Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect
Abstract
1. Introduction
2. Material and Method
2.1. Plant Material and Essential Oil Preparation
2.2. Animals
2.3. Chemical and Treatment
2.4. Inhalation Apparatus
2.5. Identification of the Constituents of Essential Oil
2.6. Behavioral Test
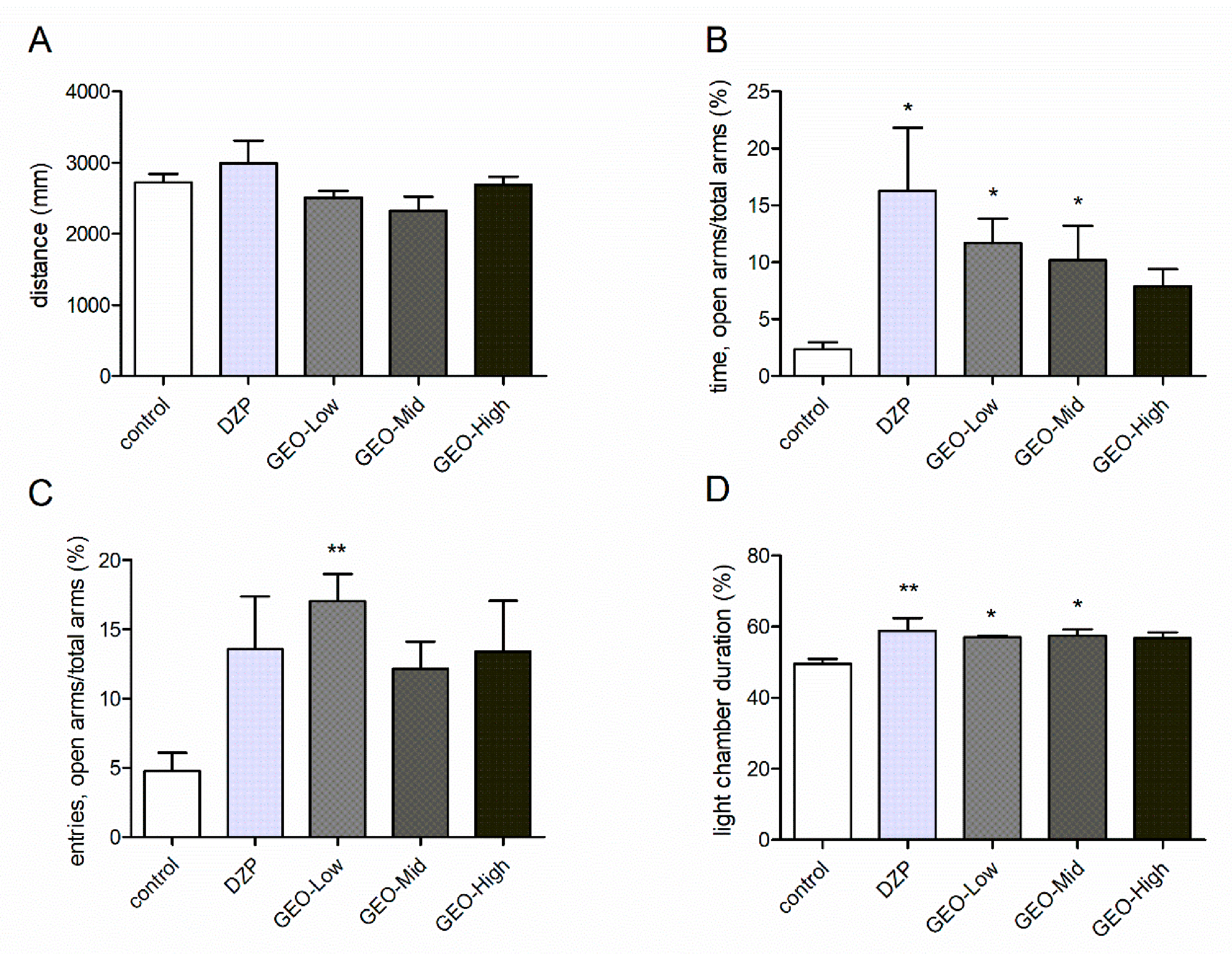
2.6.1. Open Field Test
2.6.2. Elevated Plus Maze Test
2.6.3. Light and Dark Box Test
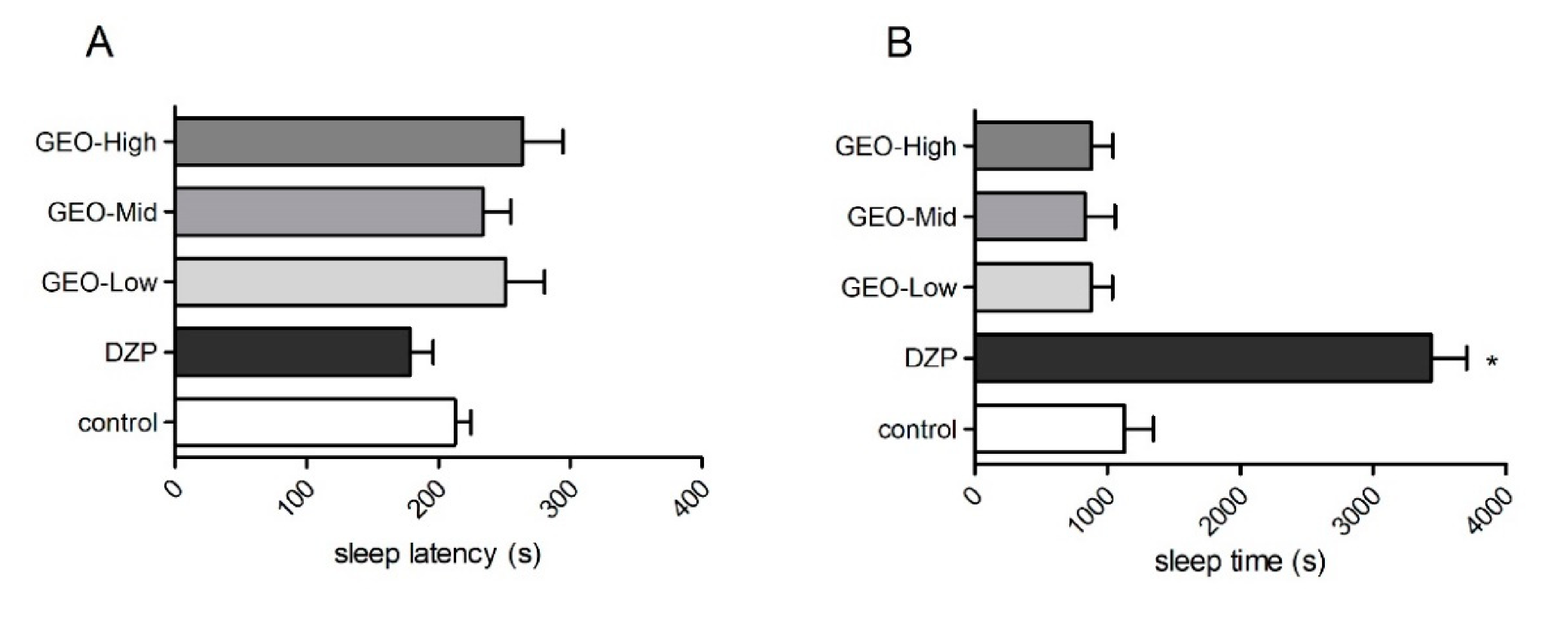
2.6.4. Pentobarbital Sodium Sleep Test
2.7. Quantification of Monoamines and Their Metabolites
2.8. Experimental Procedures
2.8.1. Effects of Odor Exposure on Anxiety Models and Pentobarbital Sodium Sleep Model
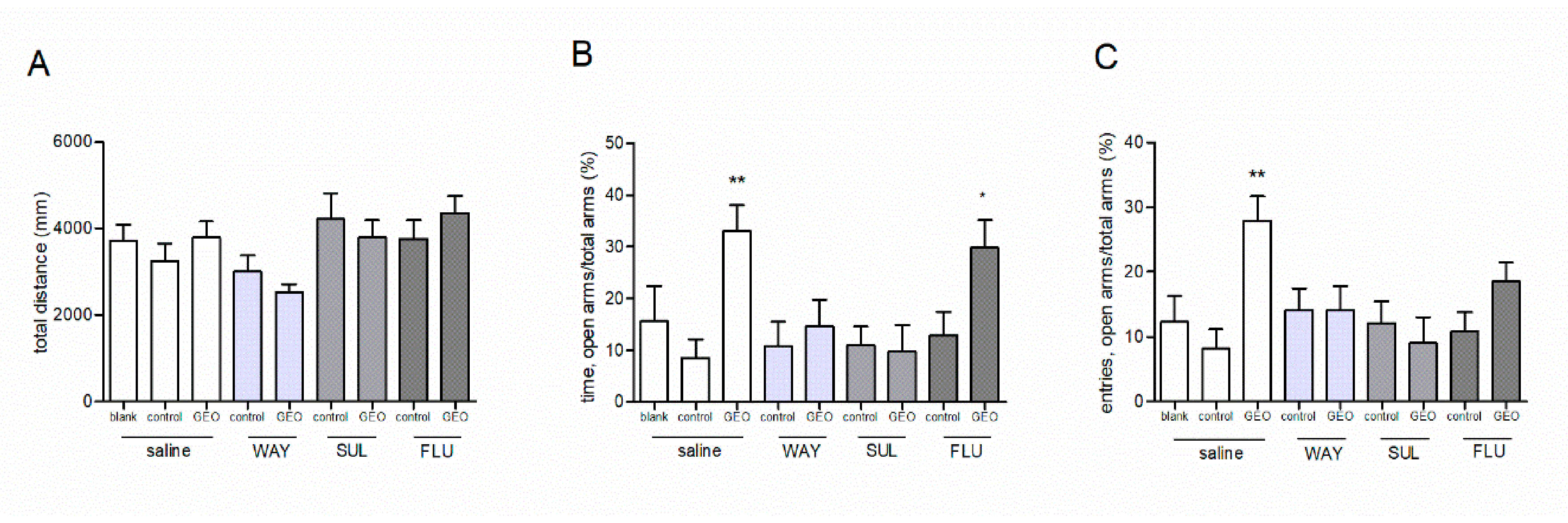
2.8.2. Study of the Involvement of Monoamine System
2.9. Statistical Analyses
3. Results
3.1. The Identification of Major Constituents of G. jasminoides Flower Essential Oil
3.2. Effects of GEO Exposure on Anxiety Animal Models
3.3. Effects of GEO Exposure in the Pentobarbital Sodium Sleep Test
3.4. Effects of Pretreatment of WAY100635, Sulpiride and Flumazenil on the Anxiolytic Effect of GEO
