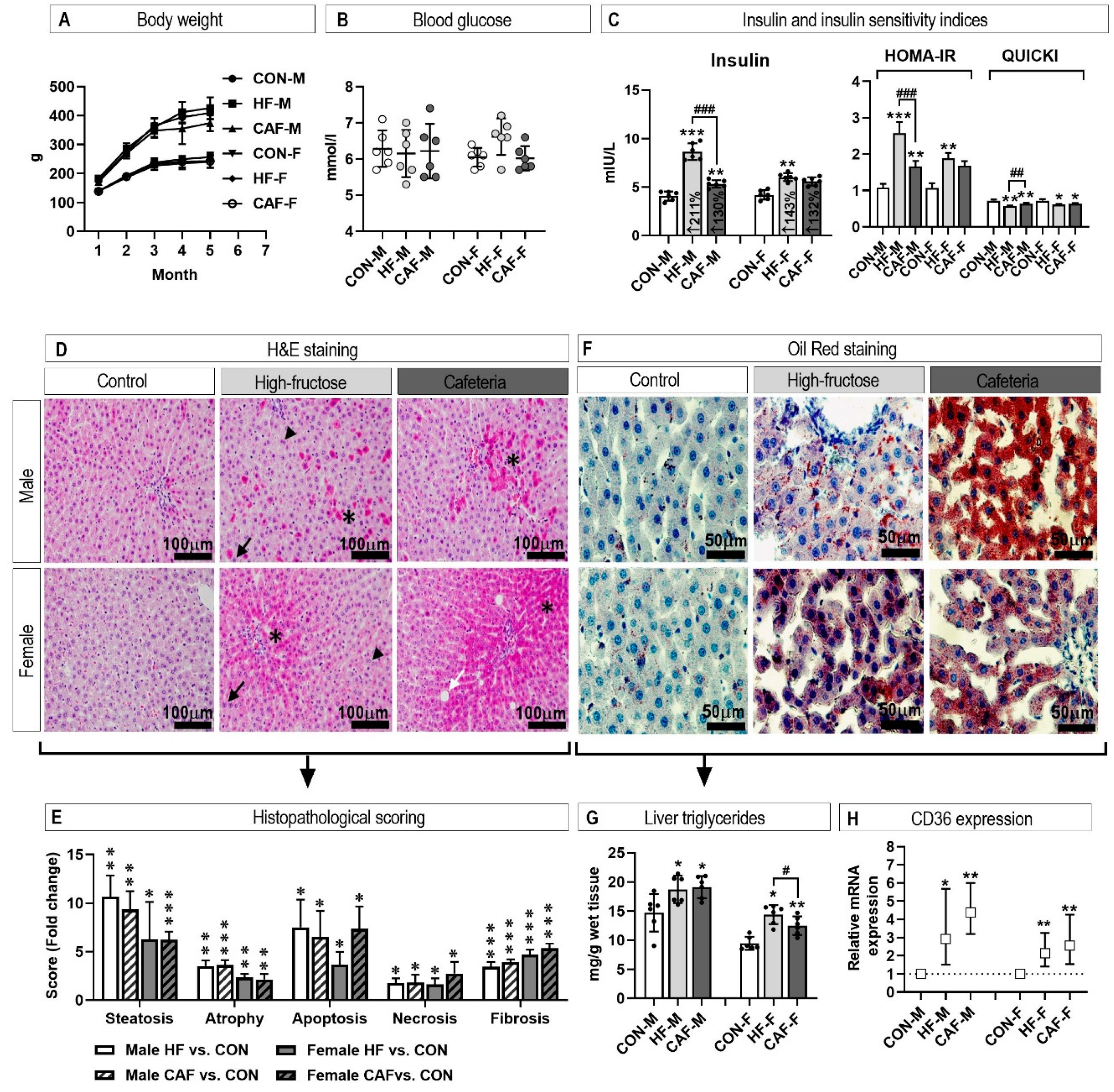
3.1. Insulin Resistance Confirmation
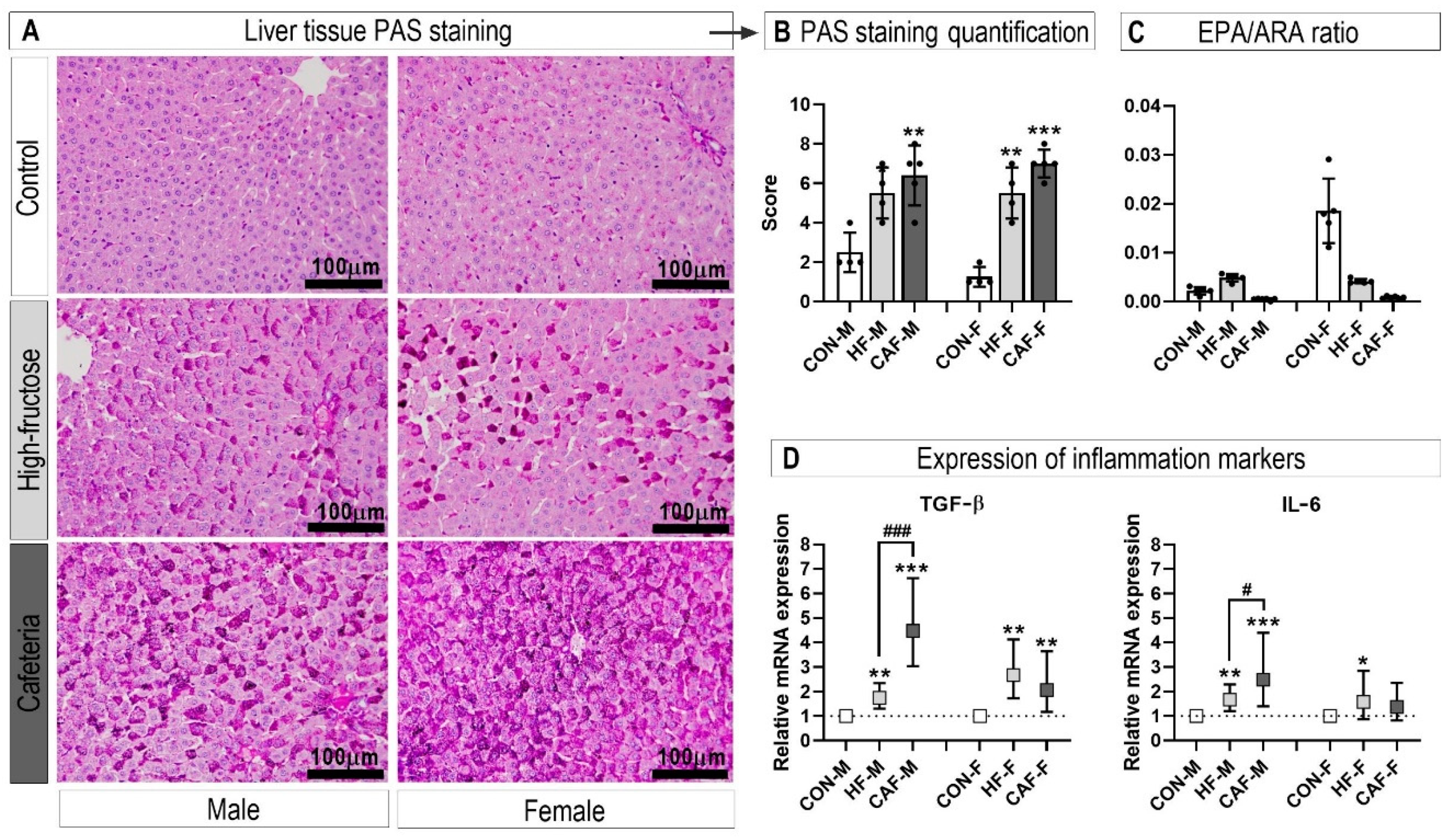
3.2. Liver Histology
3.3. Inflammation
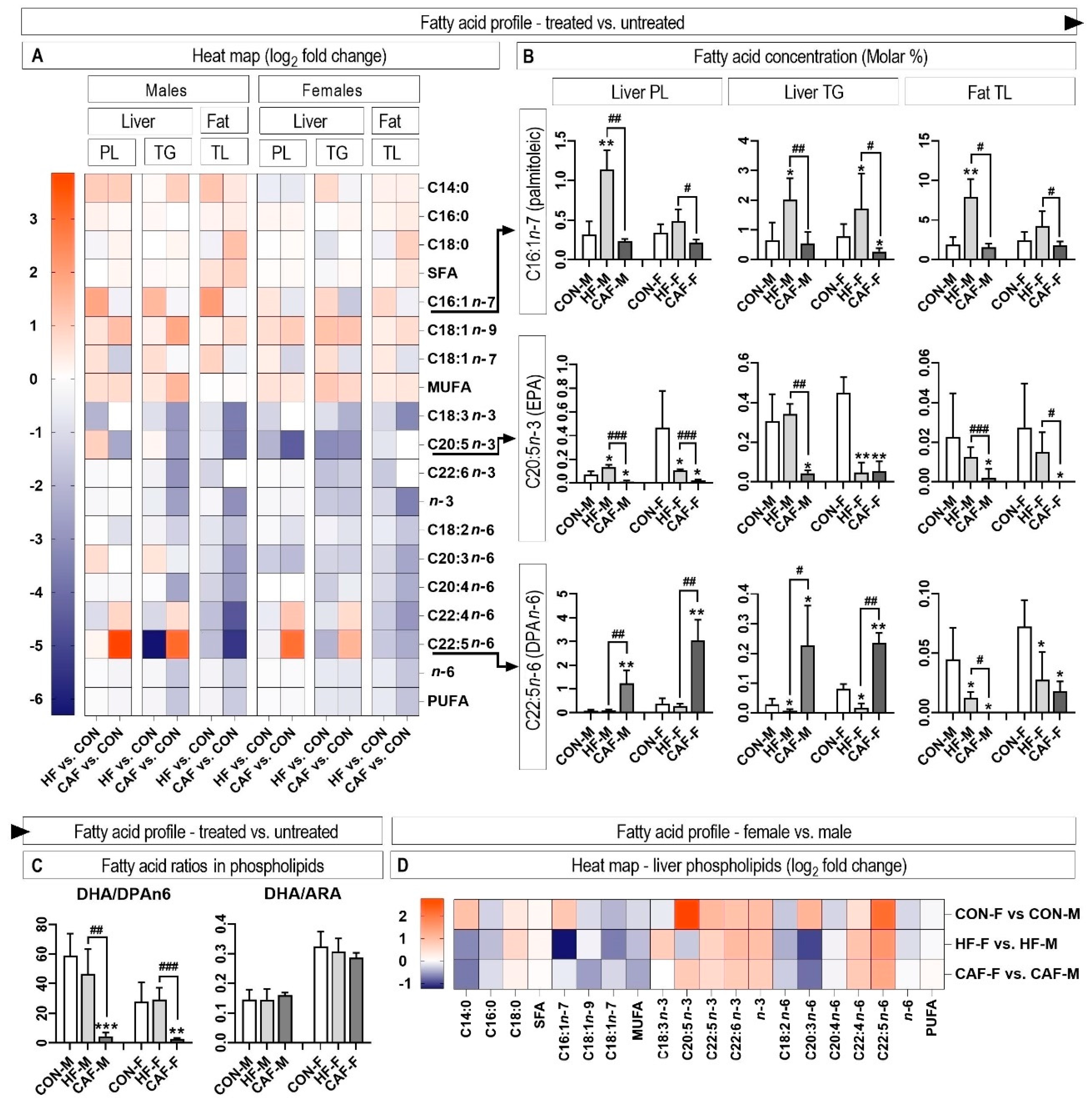
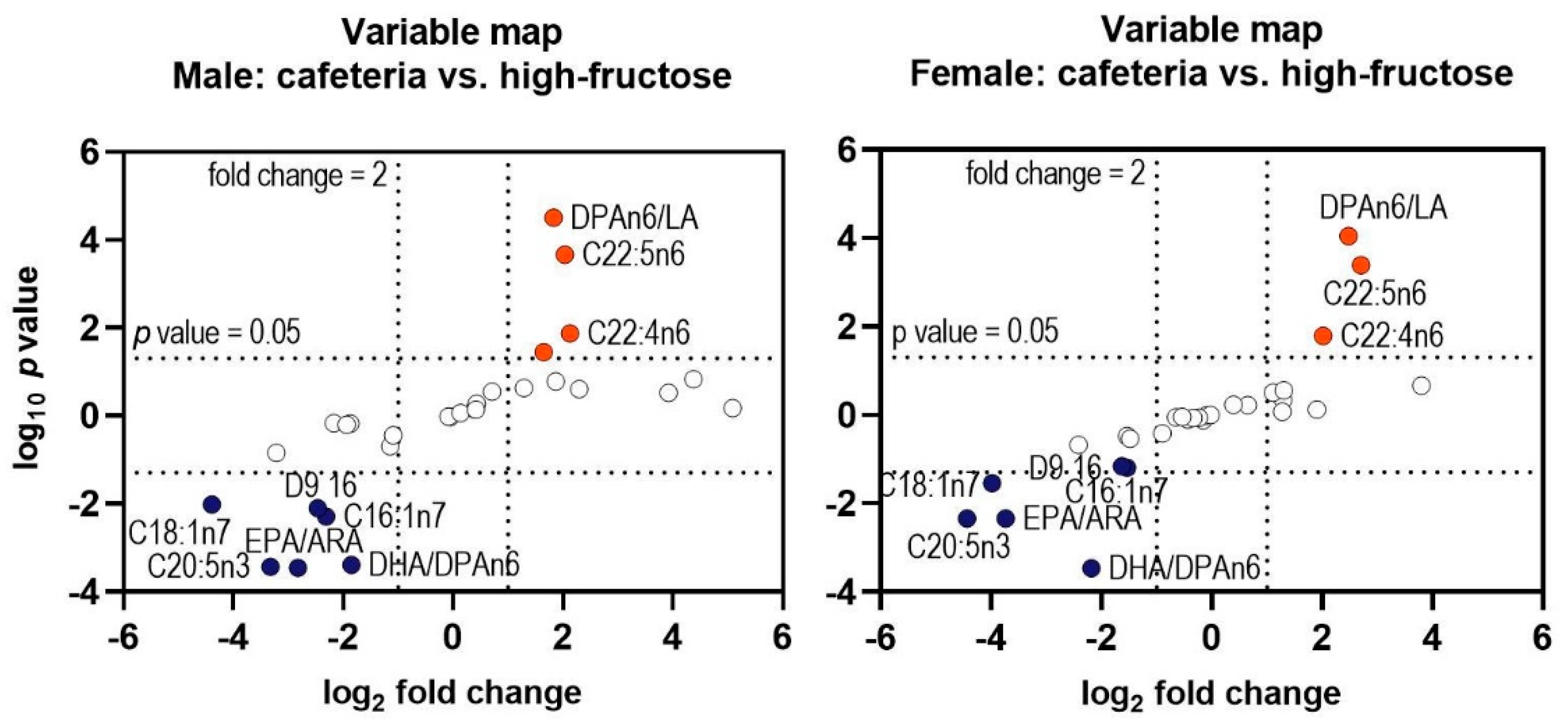
3.4. Hepatic and Perirenal Fat Fatty Acid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bertot, L.C.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver Disease-A Starter’s Guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- McManus, S.; Tejera, N.; Awwad, K.; Vauzour, D.; Rigby, N.; Fleming, I.; Cassidy, A.; Minihane, A.M. Differential effects of EPA versus DHA on postprandial vascular function and the plasma oxylipin profile in men. J. Lipid Res. 2016, 57, 1720–1727. [Google Scholar] [CrossRef]
- Allaire, J.; Vors, C.; Tremblay, A.J.; Marin, J.; Charest, A.; Tchernof, A.; Couture, P.; Lamarche, B. High-Dose DHA Has More Profound Effects on LDL-Related Features Than High-Dose EPA: The ComparED Study. J. Clin. Endocrinol. Metab. 2018, 103, 2909–2917. [Google Scholar] [CrossRef]
- Urlić, M.; Urlić, I.; Urlić, H.; Mašek, T.; Benzon, B.; Vitlov Uljević, M.; Vukojević, K.; Filipović, N. Effects of Different n6/n3 PUFAs Dietary Ratio on Cardiac Diabetic Neuropathy. Nutrients 2020, 12, 2761. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- Starčević, K.; Filipović, N.; Galan, A.; Micek, V.; Kurilj, A.G.; Mašek, T. Hepatic Lipogenesis and Brain Fatty Acid Profile in Response to Different Dietary n6/n3 Ratios and DHA/EPA Supplementation in Streptozotocin Treated Rats. