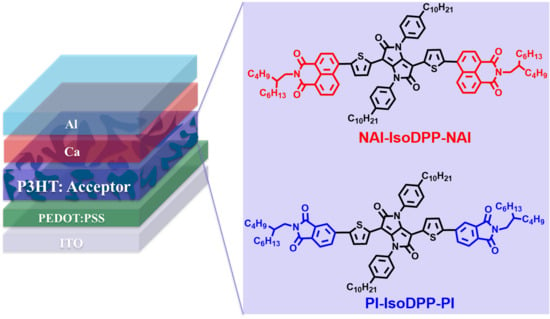
Pyrrolo[3,2-b]pyrrole-1,4-dione (IsoDPP) End Capped with Napthalimide or Phthalimide: Novel Small Molecular Acceptors for Organic Solar Cells
Abstract
1. Introduction
2. Results and Discussion
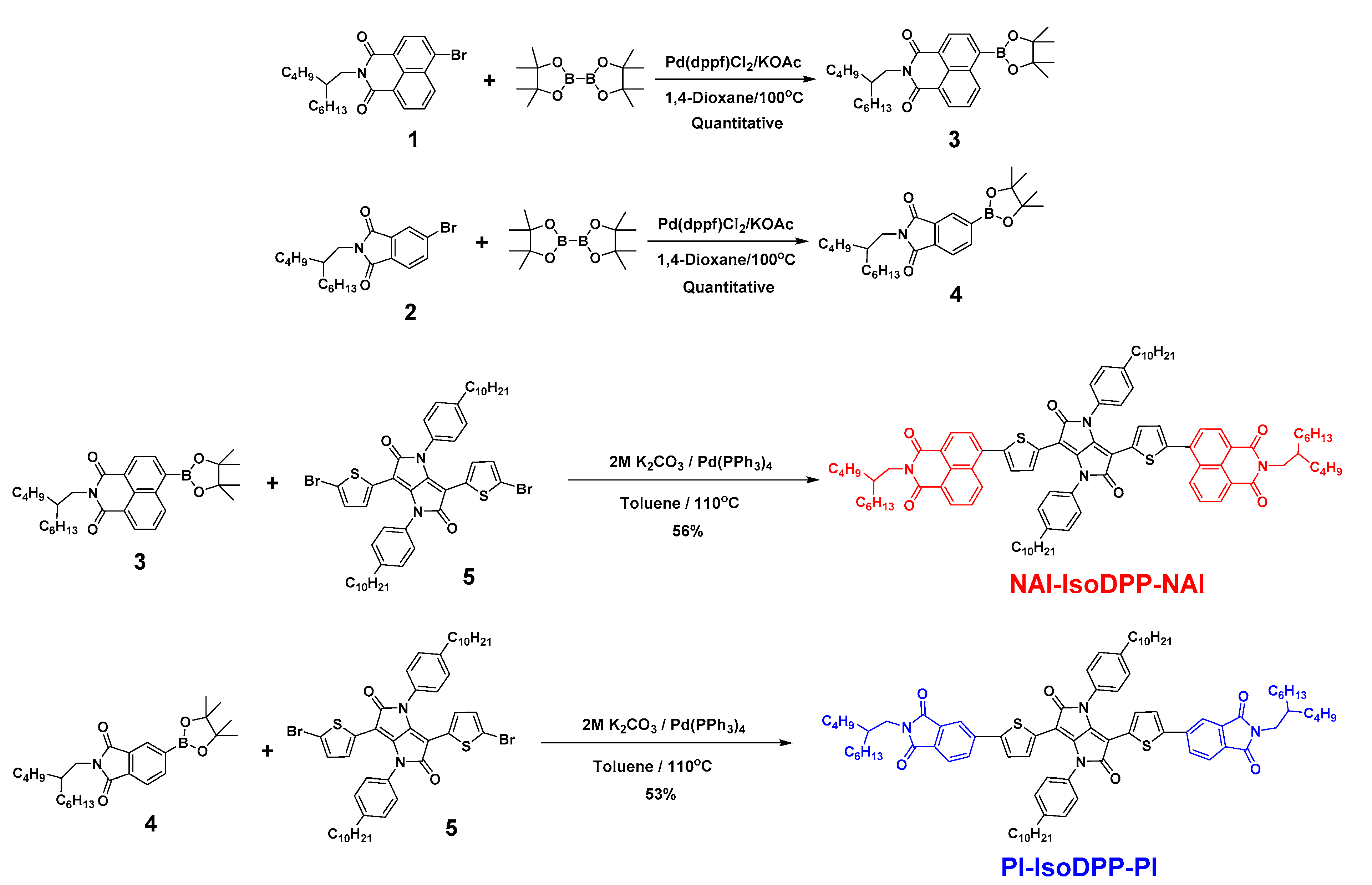
2.1. New Non-Fullerene Acceptor Molecular Design and Synthesis
2.2. Thermal Properties
2.3. Optical Properties
2.4. Electrochemical Properties
2.5. Ab Initio Calculations
2.6. Photovoltaic Properties
3. Conclusions
4. Experimental Section
4.1. Materials and Instruments
4.2. Device Fabrication and Testing
4.3. Synthesis
4.3.1. Synthesis of Compound 3
4.3.2. Synthesis of Compound 4
4.3.3. Synthesis of Compound 5
4.3.4. Synthesis of NAI-IsoDPP-NAI
4.3.5. Synthesis of PI-IsoDPP-PI
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwon, O.K.; Uddin, M.A.; Park, J.H.; Park, S.K.; Nguyen, T.L.; Woo, H.Y.; Park, S.Y. A High Efficiency Nonfullerene Organic Solar Cell with Optimized Crystalline Organizations. Adv. Mater. 2016, 28, 910–916. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganas, O.; Gao, F.; Hou, J. Free Polymer Solar Cells with over 11% Efficiency and Excellent Thermal Stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhang, D.; Li, M.; Zhong, W.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% Efficiency for Single-Junction Organic Solar Cells. Sci. China Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% Efficiency Organic Photovoltaic Cells Enabled by a Chlorinated Acceptor with Increased Open-Circuit Voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Do, T.T.; Subbiah, J.; Manzhos, S.; Jones, J.; Bell, M.; Sonar, P. Phthalimide and Naphthalimide: Effect of End-Capping Groups on Molecular Properties and Photovoltaic Performance of 9-Fluorenone Based Acceptors for Organic Solar Cells. Org. Electron. 2018, 62, 12–20. [Google Scholar] [CrossRef]
- Tan, C.H.; Gorman, J.; Wadsworth, A.; Holliday, S.; Subramaniyan, S.; Jenekhe, S.A.; Baran, D.; McCulloch, I.; Durrant, J.R. Barbiturate End-Capped Non-Fullerene Acceptors for Organic Solar Cells: Tuning Acceptor Energetics to Suppress Geminate Recombination Losses. Chem. Commun. 2018, 54, 2966–2969. [Google Scholar] [CrossRef] [PubMed]
- Schwenn, P.E.; Gui, K.; Nardes, A.M.; Krueger, K.B.; Lee, K.H.; Mutkins, K.; Rubinstein-Dunlop, H.; Shaw, P.E.; Kopidakis, N.; Burn, P.L.; et al. A Small Molecule Non-fullerene Electron Acceptor for Organic Solar Cells. Adv. Energy Mater. 2011, 1, 73–81. [Google Scholar] [CrossRef]