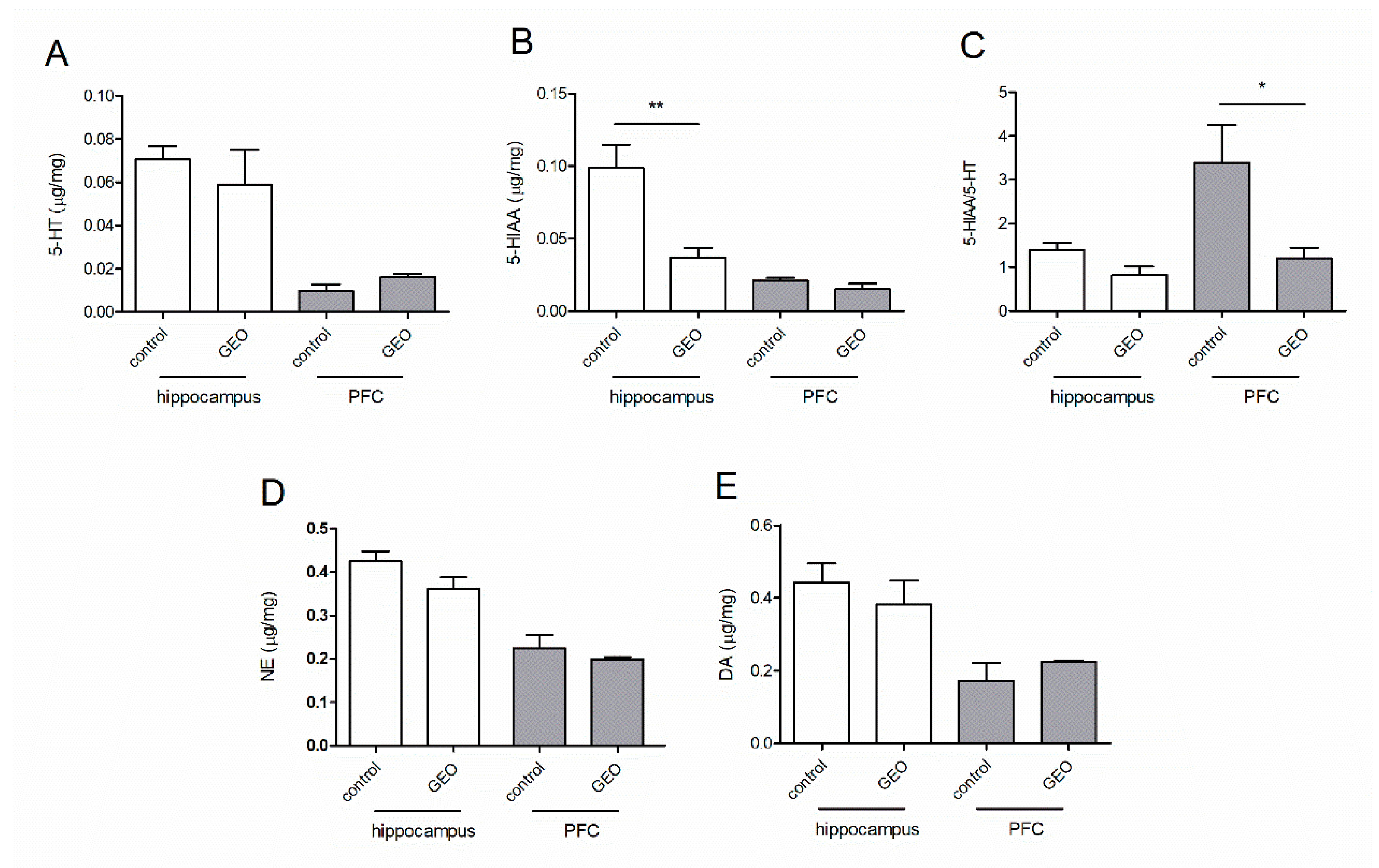
3.5. Effects of GEO on the Monoamine Neurotransmitters in Two Brain Regions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ni, Y.; Li, L.; Zhang, W.; Lu, D.; Zang, C.; Zhang, D.; Yu, Y.; Yao, X. Discovery and LC-MS characterization of new crocins in Gardeniae fructus and their neuroprotective potential. J. Agric. Food Chem. 2017, 65, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Tao, Y.; Wang, Y.Y.; Peng, I.F. Effects of Gardenia jasminoides extracts on cognition and innate immune response in an adult Drosophila model of Alzheimer’s disease. Chin. J. Nat. Med. 2017, 15, 899–904. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, W.Y.; Jiang, Y.N.; Zhang, Y.; Pan, D.B.; Zhang, D.; Yao, X.S.; Yu, Y. Two new triterpenoids from Gardenia jasminoides fruits. Nat. Prod. Res. 2019, 33, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-Isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, J.; Niwatananun, W.; Somna, S.; Vejabhikul, S.; Chansakaow, S. Volatile constituents and biological activities of Gardenia Jasminoides. J. Health Res. 2009, 23, 141–145. [Google Scholar]
