Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances
Abstract
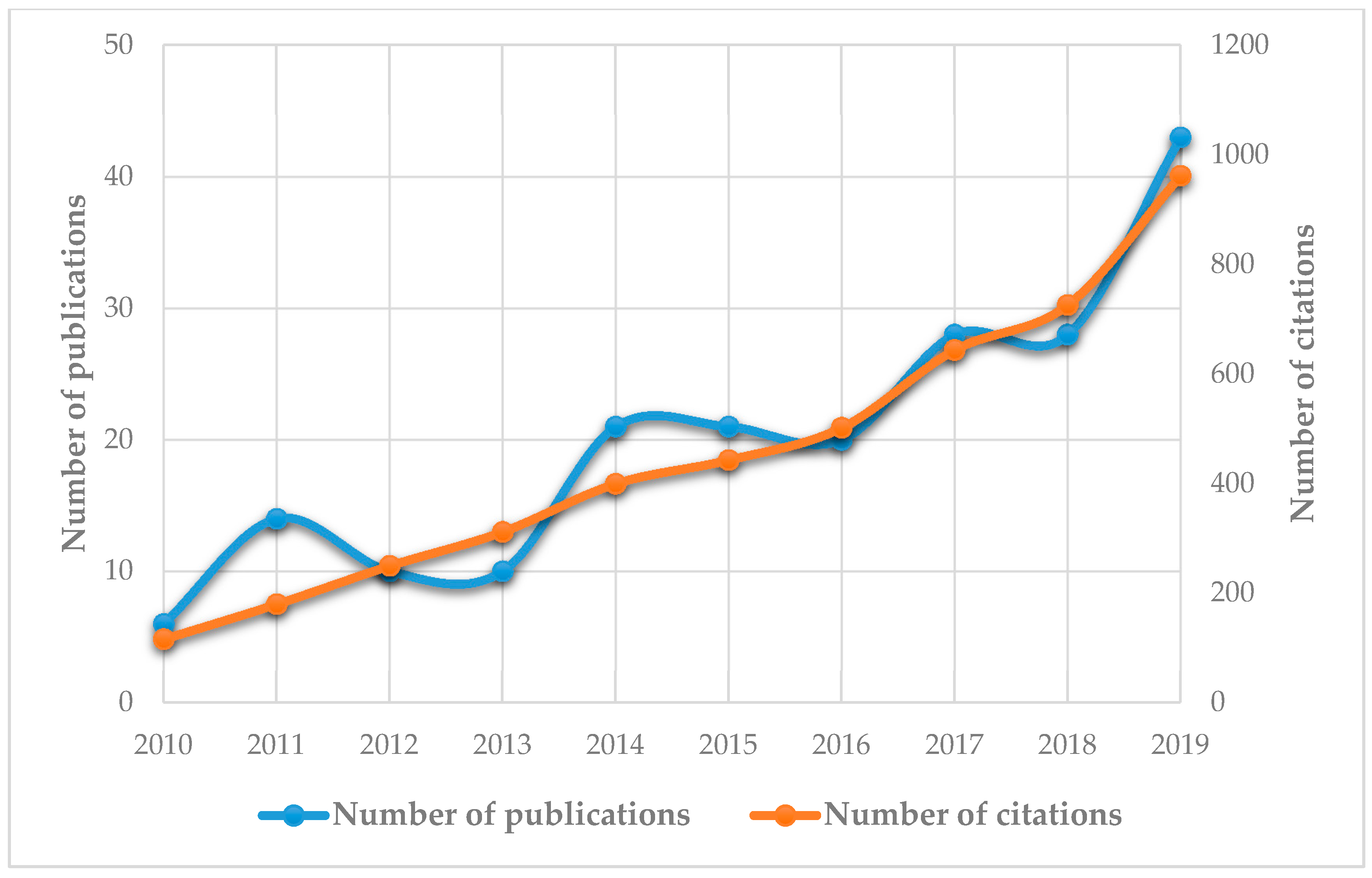
:1. Introduction
2. The Main Thermal and Non-Thermal Processing Techniques
2.1. Thermal Processing
2.2. Non-Thermal Processing
| Technology | Objective | Muscle Food Product | Main Findings | Reference |
|---|---|---|---|---|
| High pressure processing | Microbial inactivation | A. Chicken fillets | A. HPP (500 MPa/10 min) may enhance the safety and increase the shelf life (6 days at 4 °C and 2 days at 12 °C) | A. [93] |
| B. Salmon, cod, and mackerel fillets | B. Pressurizing at 500 MPa can extend shelf life; however, might have a negative effect on quality parameters | B. [94] | ||
| C. Rehydrated salted cod | C. HPP (600 MPa/5 min) of rehydrated fish prolonged the shelf life by at least 49 days | C. [95] | ||
| Structural modifications and improved functional properties | A. Chicken breast myofibrillar protein | A. Moderate HHP (200 MPa) improved MP solubility, gel hardness, WHC, and microstructures of thermal MP gel; stronger HHP treatment (≥300 MPa) weakened hardness while sharply decreasing WHC | A. [96] | |
| Ultrasound | Microbial reduction | A. Salmon, mackerel, and cod fillets | A. Ultrasound treatment for up to 45 min significantly reduced the natural microflora of salmon and mackerel | A. [97] |
| B. Chicken carcasses | B. Ultrasound bath (1200 W/130 Hz/15 min) reduced the Campylobacter count from 0.94 to 1.19 log10MPN (most probable number)/10 g | B. [98] | ||
| Physicochemical quality improvement | A. Restructured cooked ham with reduced salt | A. Ultrasound (600 W cm−2/10 min) showed good potential for production of low salt product by decreasing the total fluid release, increasing the hardness, and improving color, and sensory acceptance | A. [85] | |
| B. Meat emulsion | B. Ultrasound (25 KHz/18 min) improved the technological and reduced most of the sensory defects caused by the reduction of 50% of the phosphate level | B. [86] | ||
| Salting, curing, and marination | A. Chicken breast | A. Ultrasound-assisted sodium bicarbonate curing improved the curing rate, tenderness, and WHC | A. [99] | |
| B. Pork loins | B. The application of ultrasound increased the salt percentage without modifying the pH, shear force, or water holding capacity | B. [100] | ||
| Cold atmospheric plasma | Microbial decontamination | A. Beef jerky | A. A 2–3 Log CFU/g reduction in Escherichia coli, Listeria monocytogenes, Salmonella Typhimurium, Aspergillus flavus populations after flexible thin-layer plasma treatment for 10 min | A. [101] |
| B. Herring fillets | B. The microbial load (total aerobic mesophilic, total aerobic psychrotrophics, Pseudomonas, lactic acid bacteria and Enterobacteriaceae) were lower in the in-package treated samples compared the control | B. [102] | ||
| Curing | Ground ham | The remote infusion of CAP into meat batter rapidly generated nitrite and resulted in no difference in curing properties when compared with sodium nitrite | [103] | |
| Pulsed electric field | Tenderization | Beef briskets | PEF can be used to reduce the cooking time of tough meat cuts since it physically weakens the connective tissue and increase the collagen solubility | [104] |
| Extraction | Mussels | PEF accelerated the extraction speed and obviously improved the extraction yield of protein from mussel | [105] |
3. Conventional and Emerging Methods of Analysis of Quality Changes in Muscle Foods
3.1. Traditional Analysis Methods
3.2. Brief Overview of Spectroscopic Techniques
4. Use of Spectroscopic Techniques for Monitoring Changes in Muscle Foods during Processing
4.1. Fish and Other Seafoods
4.1.1. Thermal Treatments
4.1.2. Non-Thermal Treatments
4.2. Meat and Poultry Products
4.2.1. Thermal Treatments
4.2.2. Non-Thermal Treatments
| Muscle Foods | Analytical Technique | Chemometric Tool | Main Findings | Reference |
|---|---|---|---|---|
| Pork batters | NMR; FT-IR | ANOVA | With increasing temperatures, bonded-water converted into free water, WHC increased significantly, and α-helix transformed into β-sheets. Correlations were observed between spectral data and traditional parameters | [219] |
| Pork | NMR | PLSR | LF-NMR T2 relaxation is highly correlated to sensory attributes of pork. Reduction in juiciness and tenderness at 75 °C as compared to 65 °C was attributed to changes in the size of the pores confining the myofibrillar water within the meat in combination with an expulsion of water | [224] |
| Pork loins Longissimus dorsi | Fluorescence | ANOVA | Oxidative damages increased with increasing cooking temperatures and cooking times | [215] |
| Pork muscle | Raman; EEM | PCA, PLSR, PLS-DA | It was possible to classify stored samples according to cooking temperatures (below or above 65 °C) | [225] |
| Biceps femoris from Charolais cow | MRI | - | MRI allowed tracing thermal history of samples and provided insights into the mechanisms linking temperature, deformation, and water content during meat heating | [226] |
| Pork | LF-NMR | PCA | Heat-induced gelation of myofibrillar proteins led to diverse gel network structures. NMR T2 parameters and microstructural data were strongly correlated | [227] |
| Sheep meat | Raman | PLSR | Raman can be used to predict tenderness and cooking loss. High correlations were observed between Raman data and shear force and cooking loss | [228] |
| Porcine musculus longissimus dorsi | Fluorescence | ANCOVA | Modified atmosphere with high oxygen concentrations inhibited (quenched) the fluorescence emission of zinc protoporphyrin and protoporphyrin IX. Porphyrin fluorescence can be used as an indicator for storage-related changes | [229] |
| Beef | Raman | ANOVA, PLSR | Raman spectra can be used to predict TVC and LAB in samples packed under vacuum-packed and MAP, thus the technique could predict meat spoilage | [230] |
| Beef | NIR, MIR | PCA | Similar results were obtained from both NIR and MIR, indicating the possibility of using these techniques to support conventional techniques in determining the shelf life of minced meat | [231] |
| Chicken | Raman | ROC, MCR-ALS | Band changes of amide I were used to show protein denaturation occurring during heat treatments. The end point temperature was accurately estimated | [232] |
5. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.S. (Ed.) Handbook of Food Preservation, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2020; ISBN 9781498740487. [Google Scholar]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Luo, J.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of macro-nutrient, micro-nutrient composition and cooking conditions on in vitro digestibility of meat and aquatic dietary proteins. Food Chem. 2018, 254, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Rosnes, J.T.; Skåra, T.; Skipnes, D. Recent Advances in Minimal Heat Processing of Fish: Effects on Microbiological Activity and Safety. Food Bioprocess Technol. 2011, 4, 833–848. [Google Scholar] [CrossRef]
- Méndez, M.I.M.; Abuín, J.M.G. Thermal processing of fishery products. In Thermal Food Processing: New Technologies and Quality Issues, Second Edition; Sun, D.-W., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 249–271. ISBN 9781439876794. [Google Scholar]
- Hassoun, A.; Heia, K.; Lindberg, S.; Nilsen, H. Spectroscopic Techniques for Monitoring Thermal Treatments in Fish and Other Seafood: A Review of Recent Developments and Applications. Foods 2020, 6, 767. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W. (Ed.) Thermal Food Processing. New Technologies and Quality Issues; CRC Press Taylor@Francis Group: Boca Raton, FL, USA, 2012; ISBN 9781439876794. [Google Scholar]