- Fang, Y.; Pandey, A.K.; Lyons, D.M.; Shaw, P.E.; Watkins, S.E.; Burn, P.L.; Lo, S.C.; Meredith, P. Tuning the Optoelectronic Properties of Nonfullerene Electron Acceptors. Chem. Phys. Chem. 2015, 16, 1295–1304. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Li, Q.-S.; Li, Z.-S. End-Capped Group Manipulation of Fluorene-based Small Molecule Acceptors for Efficient Organic Solar Cells. Comput. Mater. Sci. 2019, 156, 252–259. [Google Scholar] [CrossRef]
- Cao, H.; Bauer, N.; Pang, C.; Rech, J.; You, W.; Rupar, P.A. End-cap Group Engineering of a Small Molecule Non-Fullerene Acceptor: The Influence of Benzothiophene Dioxide. ACS Appl. Mater. Interfaces 2018, 1, 7146–7152. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; et al. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Sonar, P.; Dodabalapur, A. Charge Transport Study of High Mobility Polymer Thin-Film Transistors based on Thiophene Substituted Diketopyrrolopyrrole Copolymers. Phys. Chem. Chem. Phys. 2013, 15, 9735–9741. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.; Rundel, K.; Gu, Q.; Gann, E.; Manzhos, S.; Feron, K.; Bell, J.; McNeill, C.R.; Sonar, P. 9-Fluorenone and 9,10-Anthraquinone Potential Fused Aromatic Building Blocks to Synthesize Electron Acceptors for Organic Solar Cells. New J. Chem. 2017, 41, 2899–2909. [Google Scholar] [CrossRef]
- Wang, B.; Huynh, T.P.; Wu, W.; Hayek, N.; Do, T.T.; Cancilla, J.C.; Torrecilla, J.S.; Nahid, M.M.; Colwell, J.M.; Gazit, O.M.; et al. A Highly Sensitive Diketopyrrolopyrrole-Based Ambipolar Transistor for Selective Detection and Discrimination of Xylene Isomers. Adv. Mater. 2016, 28, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sonar, P.; Murphya, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Sonar, P.; Ha, T.J.; Dodabalapur, A. Synthesis, Characterization and Organic Field Effect Transistor Performance of a Diketopyrrolopyrrole-Fluorenone Copolymer. Phys. Chem. Chem. Phys. 2013, 15, 7475–7478. [Google Scholar] [CrossRef]
- Privado, M.; de la Cruz, P.; Biswas, S.; Singhal, R.; Sharma, G.D.; Langa, F. A Non-Fullerene All Small Molecule Solar Cell Constructed with a Diketopyrrolopyrrole-based Acceptor Having a Power Conversion Efficiency Higher than 9% and an Energy Loss of 0.54 eV. J. Mater. Chem. A 2018, 6, 11714–11724. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Gao, K.; Liu, F.; Yao, H.; Yang, B.; He, C.; Russell, T.P.; Hou, J. Low Band-Gap Conjugated Polymer based on Diketopyrrolopyrrole Units and Its Application in Organic Photovoltaic Cells. J. Mater. Chem. A 2017, 5, 10416–10423. [Google Scholar] [CrossRef]