- Zhang, K.; Yao, L. The anxiolytic effect of Juniperus virginiana L. essential oil and determination of its active constituents. Physiol. Behav. 2018, 189, 50–58. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Satou, T.; Miyahara, N.; Murakami, S.; Hayashi, S.; Koike, K. Differences in the effects of essential oil fromCitrus junosand (+)-limonene on emotional behavior in mice. J. Essent. Oil Res. 2012, 24, 493–500. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef]
- Sirotin, Y.B.; Shusterman, R.; Rinberg, D. Neural coding of perceived odor intensity. eNeuro 2015, 2, 1–16. [Google Scholar] [CrossRef]
- Shaw, D.; Annett, J.M.; Doherty, B.; Leslie, J.C. Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine 2007, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lillehei, A.S.; Halcon, L.L.; Savik, K.; Reis, R. Effect of Inhaled Lavender and Sleep Hygiene on Self-Reported Sleep Issues: A Randomized Controlled Trial. J. Altern. Complement. Med. 2015, 21, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Takeuchi, T.; Harada, E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006, 172, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Matsumoto, T.; Motomura, E.; Shiroyama, T. The sleep-enhancing effect of valerian inhalation and sleep-shortening effect of lemon inhalation. Chem. Senses 2006, 31, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Piato, A.L.; Herrmann, A.P.; Dupont Birck, F.; Caramao, E.B.; Nunes, D.S.; Moreno, P.R.; Elisabetsky, E. Inhaled linalool-induced sedation in mice. Phytomedicine 2009, 16, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Yun, J. Limonene inhibits methamphetamine-induced locomotor activity via regulation of 5-HT neuronal function and dopamine release. Phytomedicine 2014, 21, 883–887. [Google Scholar] [CrossRef]
- Lima, N.G.; De Sousa, D.P.; Pimenta, F.C.; Alves, M.F.; De Souza, F.S.; Macedo, R.O.; Cardoso, R.B.; de Morais, L.C.; Melo Diniz Mde, F.; de Almeida, R.N. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef]
- Parente, M.S.R.; Custodio, F.R.; Cardoso, N.A.; Lima, M.J.A.; Melo, T.S.; Linhares, M.I.; Siqueira, R.M.P.; Nascimento, A.A.D.; Catunda Junior, F.E.A.; Melo, C.T.V. Antidepressant-Like Effect of Lippia sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 1–19. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Kadkhoda, Z.; Taghizad-Farid, R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. eJTCM 2020, 10, 327–335. [Google Scholar] [CrossRef]
- de Almeida, A.A.; de Carvalho, R.B.; Silva, O.A.; de Sousa, D.P.; de Freitas, R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014, 118, 69–78. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; de Oliveira, G.A.; de Almeida, A.A.; Islam, M.T.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of phytol: Possible involvement of GABAergic transmission. Brain Res. 2014, 1547, 34–42. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Gardenia jasminoides essential oil are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
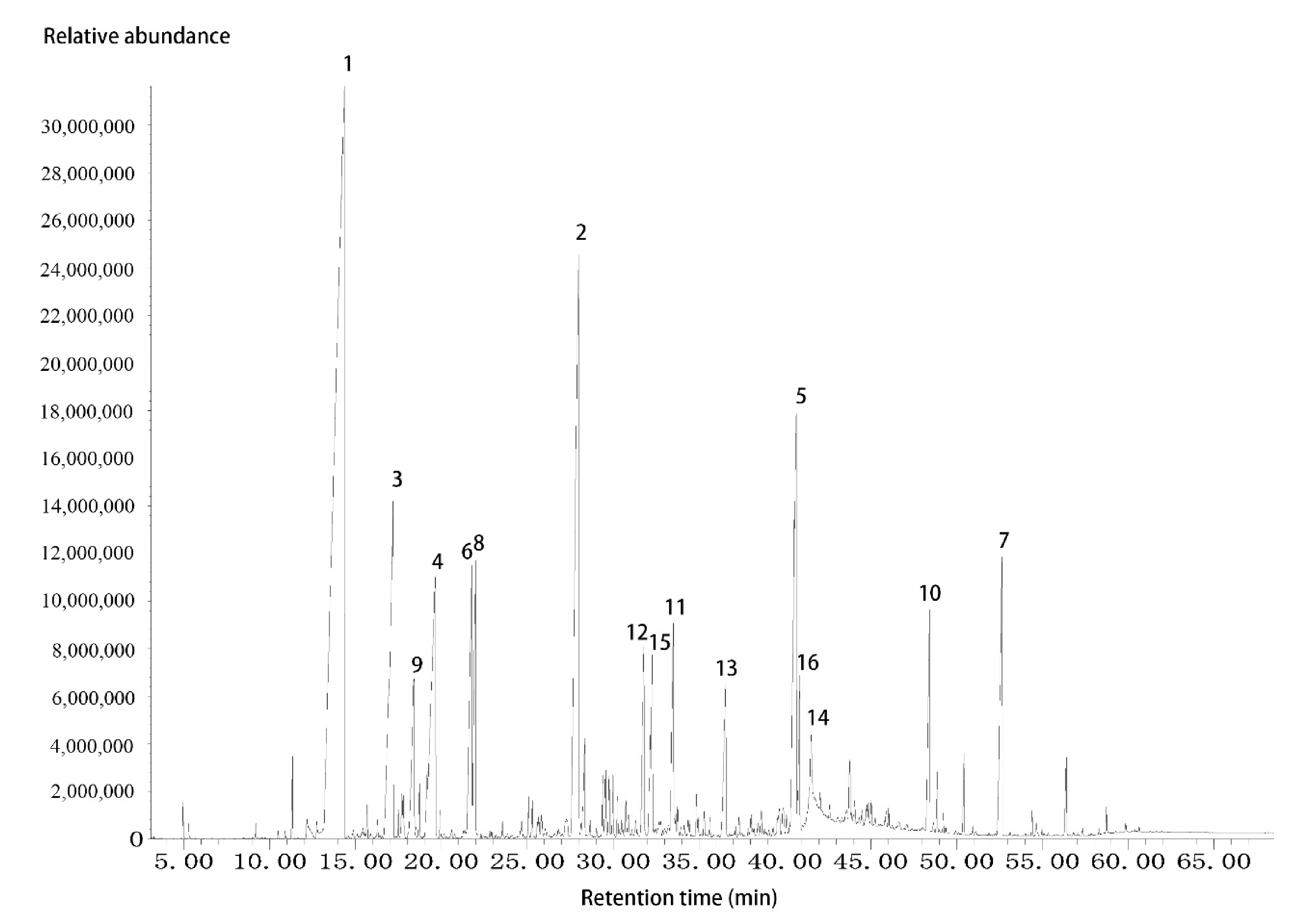
| Peak No. | Retention Time (Min) | Compound | Peak Area (%) |
|---|---|---|---|
| 1 | 14.35 | Linalool | 34.7 |
| 2 | 28.00 | α-Farnesene | 10.2 |
| 3 | 17.19 | α-Terpineol | 6.3 |
| 4 | 19.66 | Geraniol | 5.8 |
| 5 | 40.67 | Cembrene A | 5.8 |
| 6 | 21.78 | cis-3-Hexenyl tiglate | 3.1 |