- Dominguez-Hernandez, E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Cooking-Induced Protein Modifications in Meat. Compr. Rev. Food Sci. Food Saf. 2017, 16, 141–159. [Google Scholar] [CrossRef] [Green Version]
- Troy, D.J.; Ojha, K.S.; Kerry, J.P.; Tiwari, B.K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: An overview. Meat Sci. 2016, 120, 2–9. [Google Scholar] [CrossRef]
- Pasha, I.; Saeed, F.; Sultan, M.T.; Khan, M.R.; Rohi, M. Recent Developments in Minimal Processing: A Tool to Retain Nutritional Quality of Food. Crit. Rev. Food Sci. Nutr. 2014, 54, 340–351. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, R.; Zhang, B.; Erdogdu, F.; Wang, S. A comprehensive review on recent developments of radio frequency treatment for pasteurizing agricultural products. Crit. Rev. Food Sci. Nutr. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Rastogi, N.K. Recent trends and developments in infrared heating in food processing. Crit. Rev. Food Sci. Nutr. 2012, 52, 737–760. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Z.; Wang, S. Microwave processing: Effects and impacts on food components. Crit. Rev. Food Sci. Nutr. 2018, 58, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; de Alba, M.; Sun, D.W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S. Nonthermal Processes for Shelf-Life Extension of Seafoods: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.J.; Tiwari, B.K. Chemical Modifications of Lipids and Proteins by Nonthermal Food Processing Technologies. J. Agric. Food Chem. 2018, 66, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Režek Jambrak, A.; Vukušić, T.; Donsi, F.; Paniwnyk, L.; Djekic, I. Three Pillars of Novel Nonthermal Food Technologies: Food Safety, Quality, and Environment. J. Food Qual. 2018, 2018, 8619707. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Fan, K.; Mujumdar, A.S. Non-thermal Technology and Heating Technology for Fresh Food Cooking in the Central Kitchen Processing: A Review. Food Rev. Int. 2020, 9129. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Kulawik, P.; Kumar Tiwari, B. Recent advancements in the application of non-thermal plasma technology for the seafood industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 3199–3210. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Ghimire, S.; Flury, M.; Scheenstra, E.J.; Miles, C.A. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pereira, R.N.; Vicente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.P.; Marchesseau, S. Potentialities and limits of some non-thermal technologies to improve sustainability of food processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Borda, D.; Nicolau, A.I.; Raspor, P. (Eds.) Trends in Fish Processing Technologies; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781498729178. [Google Scholar]
- Ozogul, Y. (Ed.) Innovative Technologies in Seafood Processing; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780815366447. [Google Scholar]
- Cummins, E.J.; Lyng, J.G. (Eds.) Emerging Technologies in Production, Processing Production, Processing and Technology; John Wiley & Sons, Ltd.: Chichester, UK, 2017; ISBN 9781118350683. [Google Scholar]
- Bekhit, A.E.-D.A. (Ed.) Advances in Meat Processing Technology; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Fidel Toldrá, L.M.L.N. (Ed.) Advanced Technologies for Meat Processing, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781498754590. [Google Scholar]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef]
- Fan, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhou, W.; Zhang, H. Cooking evaluation of crayfish (Procambarus clarkia) subjected to microwave and conduction heating: A visualized strategy to understand the heat-induced quality changes of food. Innov. Food Sci. Emerg. Technol. 2020, 62, 102368. [Google Scholar] [CrossRef]
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Recent Applications of Spectroscopic and Hyperspectral Imaging Techniques with Chemometric Analysis for Rapid Inspection of Microbial Spoilage in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 478–490. [Google Scholar] [CrossRef]
- Feng, C.-H.; Makino, Y.; Oshita, S.; García Martín, J.F. Hyperspectral imaging and multispectral imaging as the novel techniques for detecting defects in raw and processed meat products: Current state-of-the-art research advances. Food Control 2018, 84, 165–176. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.-W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Moreno-Vilet, L.; Villanueva-Rodríguez, S.J. Current status of emerging food processing technologies in Latin America: Novel non-thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef] [Green Version]
- He, H.J.; Wu, D.; Sun, D.W. Nondestructive Spectroscopic and Imaging Techniques for Quality Evaluation and Assessment of Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, J. A Review of Hyperspectral Imaging for Chicken Meat Safety and Quality Evaluation: Application, Hardware, and Software. Compr. Rev. Food Sci. Food Saf. 2019, 18, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Kucha, C.T.; Liu, L.; Ngadi, M.O. Non-destructive spectroscopic techniques and multivariate analysis for assessment of fat quality in pork and pork products: A review. Sensors 2018, 18, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M.P.L.V.O. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. [Google Scholar] [CrossRef] [Green Version]
- Stormo, S.K.; Skåra, T.; Skipnes, D.; Sone, I.; Carlehög, M.; Heia, K.; Skjelvareid, M.H. In-Pack Surface Pasteurization of Capture-Based, Pre-Rigor Filleted Atlantic Cod (Gadus morhua). J. Aquat. Food Prod. Technol. 2018, 7, 783–794. [Google Scholar] [CrossRef]
- Stormo, S.K.; Skipnes, D.; Sone, I.; Skuland, A.; Heia, K.; Skåra, T. Modeling-assisted minimal heat processing of Atlantic cod (Gadus morhua). J. Food Process Eng. 2017, 40, e12555. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Importance of thickness in electromagnetic properties and gel characteristics of surimi during microwave heating. J. Food Eng. 2019, 248, 80–88. [Google Scholar] [CrossRef]
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic study of the effect of different cooking methods on protein oxidation in fish fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef] [Green Version]
- Lerfall, J.; Jakobsen, A.N.; Skipnes, D.; Waldenstrøm, L.; Hoel, S.; Rotabakk, B.T. Comparative Evaluation on the Quality and Shelf life of Atlantic Salmon (Salmo salar L.) Filets Using Microwave and Conventional Pasteurization in Combination with Novel Packaging Methods. J. Food Sci. 2018, 83, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, J.; Cao, A.; Zhang, Y.; Lv, Y.; Li, J. Denaturation Kinetics and Aggregation Mechanism of the Sarcoplasmic and Myofibril Proteins from Grass Carp During Microwave Processing. Food Bioprocess Technol. 2018, 11, 417–426. [Google Scholar] [CrossRef]
- Blikra, M.J.; Skipnes, D.; Feyissa, A.H. Model for heat and mass transport during cooking of cod loin in a convection oven. Food Control 2019, 102, 29–37. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. Effects of different cooking methods on the lipids and volatile components of farmed and wild European sea bass (Dicentrarchus labrax). Food Res. Int. 2018, 103, 48–58. [Google Scholar] [CrossRef]
- Dang, T.T.; Feyissa, A.H.; Gringer, N.; Jessen, F.; Olsen, K.; Bøknæs, N.; Orlien, V. Effects of high pressure and ohmic heating on shell loosening, thermal and structural properties of shrimp (Pandalus borealis). Innov. Food Sci. Emerg. Technol. 2020, 59, 102246. [Google Scholar] [CrossRef]