- Wang, H.; Yue, Q.; Nakagawa, T.; Zieleniewska, A.; Okada, H.; Ogumi, K.; Ueno, H.; Dirk, M.G.; Zhu, X.; Matsuo, Y. Star-Shaped Magnesium Tetraethynylporphyrin Bearing Four Peripheral Electron-Accepting Diketopyrrolopyrrole Functionalities for Organic Solar Cells. J. Mater. Chem. A 2019, 7, 4072–4083. [Google Scholar] [CrossRef]
- Gendron, D.; Gann, E.; Pattison, K.; Maasoumi, F.; McNeill, C.R.; Watkins, S.E.; Burn, P.L.; Powell, B.J.; Shaw, P.E. Synthesis and Properties of Pyrrolo[3,2-b]pyrrole-1,4-diones (isoDPP) Derivatives. J. Mater. Chem. C 2014, 2, 4276–4288. [Google Scholar] [CrossRef]
- Wuckelt, J.; Döring, M.; Langer, P.; Görls, H.; Beckert, R. Lactam analogues of pentalene. A new one-pot synthesis of pyrrolo[3,2-b]pyrrole-2,5-diones deriving from pulvinic acid. Tetrahedron Lett. 1997, 38, 5269–5272. [Google Scholar] [CrossRef]
- Langer, P.; Wuckelt, J.; Döring, M. New and Efficient Synthesis of Pyrrolo[3,2-b]pyrrole-2,5-diones by Double-Anion-Capture Reactions of Ester Carbanions with Bis(imidoyl)chlorides of Oxalic Acid. J. Org. Chem. 2000, 65, 729–734. [Google Scholar] [CrossRef]
- Song, S.; Ko, S.-J.; Shin, H.; Jin, Y.; Kim, I.; Kim, J.Y.; Suh, H. Pyrrolo[3,2-b]pyrrole Based Small Molecules as Donor Materials for OPVs. Sol. Energy Mater. Sol.Cells 2013, 112, 120–126. [Google Scholar] [CrossRef]
- Lu, S.; Drees, M.; Yao, Y.; Boudinet, D.; Yan, H.; Pan, H.; Wang, J.; Li, Y.; Usta, H.; Facchetti, A. 3,6-Dithiophen-2-yl-diketopyrrolo[3,2-b]pyrrole (isoDPPT) as an Acceptor Building Block for Organic Opto-Electronics. Macromolecules 2013, 46, 3895–3906. [Google Scholar] [CrossRef]
- Han, M.H.; Song, H.J.; Lee, T.H.; Lee, J.Y.; Moon, D.K.; Haw, J.R. White Polymer Light-Emitting Diode Materials with Efficient Electron Injection Backbone Containing Polyfluorene, Oxadiazole and Quinoxaline Derivatives. Synth. Met. 2012, 162, 2294–2301. [Google Scholar] [CrossRef]
- Do, T.T.; Pham, H.D.; Manzhos, S.; Bell, J.M.; Sonar, P. Molecular Engineering Strategy for High Efficiency Fullerene-Free Organic Solar Cells Using Conjugated 1,8-Naphthalimide and Fluorenone Building Blocks. ACS Appl. Mater. Interfaces 2017, 9, 16967–16976. [Google Scholar] [CrossRef]
