Influence of Hydroxyapatite Surface Functionalization on Thermal and Biological Properties of Poly(l-Lactide)- and Poly(l-Lactide-co-Glycolide)-Based Composites
Abstract
1. Introduction
2. Results and Discussion
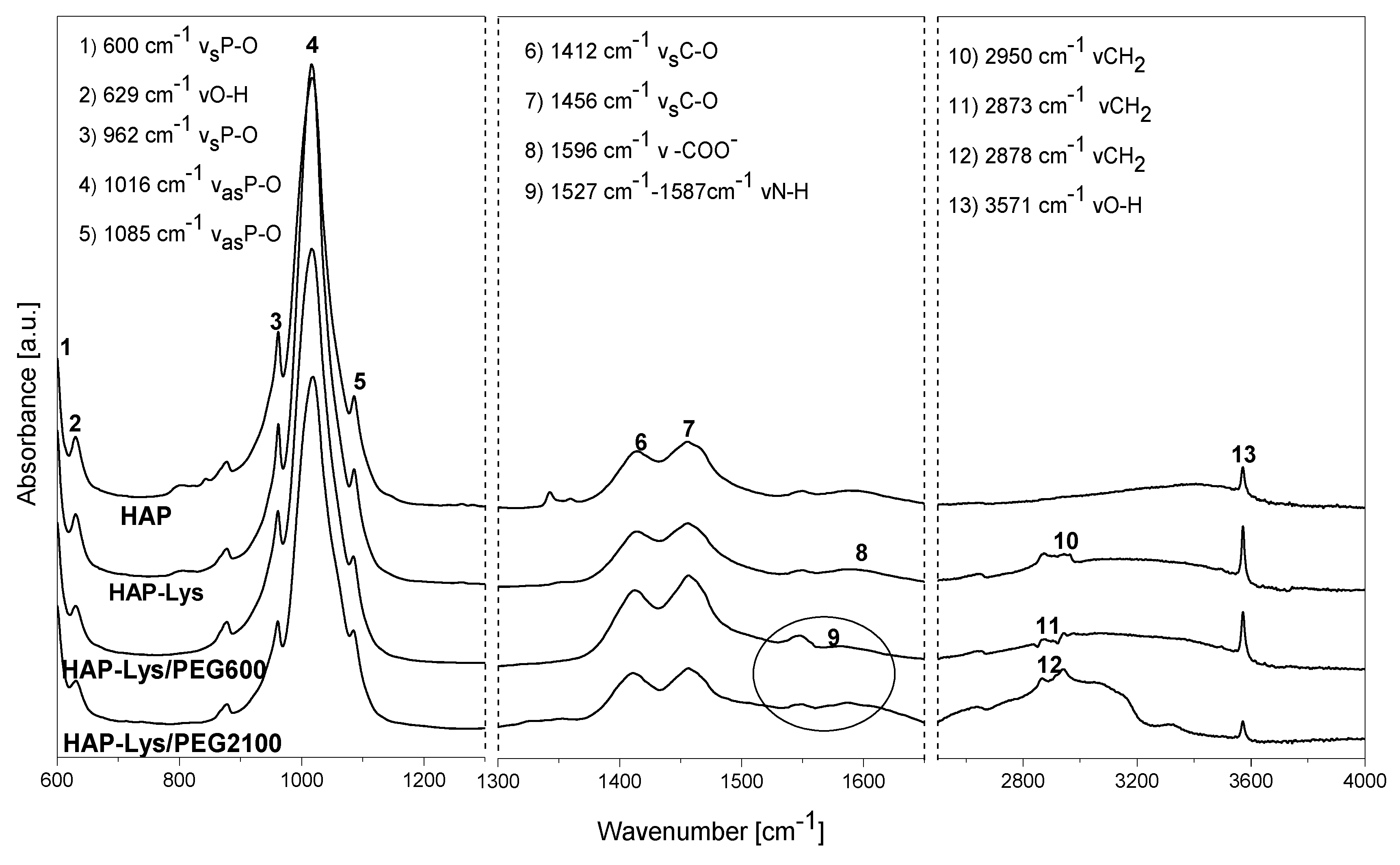