- Tian, X.; Shao, L.; Yu, Q.; Li, W.S.X.; Dai, R. Comparative analysis of quality uniformity of ohmic and water bath heating treated pork batter with different fat content. J. Food Process. Preserv. 2020, 44, e14377. [Google Scholar] [CrossRef]
- Tang, X.; Cronin, D.A.; Brunton, N.P. The effect of radio frequency heating on chemical, physical and sensory aspects of quality in turkey breast rolls. Food Chem. 2005, 93, 1–7. [Google Scholar] [CrossRef]
- Muñoz, I.; Serra, X.; Guàrdia, M.D.; Fartdinov, D.; Arnau, J.; Picouet, P.A.; Gou, P. Radio frequency cooking of pork hams followed with conventional steam cooking. LWT 2020, 123, 109104. [Google Scholar] [CrossRef]
- Rincon, A.M.; Singh, R.K. Inactivation of Shiga toxin-producing and nonpathogenic Escherichia coli in non-intact steaks cooked in a radio frequency oven. Food Control 2016, 62, 390–396. [Google Scholar] [CrossRef]
- Yildiz Turp, G.; Icier, F.; Kor, G. Influence of infrared final cooking on color, texture and cooking characteristics of ohmically pre-cooked meatball. Meat Sci. 2016, 114, 46–53. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Icier, F.; Kor, G. Effects of Combined Ohmic–Infrared Cooking Treatment on Microbiological Inactivation of Meatballs. J. Food Process Eng. 2017, 40, e12309. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, D.; Kashaninejad, M.; Ziaiifar, A.M.; Mahoonak, A.S. Effect of infrared final cooking on some physico-chemical and engineering properties of partially fried chicken nugget. Innov. Food Sci. Emerg. Technol. 2018, 47, 1–8. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Taşkıran, M.; Olum, E.; Candoğan, K. Changes in chicken meat proteins during microwave and electric oven cooking. J. Food Process. Preserv. 2020, 44, e14324. [Google Scholar] [CrossRef]
- Wang, X.; Muhoza, B.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Comparison between microwave and traditional water bath cooking on saltiness perception, water distribution and microstructure of grass crap meat. Food Res. Int. 2019, 125, 108521. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Mujumdar, A.S.; Zhang, M. New Development in Radio Frequency Heating for Fresh Food Processing: A Review. Food Eng. Rev. 2019, 11, 29–43. [Google Scholar] [CrossRef]
- Uemura, K.; Kanafusa, S.; Takahashi, C.; Kobayashi, I. Development of a radio frequency heating system for sterilization of vacuum-packed fish in water. Biosci. Biotechnol. Biochem. 2017, 81, 762–767. [Google Scholar] [CrossRef] [Green Version]
- Llave, Y.; Liu, S.; Fukuoka, M.; Sakai, N. Computer simulation of radiofrequency defrosting of frozen foods. J. Food Eng. 2015, 152, 32–42. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Q.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Radiofrequency Thawing of Frozen Minced Fish Based on the Dielectric Response Mechanism. Innov. Food Sci. Emerg. Technol. 2019, 52, 80–88. [Google Scholar] [CrossRef]
- Ángel-Rendón, S.V.; Filomena-Ambrosio, A.; Cordon-Díaz, S.; Benítez-Sastoque, E.R.; Sotelo-Díaz, L.I. Ohmic cooking: Application of a novel technology in pork and influences on water holding capacity, cooking loss and colour. Int. J. Gastron. Food Sci. 2019, 17, 100164. [Google Scholar] [CrossRef]
- Jaeger, H.; Roth, A.; Toepfl, S.; Holzhauser, T.; Engel, K.-H.; Knorr, D.; Vogel, R.F.; Bandick, N.; Kulling, S.; Heinz, V.; et al. Opinion on the use of ohmic heating for the treatment of foods. Trends Food Sci. Technol. 2016, 55, 84–97. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, Y.; Wu, W.; Lu, X.; Han, Z.; Liu, Y.; Li, X.; Dai, R. Changes in oxidation, color and texture deteriorations during refrigerated storage of ohmically and water bath-cooked pork meat. Innov. Food Sci. Emerg. Technol. 2014, 26, 341–346. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.S.; Yi-Chen, L.; Cacciola, F. A comprehensive review on infrared heating applications in food processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kor, G.; Icier, F. Thermal imaging during infrared final cooking of semi-processed cylindrical meat product. Infrared Phys. Technol. 2016, 79, 242–251. [Google Scholar] [CrossRef]
- Kendirci, P.; Icier, F.; Kor, G.; Onogur, T.A. Influence of infrared final cooking on polycyclic aromatic hydrocarbon formation in ohmically pre-cooked beef meatballs. Meat Sci. 2014, 97, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New Insights into the High-Pressure Processing of Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Stathopoulos, C.E.; Nguyen, M.H. Advances in High-Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. [Google Scholar] [CrossRef]
- Campus, M. High Pressure Processing of Meat, Meat Products and Seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Maldonado, J.A.; Schaffner, D.W.; Cuitinõ, A.M.; Karwe, M.V. In situ studies of microbial inactivation during high pressure processing. High Press. Res. 2016, 36, 79–89. [Google Scholar] [CrossRef]
- Morton, J.D.; Lee, H.Y.Y.; Pearson, R.G.; Bickerstaffe, R. The physical and biochemical effects of pre-rigor high pressure processing of beef. Meat Sci. 2018, 143, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.D.; Pearson, R.G.; Lee, H.Y.Y.; Smithson, S.; Mason, S.L.; Bickerstaffe, R. High pressure processing improves the tenderness and quality of hot-boned beef. Meat Sci. 2017, 133, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Sun, D.W.; Zhu, Z.; Wang, Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Torres, J.A.; Saraiva, J.A.; Guerra-Rodríguez, E.; Vázquez, M. Effect of high-pressure treatments applied before freezing and frozen storage on the functional and sensory properties of Atlantic mackerel (Scomber scombrus). LWT Food Sci. Technol. 2013, 53, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Tironi, V.; De Lamballerie, M.; Le-Bail, A. Quality changes during the frozen storage of sea bass (Dicentrarchus labrax) muscle after pressure shift freezing and pressure assisted thawing. Innov. Food Sci. Emerg. Technol. 2010, 11, 565–573. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Barretto, T.L.; Pollonio, M.A.R.; Telis-Romero, J.; da Silva Barretto, A.C. Improving sensory acceptance and physicochemical properties by ultrasound application to restructured cooked ham with salt (NaCl) reduction. Meat Sci. 2018, 145, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Pinton, M.B.; Correa, L.P.; Facchi, M.M.X.; Heck, R.T.; Leães, Y.S.V.; Cichoski, A.J.; Lorenzo, J.M.; dos Santos, M.; Pollonio, M.A.R.; Campagnol, P.C.B. Ultrasound: A new approach to reduce phosphate content of meat emulsions. Meat Sci. 2019, 152, 88–95. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Burgess, C.; Kerry, J.P.; Tiwari, B.K. Influence of extrinsic operational parameters on salt diffusion during ultrasound assisted meat curing. Ultrasonics 2018, 83, 164–170. [Google Scholar] [CrossRef]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. [Google Scholar] [CrossRef]
- Cooksey, K. Modified atmosphere packaging of meat, poultry and fish. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2014; pp. 475–493. [Google Scholar]