| 7 | 52.66 | Pentacosane | 3.0 |
| 8 | 21.99 | Hexyl tiglate | 2.4 |
| 9 | 18.41 | Nerol | 2.0 |
| 10 | 48.44 | Tricosane | 1.9 |
| 11 | 33.51 | Geranyl angelate | 1.9 |
| 12 | 31.79 | tau.-Cadinol | 1.8 |
| 13 | 36.57 | 8-Hydroxylinalool | 1.7 |
| 14 | 41.55 | n-Hexadecanoic acid | 1.3 |
| 15 | 32.30 | α-Terpinyl acetate | 1.2 |
| 16 | 40.86 | Verticiol | 1.0 |
| Sum | 84.0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Luo, M.; He, L.; Yao, L. Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect. Molecules 2020, 25, 4702. https://doi.org/10.3390/molecules25204702
Zhang N, Luo M, He L, Yao L. Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect. Molecules. 2020; 25(20):4702. https://doi.org/10.3390/molecules25204702
Chicago/Turabian StyleZhang, Nan, Mu Luo, Lei He, and Lei Yao. 2020. "Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect" Molecules 25, no. 20: 4702. https://doi.org/10.3390/molecules25204702
APA StyleZhang, N., Luo, M., He, L., & Yao, L. (2020). Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect. Molecules, 25(20), 4702. https://doi.org/10.3390/molecules25204702