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Hawkins, R.C. Evaluation of Roche Accu-Chek Go and Medisense Optium blood glucose meters. Clin. Chim. Acta 2005, 353, 127–131. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.J.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology-a systematic review. BMC Veter Res. 2013, 9, 123. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆ CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Quideau, S.; McIntosh, A.C.; Norris, C.E.; Lloret, E.; Swallow, M.J.; Hannam, K. Extraction and Analysis of Microbial Phospholipid Fatty Acids in Soils. J. Vis. Exp. 2016, 10, e54360. [Google Scholar] [CrossRef]
- Jouihan, H. Measurement of Liver Triglyceride Content. Bio-Protocol 2012, 2. [Google Scholar] [CrossRef]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. METABOLIC PHENOTYPING GUIDELINES: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Mašek, T.; Filipović, N.; Vuica, A.; Starčević, K. Effects of treatment with sucrose in drinking water on liver histology, lipogenesis and lipogenic gene expression in rats fed high-fiber diet. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Jensen, V.S.; Hvid, H.; Damgaard, J.; Nygaard, H.; Ingvorsen, C.; Wulff, E.M.; Lykkesfeldt, J.; Fledelius, C. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague–Dawley rats. Diabetol. Metab. Syndr. 2018, 10, 4. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Liver Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2016, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Comte, C.; Bellenger, S.; Bellenger, J.; Tessier, C.; Poisson, J.; Narce, M. Effects of streptozotocin and dietary fructose on delta-6 desaturation in spontaneously hypertensive rat liver. Biochimie 2004, 86, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Drąg, J.; Goździalska, A.; Knapik-Czajka, M.; Gawedzka, A.; Gawlik, K.; Jaśkiewicz, J. Effect of high carbohydrate diet on elongase and desaturase activity and accompanying gene expression in rat’s liver. Genes Nutr. 2017, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Karahashi, M.; Sakamoto, T.; Tsuji, Y.; Yamazaki, T.; Okazaki, M.; Mitsumoto, A.; Kudo, N.; Kawashima, Y. Fatty Acid β-Oxidation Plays a Key Role in Regulating cis-Palmitoleic Acid Levels in the Liver. Biol. Pharm. Bull. 2016, 39, 1995–2008. [Google Scholar] [CrossRef]
- Yamada, K.; Mizukoshi, E.; Seike, T.; Horii, R.; Terashima, T.; Iida, N.; Kitahara, M.; Sunagozaka, H.; Arai, K.; Yamashita, T.; et al. Serum C16:1n7/C16:0 ratio as a diagnostic marker for non-alcoholic steatohepatitis Diagnosis of NASH using serum fatty acid. J. Gastroenterol. Hepatol. 2019, 34, 1829–1835. [Google Scholar] [CrossRef]