- Belcher, J.N. Industrial packaging developments for the global meat market. Meat Sci. 2006, 74, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Spanos, D.; Tørngren, M.A.; Christensen, M.; Baron, C.P. Effect of oxygen level on the oxidative stability of two different retail pork products stored using modified atmosphere packaging (MAP). Meat Sci. 2016, 113, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyri, A.A.; Papadopoulou, O.S.; Nisiotou, A.; Tassou, C.C.; Chorianopoulos, N. Effect of high pressure processing on the survival of Salmonella Enteritidis and shelf-life of chicken fillets. Food Microbiol. 2018, 70, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Hovda, M.B. High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control 2016, 70, 242–248. [Google Scholar] [CrossRef]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Innov. Food Sci. Emerg. Technol. 2020, 136126, in press. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Pedrós-Garrido, S.; Condón-Abanto, S.; Beltrán, J.A.; Lyng, J.G.; Brunton, N.P.; Bolton, D.; Whyte, P. Assessment of high intensity ultrasound for surface decontamination of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. merluccius) fillets, and its impact on fish quality. Innov. Food Sci. Emerg. Technol. 2017, 41, 64–70. [Google Scholar] [CrossRef]
- Vetchapitak, T.; Shinki, T.; Sasaki, S.; Taniguchi, T.; Luangtongkum, T.; Misawa, N. Evaluation of chemical treatment combined with vacuum and ultrasonication with a water resonance system for reducing Campylobacter on naturally contaminated chicken carcasses. Food Control 2020, 112, 107087. [Google Scholar] [CrossRef]
- Xiong, G.; Fu, X.; Pan, D.; Qi, J.; Xu, X.; Jiang, X. Influence of ultrasound-assisted sodium bicarbonate marination on the curing efficiency of chicken breast meat. Ultrason. Sonochem. 2020, 60, 104808. [Google Scholar] [CrossRef]
- Contreras-Lopez, G.; Carnero-Hernandez, A.; Huerta-Jimenez, M.; Alarcon-Rojo, A.D.; Garcia-Galicia, I.; Carrillo-López, L.M. High-intensity ultrasound applied on cured pork: Sensory and physicochemical characteristics. Food Sci. Nutr. 2020, 8, 786–795. [Google Scholar] [CrossRef]
- Yong, H.I.; Lee, H.; Park, S.; Park, J.; Choe, W.; Jung, S.; Jo, C. Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat’s physicochemical properties. Meat Sci. 2017, 123, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Martin-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, K.S.; Bourke, P.; Rico, D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 85–91. [Google Scholar] [CrossRef]
- Jo, K.; Lee, J.; Lee, S.; Lim, Y.; Choi, Y.S.; Jo, C.; Jung, S. Curing of ground ham by remote infusion of atmospheric non-thermal plasma. Food Chem. 2020, 309, 125643. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Oey, I.; Bremer, P.; Silcock, P. Process optimisation of pulsed electric fields pre-treatment to reduce the sous vide processing time of beef briskets. Int. J. Food Sci. Technol. 2019, 54, 823–834. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Q.; Zhou, D. Optimization Extraction of Protein from Mussel by High-Intensity Pulsed Electric Fields. J. Food Process. Preserv. 2017, 41, e12962. [Google Scholar] [CrossRef]
- Hultmann, L.; Rustad, T. Iced storage of Atlantic salmon (Salmo salar)—Effects on endogenous enzymes and their impact on muscle proteins and texture. Food Chem. 2004, 87, 31–41. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Jensen, W.B. The Origin of the Soxhlet Extractor. J. Chem. Educ. 2007, 84, 1913. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007, 105, 1067–1075. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Aftret, K.C.; Rustad, T. The Effect of Sous-Vide Cooking Parameters, Chilled Storage and Antioxidants on Quality Characteristics of Atlantic Mackerel (Scomber scombrus) in Relation to Structural Changes in Proteins. Food Technol. Biotechnol. 2019, 57, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hultmann, L.; Rørå, A.M.B.; Steinsland, I.; Skåra, T.; Rustad, T. Proteolytic activity and properties of proteins in smoked salmon (Salmo salar)—Effects of smoking temperature. Food Chem. 2004, 85, 377–387. [Google Scholar] [CrossRef]
- Nilsson, K.; Ekstrand, B. Enzyme leakage in muscle-tissue of rainbow-trout (oncorhynchus-mykiss) related to various thawing treatments. Z. Lebensm. Unters. Forsch. 1994, 198, 253–257. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrason. Sonochem. 2020, 65, 105063. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Karoui, R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chem. 2016, 200, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S. Synergistic effect of tannic acid and modified atmospheric packaging on the prevention of lipid oxidation and quality losses of refrigerated striped catfish slices. Food Chem. 2010, 121, 29–38. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; Yan, L.; Li, R. Effects of microwave heating on physicochemical properties, microstructure and volatile profiles of yak meat. J. Appl. Anim. Res. 2019, 47, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Hultmann, L.; Rustad, T. Textural Changes During Iced Storage of Salmon (Salmo salar) and Cod (Gadus morhua). J. Aquat. Food Prod. Technol. 2002, 11, 105–123. [Google Scholar] [CrossRef]
- Ofstad, R.; Kidman, S.; Myklebust, R.; Hermansson, A.-M. Liquid Holding Capacity and Structural Changes During Heating of Fish Muscle: Cod (Gadus morhua L.) and Salmon (Salmo salar). Food Struct. 1993, 12, 163–174. [Google Scholar]
- Sigurgisladottir, S.; Torrissen, O.; Lie, Ø.; Thomassen, M.; Hafsteinsson, H. Salmon quality: Methods to determine the quality parameters. Rev. Fish. Sci. 1997, 5, 223–252. [Google Scholar] [CrossRef]
- Ofstad, R.; Kidman, S.; Myklebust, R.; Olsen, R.L.; Hermansson, A.M. Liquid-holding capacity and structural changes in comminuted salmon (Salmo salar) muscle as influenced by pH, salt and temperature. LWT Food Sci. Technol. 1995, 28, 329–339. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. The Influence of Cooking Parameters and Chilled Storage Time on Quality of Sous-Vide Atlantic Mackerel (Scomber scombrus). J. Aquat. Food Prod. Technol. 2019, 28, 505–518. [Google Scholar] [CrossRef]
- Hultmann, L.; Phu, T.M.; Tobiassen, T.; Aas-Hansen, O.; Rustad, T. Effects of pre-slaughter stress on proteolytic enzyme activities and muscle quality of farmed Atlantic cod (Gadus morhua). Food Chem. 2012, 134, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.A.; Velazquez, G.; Cando, D.; Núñez-Flores, R.; Borderías, A.J.; Moreno, H.M. Effects of high pressure processing on protein fractions of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 323–329. [Google Scholar] [CrossRef]
- Schubring, R.; Meyer, C.; Schlüter, O.; Boguslawski, S.; Knorr, D. Impact of high pressure assisted thawing on the quality of fillets from various fish species. Innov. Food Sci. Emerg. Technol. 2003, 4, 257–267. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Neto, O.C.; dos Santos, L.M.R.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- Roberts, J.J.; Cozzolino, D. An Overview on the Application of Chemometrics in Food Science and Technology—An Approach to Quantitative Data Analysis. Food Anal. Methods 2016, 9, 3258–3267. [Google Scholar] [CrossRef]
- Wang, L.; Sun, D.W.; Pu, H.; Cheng, J.H. Quality analysis, classification, and authentication of liquid foods by near-infrared spectroscopy: A review of recent research developments. Crit. Rev. Food Sci. Nutr. 2017, 57, 1524–1538. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared spectroscopy coupled with chemometrics: A tool for the analysis of intact food systems and the exploration of their molecular structure-quality relationships—A review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Bochko, V.; Martinkauppi, B.; Saranwong, S.; Mantere, T. A Review of Optical Nondestructive Visual and Near-Infrared Methods for Food Quality and Safety. Int. J. Spectrosc. 2013, 2013, 341402. [Google Scholar] [CrossRef]