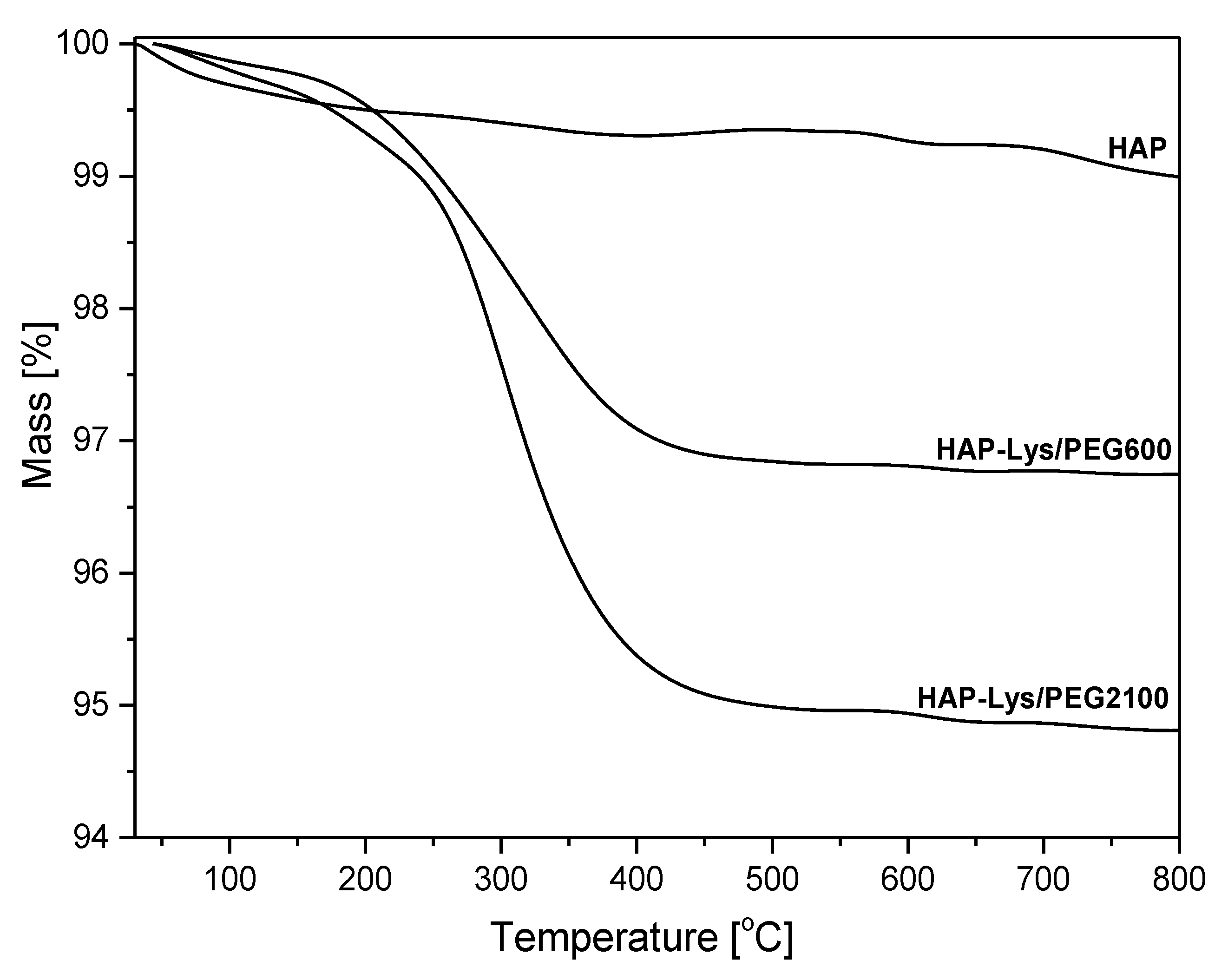
2.1. Characterization of Hydroxyapatite Surface Modification
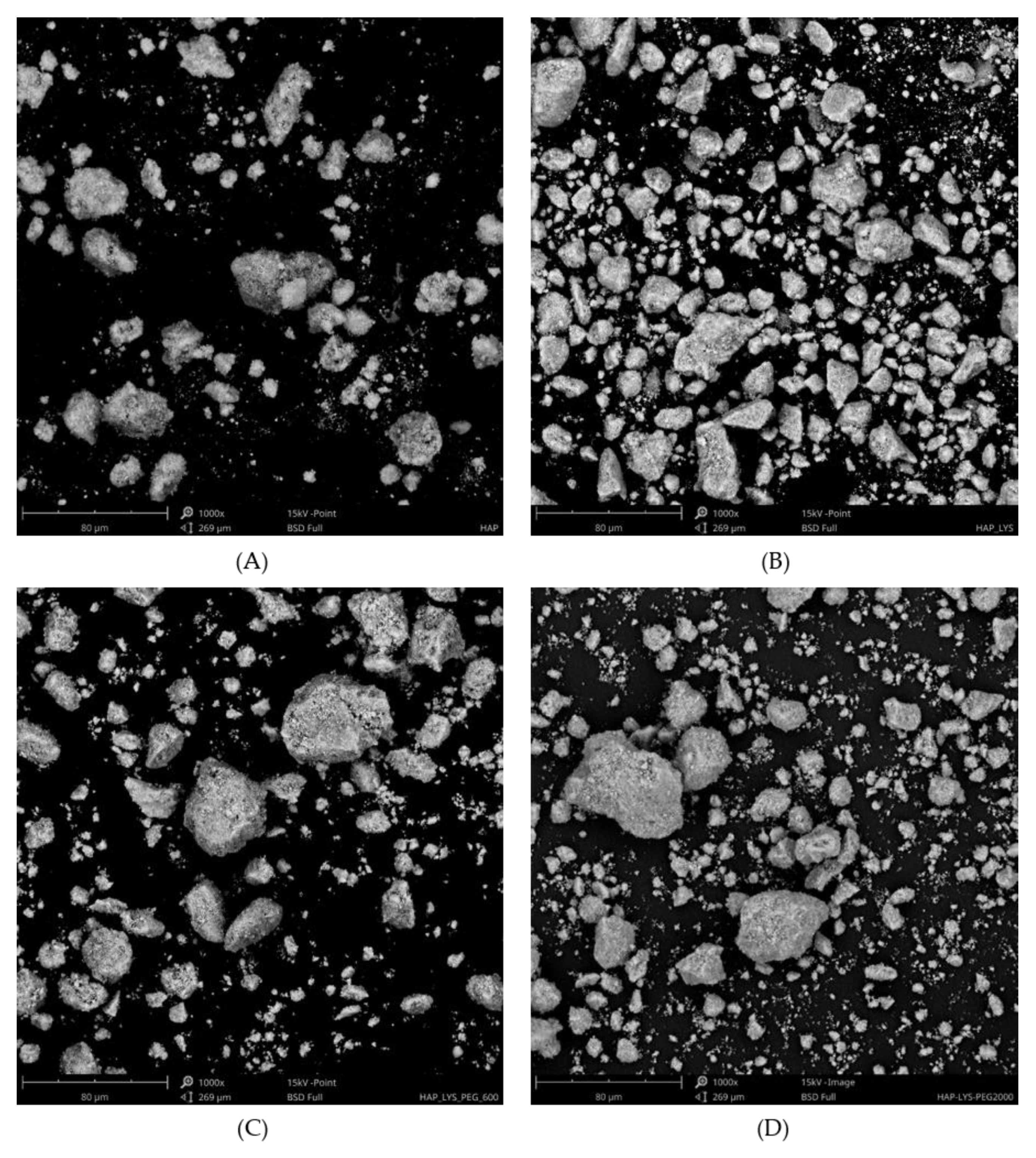