- Montanaro, M.A.; Bernasconi, A.M.; González, M.S.; Rimoldi, O.J.; Brenner, R.R. Effects of fenofibrate and insulin on the biosynthesis of unsaturated fatty acids in streptozotocin diabetic rats. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 369–378. [Google Scholar] [CrossRef]
- Brenner, R.R.; Rimoldi, O.J.; Lombardo, Y.B.; González, M.S.; Bernasconi, A.M.; Chicco, A.; Basabe, J.C. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 2003, 38, 733–742. [Google Scholar] [CrossRef]
- El Hafidi, M.; Cuéllar, A.; Ramírez, J.; Baños, G. Effect of sucrose addition to drinking water, that induces hypertension in the rats, on liver microsomal ∆9 and Δ5-desaturase activities. J. Nutr. Biochem. 2001, 12, 396–403. [Google Scholar] [CrossRef]
- Pérez, I.; El Hafidi, M.; Carvajal, K.; Baños, G. Castration modifies aortic vasoreactivity and serum fatty acids in a sucrose-fed rat model of metabolic syndrome. Hear. Vessel. 2009, 24, 147–155. [Google Scholar] [CrossRef]
- Fard, S.G.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2018, 59, 1684–1727. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Satoh-Asahara, N.; Yamakage, H.; Sasaki, Y.; Odori, S.; Kono, S.; Wada, H.; Suganami, T.; Ogawa, Y.; Hasegawa, K.; et al. An Increase in the EPA/AA Ratio is Associated with Improved Arterial Stiffness in Obese Patients with Dyslipidemia. J. Atheroscler. Thromb. 