- Sorak, D.; Herberholz, L.; Iwascek, S.; Altinpinar, S.; Pfeifer, F.; Siesler, H.W. New developments and applications of handheld raman, mid-infrared, and near-infrared spectrometers. Appl. Spectrosc. Rev. 2012, 47, 83–115. [Google Scholar] [CrossRef]
- Cozzolino, D. The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods. Anal. Methods 2015, 7, 9390–9400. [Google Scholar] [CrossRef]
- Rolinger, L.; Rüdt, M.; Hubbuch, J. A critical review of recent trends, and a future perspective of optical spectroscopy as PAT in biopharmaceutical downstream processing. Anal. Bioanal. Chem. 2020, 412, 2047–2064. [Google Scholar] [CrossRef] [Green Version]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Khakimov, B.; Bak, S.; Engelsen, S.B. High-throughput cereal metabolomics: Current analytical technologies, challenges and perspectives. J. Cereal Sci. 2014, 59, 393–418. [Google Scholar] [CrossRef]
- Cattaneo, T.M.P.; Stellari, A. Review: NIR spectroscopy as a suitable tool for the investigation of the horticultural field. Agronomy 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Li, Y.S.; Church, J.S. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J. Food Drug Anal. 2014, 22, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; He, L. Surface-Enhanced Raman Spectroscopy for the Chemical Analysis of Food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Chao, K.; Cho, B. Raman Chemical Imaging Technology for Food and Agricultural Applications. J. Biosyst. Eng. 2017, 42, 170–189. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghi, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR News 2020, 31, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Kim, M.S.; Chao, K.; Schmidt, W.F.; Dhakal, S.; Cho, B.-K.; Peng, Y.; Huang, M. Subsurface inspection of food safety and quality using line-scan spatially offset Raman spectroscopy technique. Food Control 2017, 75, 246–254. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, D.; Roberts, J. Applications and developments on the use of vibrational spectroscopy imaging for the analysis, monitoring and characterisation of crops and plants. Molecules 2016, 21, 755. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Roohinejad, S.; Ishikawa, K.; Leong, S.Y.; El-Din A Bekhit, A.; Saraiva, J.A.; Lebovka, N. Electron spin resonance as a tool to monitor the influence of novel processing technologies on food properties. Trends Food Sci. Technol. 2020, 100, 77–87. [Google Scholar] [CrossRef]
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online Low-field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI) for Food Quality Optimization in Food Processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- Hassoun, A.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö.E.; Guðjónsdóttir, M.; Barba, F.J.; Marti-Quijal, F.J.; et al. Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review. Antioxidants 2020, 9, 882. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Wold, J.P. On-line and non-destructive measurement of core temperature in heat treated fish cakes by NIR hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Feng, X.; Li, W.; Yuan, L.; Ge, J.; Lu, D.; Chen, B.; Yu, G. Changes in properties of white shrimp (Litopenaeus vannamei) protein during thermal denaturation. Food Sci. Biotechnol. 2016, 25, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Bouraoui, M.; Nakai, S.; Li-Chan, E. In situ investigation of protein structure in Pacific whiting surimi and gels using Raman spectroscopy. Food Res. Int. 1997, 30, 65–72. [Google Scholar] [CrossRef]
- Lin, X.; Yang, W.; Xu, D.; Wang, L. Effect of electron irradiation and heat on the structure of hairtail surimi. Radiat. Phys. Chem. 2015, 114, 50–54. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, X. Changes of protein secondary structures of pollock surimi gels under high-temperature (100 °C and 120 °C) treatment. J. Food Eng. 2016, 171, 159–163. [Google Scholar] [CrossRef]
- Uddin, M.; Ishizaki, S.; Okazaki, E.; Tanaka, M. Near-infrared reflectance spectroscopy for determining end-point temperature of heated fish and shellfish meats. J. Sci. Food Agric. 2002, 82, 286–292. [Google Scholar] [CrossRef]
- Skåra, T.; Stormo, S.K.; Skipnes, D.; Kondjoyan, A.; Sivertsen, A.; Gins, G.; Van Derlinden, E.; Valdramidis, V.P.; Van Impe, J.F.M. Estimation of surface temperature and thermal load in short-time heat treatment of surimi through reflectance spectroscopy and heat transfer modeling. J. Food Eng. 2014, 120, 75–80. [Google Scholar] [CrossRef]
- Uddin, M.; Okazaki, E.; Uddin Ahmad, M.; Fukuda, Y.; Tanaka, M. Noninvasive NIR spectroscopy to verify endpoint temperature of kamaboko gel. LWT Food Sci. Technol. 2005, 38, 809–814. [Google Scholar] [CrossRef]
- Stormo, S.K.; Sivertsen, A.H.; Heia, K.; Skipnes, D. Endpoint temperature of heat-treated surimi can be measured by visible spectroscopy. Food Control 2012, 26, 92–97. [Google Scholar] [CrossRef]
- Uddin, M.; Okazaki, E.; Ahmad, M.U.; Fukuda, Y.; Tanaka, M. NIR spectroscopy: A non-destructive fast technique to verify heat treatment of fish-meat gel. Food Control 2006, 17, 660–664. [Google Scholar] [CrossRef]
- Elmasry, G.; Nakauchi, S. Noninvasive sensing of thermal treatments of Japanese seafood products using imaging spectroscopy. Int. J. Food Sci. Technol. 2015, 50, 1960–1971. [Google Scholar] [CrossRef]
- Ovissipour, M.; Rasco, B.; Tang, J.; Sablani, S. Kinetics of Protein Degradation and Physical Changes in Thermally Processed Atlantic Salmon (Salmo salar). Food Bioprocess Technol. 2017, 10, 1865–1882. [Google Scholar] [CrossRef]
- Ovissipour, M.; Shiroodi, S.G.; Rasco, B.; Tang, J.; Sablani, S.S. Electrolyzed water and mild-thermal processing of Atlantic salmon (Salmo salar): Reduction of Listeria monocytogenes and changes in protein structure. Int. J. Food Microbiol. 2018, 276, 10–19. [Google Scholar] [CrossRef]
- Shaikh, S.; O’Donnell, C. Applications of fluorescence spectroscopy in dairy processing: A review. Curr. Opin. Food Sci. 2017, 17, 16–24. [Google Scholar] [CrossRef]
- Sahar, A.; ur Rahman, U.; Kondjoyan, A.; Portanguen, S.; Dufour, E. Monitoring of thermal changes in meat by synchronous fluorescence spectroscopy. J. Food Eng. 2016, 168, 160–165. [Google Scholar] [CrossRef]
- Zhu, D.; Ji, B.; Eum, H.L.; Zude, M. Evaluation of the non-enzymatic browning in thermally processed apple juice by front-face fluorescence spectroscopy. Food Chem. 2009, 113, 272–279. [Google Scholar] [CrossRef]
- Ali, H.; Saleem, M.; Ullah, R.; Khan, S.; Atta, B.M.; Bilal, M. Thermal Effects on Biochemical Signatures of UHT, Pasteurized and Domestically Boiled Buffalo Milk Detected by Synchronous Fluorescence Spectroscopy. J. Fluoresc. 2019, 29, 485–493. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Simpson, B.K.; Simpson, M.V. Pulsed electric field induced structural modification of soy protein isolate as studied by fluorescence spectroscopy. J. Food Process. Preserv. 2011, 35, 563–570. [Google Scholar] [CrossRef]
- Hu, L.; Ren, S.; Shen, Q.; Ye, X.; Chen, J.; Ling, J. Protein oxidation and proteolysis during roasting and in vitro digestion of fish (Acipenser gueldenstaedtii). J. Sci. Food Agric. 2018, 98, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Semedo Tavares, W.P.; Dong, S.; Jin, W.; Yang, Y.; Han, K.; Zha, F.; Zhao, Y.; Zeng, M. Effect of different cooking conditions on the profiles of Maillard reaction products and nutrient composition of hairtail (Thichiurus lepturus) fillets. Food Res. Int. 2018, 103, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. A non-invasive approach to assess texture changes in sous-vide cooked Atlantic mackerel during chilled storage by fluorescence imaging. Food Control 2018, 92, 216–224. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. Assessment of lipid oxidation in Atlantic mackerel (Scomber scombrus) subjected to different antioxidant and sous-vide cooking treatments by conventional and fluorescence microscopy methods. Food Control 2019, 104, 1–8. [Google Scholar] [CrossRef]