- Welterlich, I.; Charov, O.; Tieke, B. Deeply Colored Polymers Containing 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (IsoDPP) Units in the Main Chain. Macromolecules 2012, 45, 4511–4519. [Google Scholar] [CrossRef]
- Pham, H.D.; Jain, S.M.; Li, M.; Manzhos, S.; Feron, K.; Pitchaimuthu, S.; Liu, Z.; Motta, N.; Wang, H.; Durrant, J.R.; et al. Dopant-free Novel Hole-Transporting Materials Based on Quinacridone Dye for High-Performance and Humidity-Stable Mesoporous Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 5315–5323. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.-P.; Rutledge, L.R.; Hill, I.G.; Welch, G.C. Electron Deficient Diketopyrrolopyrrole Dyes for Organic Electronics: Synthesis by Direct Arylation, Optoelectronic Characterization, and Charge Carrier Mobility. J. Mater. Chem. A 2014, 2, 4198–4207. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, X.; Tian, H.; Geng, Y.; Wang, F. Donor–Acceptor–Donor Conjugated Oligomers Based on Isoindigo and Anthra[1,2-b]thieno[2,3-d]-thiophene for Organic Thin-Film Transistors: The Effect of The Alkyl Side Chain Length on Semiconducting Properties. J. Mater. Chem. C 2015, 3, 7567–7574. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Burkhart, B.; Thompson, B.C. Efficient Ternary Blend Bulk Heterojunction Solar Cells with Tunable Open-Circuit Voltage. J. Am. Chem. Soc. 2011, 133, 14534–14537. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Armin, A.; Yazmaciyan, A.; Hambsch, M.; Li, J.; Burn, P.L.; Meredith, P. Electro-Optics of Conventional and Inverted Thick Junction Organic Solar Cells. ACS Photonics 2015, 2, 1745–1754. [Google Scholar] [CrossRef]
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2011, 45, 723–733. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open Circuit Voltage of Organic Solar Cells: An in-Depth Review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Fei, Z.; Boufflet, P.; Wood, S.; Wade, J.; Moriarty, J.; Gann, E.; Ratcliff, E.L.; McNeill, C.R.; Sirringhaus, H.; Kim, J.S.; et al. Influence of Backbone Fluorination in Regioregular Poly(3-alkyl-4-fluoro)thiophenes. J. Am. Chem. Soc. 2015, 137, 6866–6879. [Google Scholar] [CrossRef]
- Sartorio, C.; Campisciano, V.; Chiappara, C.; Cataldo, S.; Scopelliti, M.; Gruttadauria, M.; Giacalone, F.; Pignataro, B. Enhanced Power-conversion Efficiency in Organic Solar Cells Incorporating Copolymeric Phase-Separation Modulators. J. Mater. Chem. A 2018, 6, 3884–3894. [Google Scholar] [CrossRef]