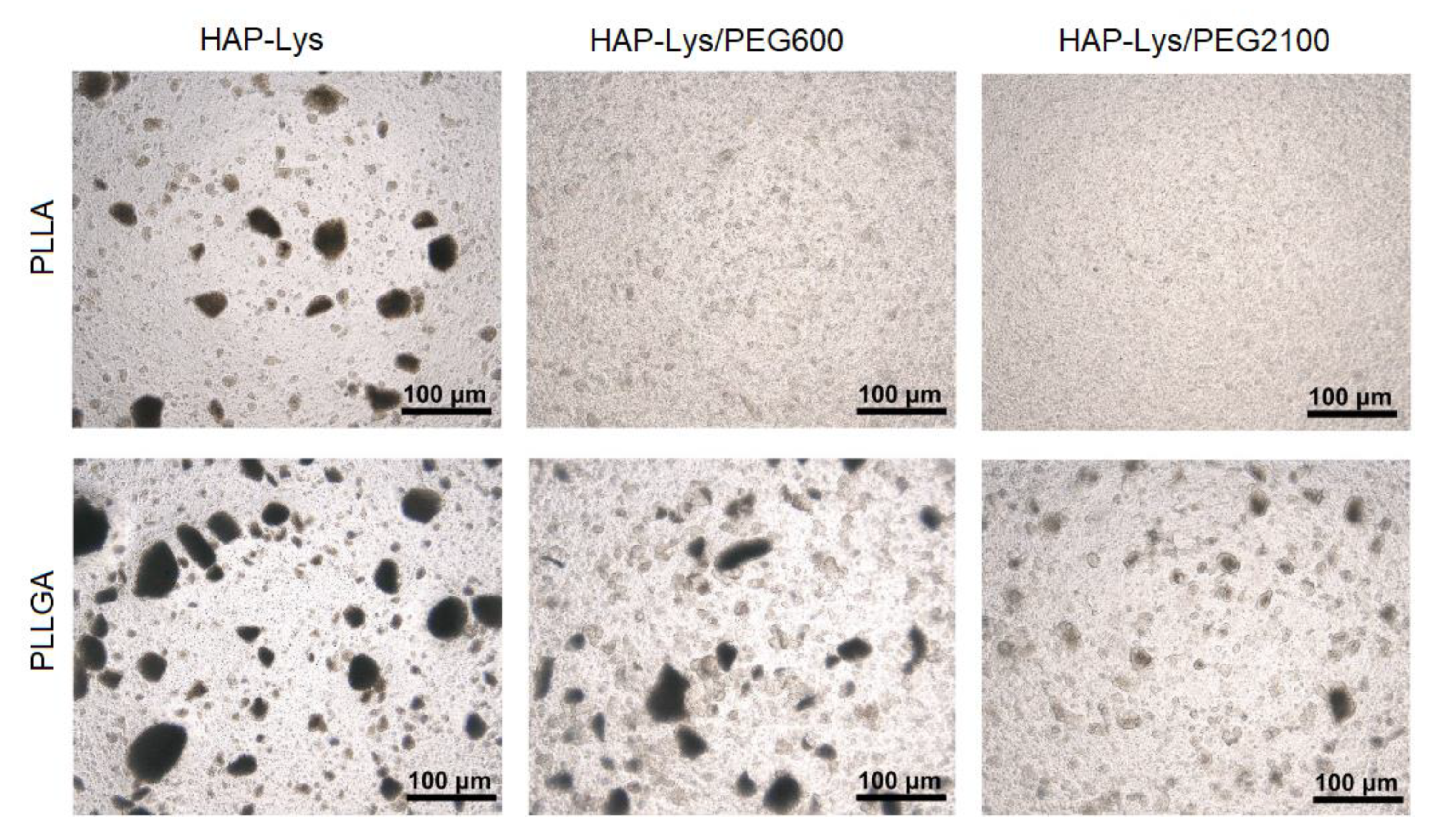
2.2. Morphology Characterization of Hydroxyapatite Particles and Dispersion