- Hassoun, A.; Cropotova, J.; Rustad, T.; Heia, K.; Lindberg, S.-K.; Nilsen, H. Use of Spectroscopic Techniques for a Rapid and Non-Destructive Monitoring of Thermal Treatments and Storage Time of Sous-Vide Cooked Cod Fillets. Sensors 2020, 20, 2410. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H.; et al. Intervention of transglutaminase in surimi gel under microwave irradiation. Food Chem. 2018, 268, 378–385. [Google Scholar] [CrossRef]
- Tsakanikas, P.; Karnavas, A.; Panagou, E.Z.; Nychas, G.J. A machine learning workflow for raw food spectroscopic classification in a future industry. Sci. Rep. 2020, 10, 11212. [Google Scholar] [CrossRef]
- Bi, J.; Li, Y.; Cheng, S.; Dong, X.; Kamal, T.; Zhou, D.; Li, D.; Jiang, P.; Zhu, B.W.; Tan, M. Changes in Body Wall of Sea Cucumber (Stichopus japonicus) during a two-Step Heating Process Assessed by Rheology, LF-NMR, and Texture Profile Analysis. Food Biophys. 2016, 11, 257–265. [Google Scholar] [CrossRef]
- Xia, K.; Xu, W.; Huang, L.; Song, Y.; Zhu, B.W.; Tan, M. Water dynamics of turbot flesh during frying, boiling, and stewing processes and its relationship with color and texture properties: Low-field NMR and MRI studies. J. Food Process. Preserv. 2018, 42, e13338. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Wang, S.; Lin, R.; Cheng, S.; Yuan, B.; Wang, Z.; Tan, M. Effect of Different Cooking Methods on Proton Dynamics and Physicochemical Attributes in Spanish Mackerel Assessed by Low-Field NMR. Foods 2020, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, R.; Cheng, S.; Tan, M. Water dynamics changes and protein denaturation in surf clam evaluated by two-dimensional LF-NMR T1-T2 relaxation technique during heating process. Food Chem. 2020, 320, 126622. [Google Scholar] [CrossRef] [PubMed]
- Tintchev, F.; Kuhlmann, U.; Wackerbarth, H.; Töpfl, S.; Heinz, V.; Knorr, D.; Hildebrandt, P. Redox processes in pressurised smoked salmon studied by resonance Raman spectroscopy. Food Chem. 2009, 112, 482–486. [Google Scholar] [CrossRef]
- Saraiva, C.; Vasconcelos, H.; de Almeida, J.M.M.M. A chemometrics approach applied to Fourier transform infrared spectroscopy (FTIR) for monitoring the spoilage of fresh salmon (Salmo salar) stored under modified atmospheres. Int. J. Food Microbiol. 2017, 241, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ojagh, S.M.; Núñez-Flores, R.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Lessening of high-pressure-induced changes in Atlantic salmon muscle by the combined use of a fish gelatin–lignin film. Food Chem. 2011, 125, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Wang, J.; Raghavan, V. Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innov. Food Sci. Emerg. Technol. 2020, 65, 102441. [Google Scholar] [CrossRef]
- Martínez-Maldonado, M.A.; Velazquez, G.; Ramírez de León, J.A.; Borderías, A.J.; Moreno, H.M. Effect of high pressure processing on heat-induced gelling capacity of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2020, 59, 102253. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Moreno-Osorio, L.; Villalobos-Carvajal, R.; Pérez-Won, M. Protein Changes Caused by High Hydrostatic Pressure (HHP): A Study Using Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2015, 7, 222–230. [Google Scholar] [CrossRef]
- Sone, I.; Olsen, R.L.; Sivertsen, A.H.; Eilertsen, G.; Heia, K. Classification of fresh Atlantic salmon (Salmo salar L.) fillets stored under different atmospheres by hyperspectral imaging. J. Food Eng. 2012, 109, 482–489. [Google Scholar] [CrossRef]
- Ivorra, E.; Girón, J.; Sánchez, A.J.; Verdú, S.; Barat, J.M.; Grau, R. Detection of expired vacuum-packed smoked salmon based on PLS-DA method using hyperspectral images. J. Food Eng. 2013, 117, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Shen, Y.; Liu, W.; Mei, J.; Xie, J. Low-field NMR and MRI to analyze the effect of edible coating incorporated with MAP on qualities of half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets during refrigerated storage. Appl. Sci. 2018, 8, 1391. [Google Scholar] [CrossRef] [Green Version]
- De Aguiar Saldanha Pinheiro, A.C.; Urbinati, E.; Tappi, S.; Picone, G.; Patrignani, F.; Lanciotti, R.; Romani, S.; Rocculi, P. The impact of gas mixtures of Argon and Nitrous oxide (N2O) on quality parameters of sardine (Sardina pilchardus) fillets during refrigerated storage. Food Res. Int. 2019, 115, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Martín-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Álvarez, C.; Rico, D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Carneiro, C.D.S.; Mársico, E.T.; Ribeiro, R.D.O.R.; Conte-Júnior, C.A.; Mano, S.B.; Augusto, C.J.C.; Oliveira De Jesus, E.F. Low-Field Nuclear Magnetic Resonance (LF NMR 1H) to assess the mobility of water during storage of salted fish (Sardinella brasiliensis). J. Food Eng. 2016, 169, 321–325. [Google Scholar] [CrossRef]
- Collewet, G.; Bugeon, J.; Idier, J.; Quellec, S.; Quittet, B.; Cambert, M.; Haffray, P. Rapid quantification of muscle fat content and subcutaneous adipose tissue in fish using MRI. Food Chem. 2013, 138, 2008–2015. [Google Scholar] [CrossRef]
- Herrero, A.; Carmona, P.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Application of Vibrational Spectroscopy to Elucidate Protein Conformational Changes Promoted by Thermal Treatment in Muscle-Based Food. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A., Raj, B., Eds.; Wiley-Scrivener: Beverly, MA, USA, 2015; pp. 467–482. [Google Scholar]
- Shao, J.-H.; Zou, Y.-F.; Xu, X.-L.; Wu, J.-Q.; Zhou, G.-H. Evaluation of structural changes in raw and heated meat batters prepared with different lipids using Raman spectroscopy. Food Res. Int. 2011, 44, 2955–2961. [Google Scholar] [CrossRef]
- Wang, S.F.; Smith, D.M. Dynamic Rheological Properties and Secondary Structure of Chicken Breast Myosin As Influenced by Isothermal Heating. J. Agric. Food Chem. 1994, 42, 1434–1439. [Google Scholar] [CrossRef]
- Xu, X.L.; Han, M.Y.; Fei, Y.; Zhou, G.H. Raman spectroscopic study of heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristic. Meat Sci. 2011, 87, 159–164. [Google Scholar] [CrossRef]
- Berhe, D.T.; Engelsen, S.B.; Hviid, M.S.; Lametsch, R. Raman spectroscopic study of effect of the cooking temperature and time on meat proteins. Food Res. Int. 2014, 66, 123–131. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Ellekjær, M.R.; Isaksson, T. Assessment of maximum cooking temperatures in previously heat treated beef. Part 1: Near infrared spectroscopy. J. Sci. Food Agric. 1992, 59, 335–343. [Google Scholar] [CrossRef]
- González-Mohino, A.; Antequera, T.; Ventanas, S.; Caballero, D.; Mir-Bel, J.; Pérez-Palacios, T. Near-infrared spectroscopy-based analysis to study sensory parameters on pork loins as affected by cooking methods and conditions. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Biancolillo, A.; Boqué, R.; Cocchi, M.; Marini, F. Data Fusion Strategies in Food Analysis. In Data Handling in Science and Technology; Cocchi, M., Ed.; Elsevier: Amsterdam, The Nederlands, 2019; Volume 31, pp. 271–310. ISBN 9780444639844. [Google Scholar]