- Sonar, P.; Ha, T.J.; Dodabalapur, A. A Fluorenone Based Low Band Gap Solution Processable Copolymer for Air Stable and High Mobility Organic Field Effect Transistors. Chem. Commun. 2013, 49, 1588–1590. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds NAI-IsoDPP-NAI, and PI-IsoDPP-PI are available from the authors. |








| Compound | λmax (nm) | IP (eV) | EA (eV) | |||
|---|---|---|---|---|---|---|
| Solution | Film | |||||
| NAI-IsoDPP-NAI | 464 | 514 | 2.0 | 2.0 | 5.8 | 3.8 |
| PI-IsoDPP-PI | 469 | 486 | 2.1 | 2.0 | 5.8 | 3.8 |
| Active Layer Blend | Acceptor Loading (%) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| P3HT: NAI-IsoDPP-NAI | 50 | 1.17 ± 0.107 | 0.80 ± 0.012 | 43 ± 1.3 | 0.40 ± 0.030 |
| 75 | 1.13 ± 0.060 | 0.88 ± 0.001 | 62 ± 1.7 | 0.62 ± 0.027 | |
| 80 | 1.57 ± 0.051 | 0.92 ± 0.006 | 63 ± 1.4 | 0.91 ± 0.050 | |
| P3HT: PI-IsoDPP-PI | 50 | 1.33 ± 0.136 | 0.92 ± 0.012 | 39 ± 0.9 | 0.47 ± 0.051 |
| 75 | 0.81 ± 0.076 | 0.98 ± 0.002 | 50 ± 0.6 | 0.39 ± 0.033 | |
| 80 | 0.68 ± 0.055 | 0.95 ± 0.024 | 43 ± 3.0 | 0.28 ± 0.045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.T.; Stephen, M.; Chan, K.L.; Manzhos, S.; Burn, P.L.; Sonar, P. Pyrrolo[3,2-b]pyrrole-1,4-dione (IsoDPP) End Capped with Napthalimide or Phthalimide: Novel Small Molecular Acceptors for Organic Solar Cells. Molecules 2020, 25, 4700. https://doi.org/10.3390/molecules25204700
Do TT, Stephen M, Chan KL, Manzhos S, Burn PL, Sonar P. Pyrrolo[3,2-b]pyrrole-1,4-dione (IsoDPP) End Capped with Napthalimide or Phthalimide: Novel Small Molecular Acceptors for Organic Solar Cells. Molecules. 2020; 25(20):4700. https://doi.org/10.3390/molecules25204700
Chicago/Turabian StyleDo, Thu Trang, Meera Stephen, Khai Leok Chan, Sergei Manzhos, Paul L. Burn, and Prashant Sonar. 2020. "Pyrrolo[3,2-b]pyrrole-1,4-dione (IsoDPP) End Capped with Napthalimide or Phthalimide: Novel Small Molecular Acceptors for Organic Solar Cells" Molecules 25, no. 20: 4700. https://doi.org/10.3390/molecules25204700
APA StyleDo, T. T., Stephen, M., Chan, K. L., Manzhos, S., Burn, P. L., & Sonar, P. (2020). Pyrrolo[3,2-b]pyrrole-1,4-dione (IsoDPP) End Capped with Napthalimide or Phthalimide: Novel Small Molecular Acceptors for Organic Solar Cells. Molecules, 25(20), 4700. https://doi.org/10.3390/molecules25204700