2014, 21, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, Y.; Motoyama, S.; Sarai, M.; Ito, H.; Kawai, H.; Takakuwa, Y.; Miyagi, M.; Shibata, D.; Takahashi, H.; Naruse, H.; et al. Eicosapentaenoic acid to arachidonic acid (EPA/AA) ratio as an associated factor of high risk plaque on coronary computed tomography in patients without coronary artery disease. Atherosclerosis 2016, 250, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Park, T.; Barnes, J.; Kevala, K.; Chen, H.; Kim, H.-Y. Reduced acute neuroinflammation and improved functional recovery after traumatic brain injury by α-linolenic acid supplementation in mice. J. Neuroinflamm. 2016, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mašek, T.; Barišić, J.; Micek, V.; Starčević, K. Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex. Nutrients 2020, 12, 3339. https://doi.org/10.3390/nu12113339
Mašek T, Barišić J, Micek V, Starčević K. Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex. Nutrients. 2020; 12(11):3339. https://doi.org/10.3390/nu12113339
Chicago/Turabian StyleMašek, Tomislav, Josip Barišić, Vedran Micek, and Kristina Starčević. 2020. "Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex" Nutrients 12, no. 11: 3339. https://doi.org/10.3390/nu12113339
APA StyleMašek, T., Barišić, J., Micek, V., & Starčević, K. (2020). Cafeteria Diet and High-Fructose Rodent Models of NAFLD Differ in the Metabolism of Important PUFA and Palmitoleic Acid without Additional Influence of Sex. Nutrients, 12(11), 3339. https://doi.org/10.3390/nu12113339