- Calabrò, E.; Magazù, S. Comparison Between Conventional Convective Heating and Microwave Heating: An FTIR Spectroscopy Study of the Effects of Microwave Oven Cooking of Bovine Breast Meat. J. Electromagn. Anal. Appl. 2012, 04, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Perez-Palacios, T.; Caballero, D.; González-Mohíno, A.; Mir-Bel, J.; Antequera, T. Near Infrared Reflectance spectroscopy to analyse texture related characteristics of sous vide pork loin. J. Food Eng. 2019, 263, 417–423. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Lee, H.; Kim, M.S.; Mo, C.; Cho, B.K. Hyperspectral reflectance imaging technique for visualization of moisture distribution in cooked chicken breast. Sensors 2013, 13, 13289–13300. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, D.; Cheng, J.-H.; Han, Z. Hyperspectral Imaging Sensing of Changes in Moisture Content and Color of Beef During Microwave Heating Process. Food Anal. Methods 2018, 11, 2472–2484. [Google Scholar] [CrossRef]
- O’Farrell, M.; Bakke, K.A.H.; Tschudi, J.; Wold, J.P. Near-infrared (NIR) interactance system for non-contact monitoring of the temperature profile of baked liver pâté. Appl. Spectrosc. 2011, 65, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Wold, J.P.; O’Farrell, M.; Tschudi, J.; Eskildsen, C.E.; Andersen, P.V.; Ottestad, S. In-line and non-destructive monitoring of core temperature in sausages during industrial heat treatment by NIR interaction spectroscopy. J. Food Eng. 2020, 277, 109921. [Google Scholar] [CrossRef]
- Sahar, A.; Boubellouta, T.; Portanguen, S.; Kondjoyan, A.; Dufour, É. Synchronous front-face fluorescence spectroscopy coupled with parallel factors (PARAFAC) analysis to study the effects of cooking time on meat. J. Food Sci. 2009, 74, E534–E539. [Google Scholar] [CrossRef]
- Sahar, A.; Portanguen, S.; Dufour, A.K.É. Potential of synchronous fuorescence spectroscopy coupled with chemometrics to determine the heterocyclic aromatic amines in grilled meat. Eur. Food Res. Technol. 2010, 231, 803–812. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Nomi, Y.; Homma, T.; Kasai, M.; Otsuka, Y. Determination of furosine and fluorescence as markers of the maillard reaction for the evaluation of meat products during actual cooking conditions. Food Sci. Technol. Res. 2012, 18, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Mitra, B.; Lametsch, R.; Akcan, T.; Ruiz-Carrascal, J. Pork proteins oxidative modifications under the influence of varied time-temperature thermal treatments: A chemical and redox proteomics assessment. Meat Sci. 2018, 140, 134–144. [Google Scholar] [CrossRef]
- Trevisan, A.J.B.; de Almeida Lima, D.; Sampaio, G.R.; Soares, R.A.M.; Markowicz Bastos, D.H. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016, 196, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.C.S.; Morcuende, D.; Madruga, M.S.; Silva, F.A.P.; Estévez, M. Role of protein oxidation in the nutritional loss and texture changes in ready-to-eat chicken patties. Int. J. Food Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef]
- Sun, H.X.; Huang, F.; Ding, Z.J.; Zhang, C.J.; Zhang, L.; Zhang, H. Low-field nuclear magnetic resonance analysis of the effects of heating temperature and time on braised beef. Int. J. Food Sci. Technol. 2017, 52, 1193–1202. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, J.; Zheng, J.; Li, X.; Shao, J.H. The study of protein conformation and hydration characteristics of meat batters at various phase transition temperatures combined with Low-field nuclear magnetic resonance and Fourier transform infrared spectroscopy. Food Chem. 2019, 280, 263–269. [Google Scholar] [CrossRef]
- Wackerbarth, H.; Kuhlmann, U.; Tintchev, F.; Heinz, V.; Hildebrandt, P. Structural changes of myoglobin in pressure-treated pork meat probed by resonance Raman spectroscopy. Food Chem. 2009, 115, 1194–1198. [Google Scholar] [CrossRef]
- Sazonova, S.; Grube, M.; Shvirksts, K.; Galoburda, R.; Gramatina, I. FTIR spectroscopy studies of high pressure-induced changes in pork macromolecular structure. J. Mol. Struct. 2019, 1186, 377–383. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Nychas, G.J.E.; Tassou, C.; Chorianopoulos, N. Use of Fourier transform infrared spectroscopy for monitoring the shelf life of ham slices packed with probiotic supplemented edible films after treatment with high pressure processing. Food Res. Int. 2018, 106, 1061–1068. [Google Scholar] [CrossRef]
- Ammor, M.S.; Argyri, A.; Nychas, G.J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef]
- Bertram, H.C.; Aaslyng, M.D.; Andersen, H.J. Elucidation of the relationship between cooking temperature, water distribution and sensory attributes of pork—A combined NMR and sensory study. Meat Sci. 2005, 70, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Berhe, D.T.; Lawaetz, A.J.; Engelsen, S.B.; Hviid, M.S.; Lametsch, R. Accurate determination of endpoint temperature of cooked meat after storage by Raman spectroscopy and chemometrics. Food Control 2015, 52, 119–125. [Google Scholar] [CrossRef]
- Bouhrara, M.; Clerjon, S.; Damez, J.L.; Kondjoyan, A.; Bonny, J.M. In situ imaging highlights local structural changes during heating: The case of meat. J. Agric. Food Chem. 2012, 60, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Schmidt, H.; Scheier, R.; Hopkins, D.L. Preliminary investigation on the relationship of Raman spectra of sheep meat with shear force and cooking loss. Meat Sci. 2013, 93, 138–143. [Google Scholar] [CrossRef]
- Durek, J.; Bolling, J.S.; Knorr, D.; Schwägele, F.; Schlüter, O. Effects of different storage conditions on quality related porphyrin fluorescence signatures of pork slices. Meat Sci. 2012, 90, 252–258. [Google Scholar] [CrossRef]
- Yang, H.; Hopkins, D.L.; Zhang, Y.; Zhu, L.; Dong, P.; Wang, X.; Mao, Y.; Luo, X.; Fowler, S.M. Preliminary investigation of the use of Raman spectroscopy to predict beef spoilage in different types of packaging. Meat Sci. 2020, 165, 108136. [Google Scholar] [CrossRef]
- Sinelli, N.; Limbo, S.; Torri, L.; Di Egidio, V.; Casiraghi, E. Evaluation of freshness decay of minced beef stored in high-oxygen modified atmosphere packaged at different temperatures using NIR and MIR spectroscopy. Meat Sci. 2010, 86, 748–752. [Google Scholar] [CrossRef]
- Miyaoka, R.; Ando, M.; Harada, R.; Osaka, H.; Samuel, A.Z.; Hosokawa, M.; Takeyama, H. Rapid inspection method for investigating the heat processing conditions employed for chicken meat using Raman spectroscopy. J. Biosci. Bioeng. 2020, 129, 700–705. [Google Scholar] [CrossRef]
- Spanos, D.; Christensen, M.; Tørngren, M.A.; Baron, C.P. Visible spectroscopy as a tool for the assessment of storage conditions of fresh pork packaged in modified atmosphere. Meat Sci. 2016, 113, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Sarangapani, C.; Ryan Keogh, D.; Dunne, J.; Bourke, P.; Cullen, P.J. Characterisation of cold plasma treated beef and dairy lipids using spectroscopic and chromatographic methods. Food Chem. 2017, 235, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.C.; Zou, Y.H.; Cheng, Y.P.; Xing, L.J.; Zhou, G.H.; Zhang, W.G. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- Flores, D.R.M.; Brasil, C.C.B.; Campagnol, P.C.B.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Menezes, C.R.; Barin, J.S.; Flores, E.M.M.; Cichoski, A.J. Application of ultrasound in chicken breast during chilling by immersion promotes a fast and uniform cooling. Food Res. Int. 2018, 109, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, X.; Chen, X.; Fang, R.; Zou, Y.; Wang, D.; Xu, W. How ultrasound combined with potassium alginate marination tenderizes old chicken breast meat: Possible mechanisms from tissue to protein. Food Chem. 2020, 328, 127144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, R.; Zhao, W.; Liang, Q.; Zhang, Z. The first ESR observation of radical species generated under pulsed electric fields processing. LWT Food Sci. Technol. 2011, 44, 1233–1235. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Mechanisms of radical formation in beef and chicken meat during high pressure processing evaluated by electron spin resonance detection and the addition of antioxidants. Food Chem. 2014, 150, 422–428. [Google Scholar] [CrossRef]
- Bolumar, T.; Skibsted, L.H.; Orlien, V. Kinetics of the formation of radicals in meat during high pressure processing. Food Chem. 2012, 134, 2114–2120. [Google Scholar] [CrossRef]
- O’Dowd, L.P.; Arimi, J.M.; Noci, F.; Cronin, D.A.; Lyng, J.G. An assessment of the effect of pulsed electrical fields on tenderness and selected quality attributes of post rigour beef muscle. Meat Sci. 2013, 93, 303–309. [Google Scholar] [CrossRef]
- Mungure, T.E.; Farouk, M.M.; Birch, E.J.; Carne, A.; Staincliffe, M.; Stewart, I.; Bekhit, A.E.-D.A. Effect of PEF treatment on meat quality attributes, ultrastructure and metabolite profiles of wet and dry aged venison Longissimus dorsi muscle. Innov. Food Sci. Emerg. Technol. 2020, 65, 102457. [Google Scholar] [CrossRef]
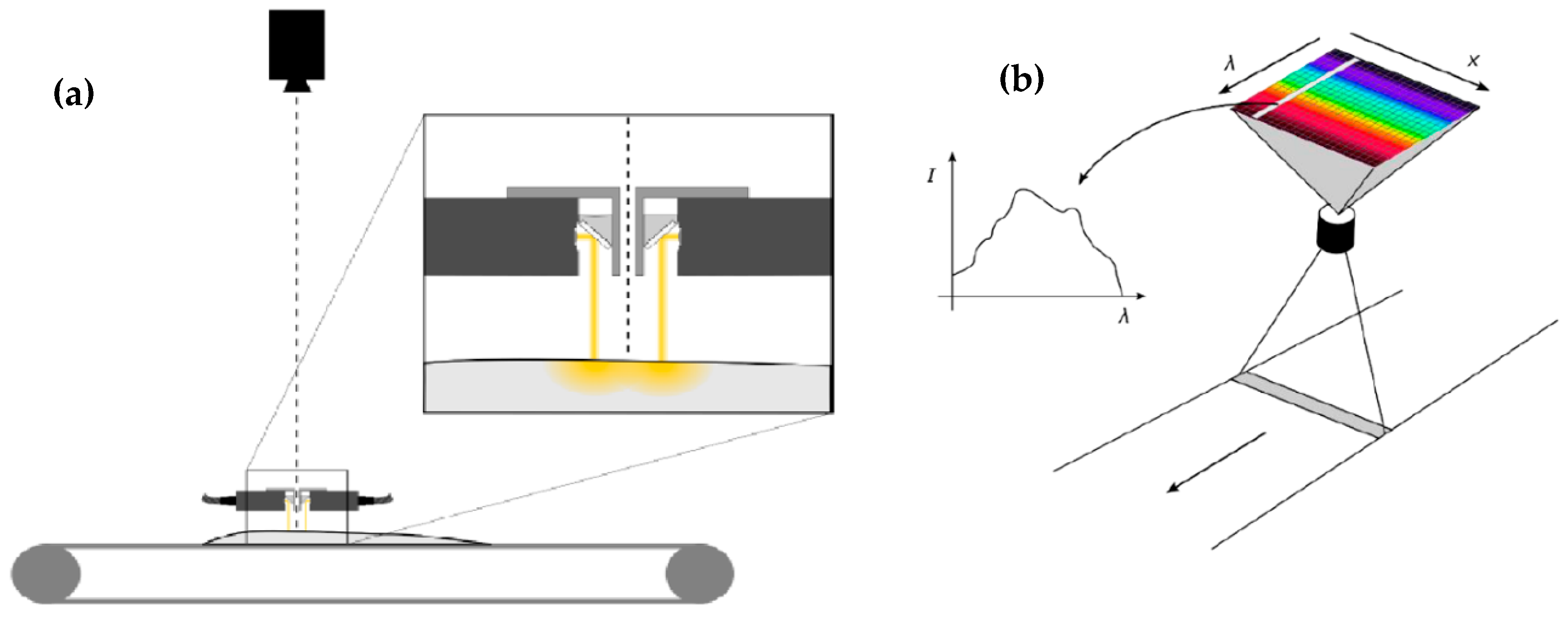
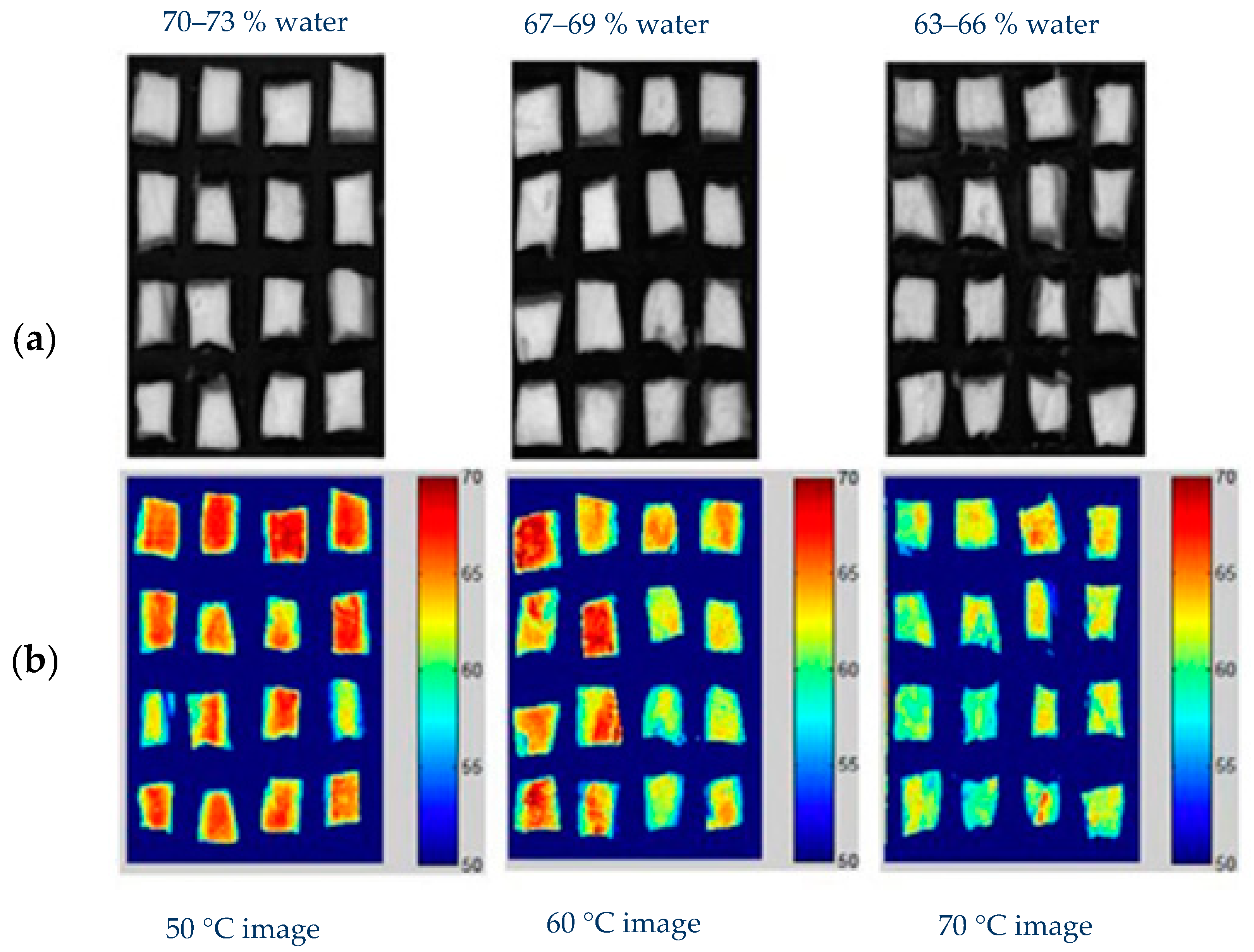
| Muscle Food Product | Thermal Treatments | Main Findings | Reference |
|---|---|---|---|
| Sea bream surimi | Microwave | Microwave- and water bath-treated samples demonstrated better gel properties (stronger gel with finer texture and increased WHC, etc.) than samples heated only with microwave | [47] |
| Sea bream surimi | Microwave | Surimi paste with a thickness of 2 cm of surimi gave the highest WHC, lowest cooking loss, uniformity of temperature distribution, higher tensile force, and whiteness | [48] |
| Sturgeon | Microwaving and other traditional methods | Higher proteins and lipid oxidation in roasted and fried samples than those cooked under less intense cooking conditions (boiling, steaming, and microwaving) | [49] |
| Atlantic salmon | Microwave and conventional pasteurization | Combination of CO2 with heating increased shelf life compared to heating (with microwave or conventional pasteurization) without presence of CO2 in vacuum-package | [50] |
| Grass carp | Microwave | Increased microwave power and treatment time induced a decrease in solubility and an increase in turbidity, indicating a more aggregation of proteins | [51] |
| Cod | Convection oven | Development of a model to predict temperature and moisture concentration based on both heat and mass transfer during cooking | [52] |
| European sea bass | Microwave and conventional oven | Conventional oven backing gave higher abundances of volatile compounds than microwave cooking and salt-crusted oven baking | [53] |
| Shrimp | Ohmic heating | Ohmic heating was applied as a blanching method prior to maturation. The impact of ohmic heating and high pressure on peelability, thermal and structural properties of shell and meat of shrimp were discussed | [54] |
| Pork batters | Ohmic heating | Ohmic heated samples had a more uniform temperature distribution compared to those heated in a traditional water bath, and the overall results suggested that ohmic heating was suitable for meat processing | [55] |
| Turkey breast rolls | Radio frequency heating | Radio frequency heating allowed a reduction of processing time from 150 min (traditional steam processing) to only 40 min. Radio frequency heated samples had lower redness values and lipid oxidation rates compared to the other samples | [56] |
| Pork hams | Radio frequency heating | Radio frequency heating enabled 50% reduction of processing time. The best results were obtained by combining traditional steam heating with radio frequency method, with little differences in sensory quality being observed compared with the traditional steam process | [57] |
| Beef steaks | Radio frequency heating | The developed cooking protocol was applicable to the whole steaks under real processing conditions. Thermal inactivation of Shiga toxin-producing Escherichia coli was more effective at 65 °C with a 5.0 log reduction | [58] |
| Beef meatballs | Ohmic-infrared heating | Infrared heating improved the appearance of the ohmically precooked meatball samples, and the obtained results were affected by the power applied and the distance between the infrared source and the sample surface | [59] |
| Beef meatballs | Ohmic-infrared heating | Ohmically precooked samples followed by infrared heating could improve the microbiological safety and reduce the risk of foodborne illnesses | [60] |
| Chicken nugget | Infrared heating and frying | Compared to deep fat frying method, infrared heated samples had significantly lower fat content, while the color, texture, and other sensory properties were similar to those cooked with the deep fat frying | [61] |
| Spectroscopic Technique | Wavelength Limits | Type of Transition | Advantages | Limitations |
|---|---|---|---|---|
| Fluorescence | 250–750 nm | Bonding electrons in molecules | Rapid, high accuracy, sensitivity, relatively low cost | Limited to samples containing fluorophores, sample surface technique, inner filter effect |
| Near-infrared | 750–2500 nm | Overtones and combinations of fundamental bands | Less sample preparation requirement, high sensitivity to physical structure and presence of water | Requires reliable reference methods, low specificity, overlapped and complex spectra |
| Hyperspectral imaging | 400–1000 nm (most common) | - | Providing spatial information (pixel-to-pixel signal) | Huge amount of data and data processing, costs |
| Mid-infrared | 2500–25,000 nm | Fundamental stretching, bending, and rotating | High sensitivity to chemical compositions, distinct absorption peaks | Water interference; limited suitability of moist samples. Low light penetration |
| Raman | 750–1064 nm (excitation), 2500–200,000 nm | Vibrational transitions | Provides structural and qualitative information, low sensitivity to water | Interference from biological fluorescence background signals, small part of the sample is irradiated (laser spot), low sensitivity, and complex instrumentation |
| Nuclear magnetic resonance | 1–1000 m | Nuclei orientation into a magnetic field | Accuracy, determination of precise structures, minimal sample preparation, spatial information (magnetic resonance imaging: MRI) | Expensive equipment, low sensitivity, overlapping signal, especially when analyzing complex mixtures |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; Bekhit, A.E.-D.; Biancolillo, A.; Wold, J.P. Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Appl. Sci. 2020, 10, 6802. https://doi.org/10.3390/app10196802
Hassoun A, Ojha S, Tiwari B, Rustad T, Nilsen H, Heia K, Cozzolino D, Bekhit AE-D, Biancolillo A, Wold JP. Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Applied Sciences. 2020; 10(19):6802. https://doi.org/10.3390/app10196802
Chicago/Turabian StyleHassoun, Abdo, Shikha Ojha, Brijesh Tiwari, Turid Rustad, Heidi Nilsen, Karsten Heia, Daniel Cozzolino, Alaa El-Din Bekhit, Alessandra Biancolillo, and Jens Petter Wold. 2020. "Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances" Applied Sciences 10, no. 19: 6802. https://doi.org/10.3390/app10196802
APA StyleHassoun, A., Ojha, S., Tiwari, B., Rustad, T., Nilsen, H., Heia, K., Cozzolino, D., Bekhit, A. E.-D., Biancolillo, A., & Wold, J. P. (2020). Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Applied Sciences, 10(19), 6802. https://doi.org/10.3390/app10196802