Effect in Composite Films
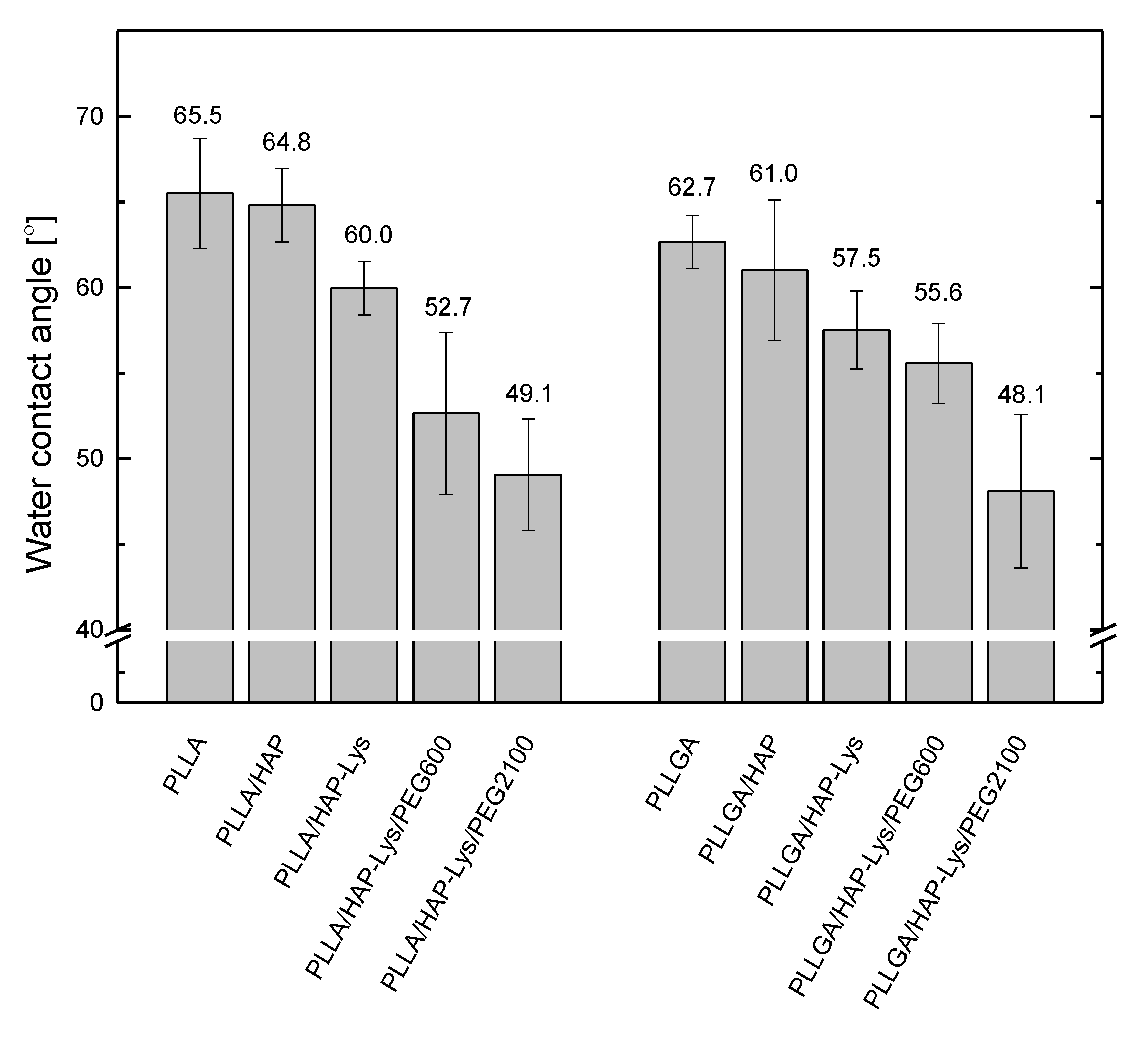
2.3. Water Contact Angle of PLLA, PLLGA, and Composite Film
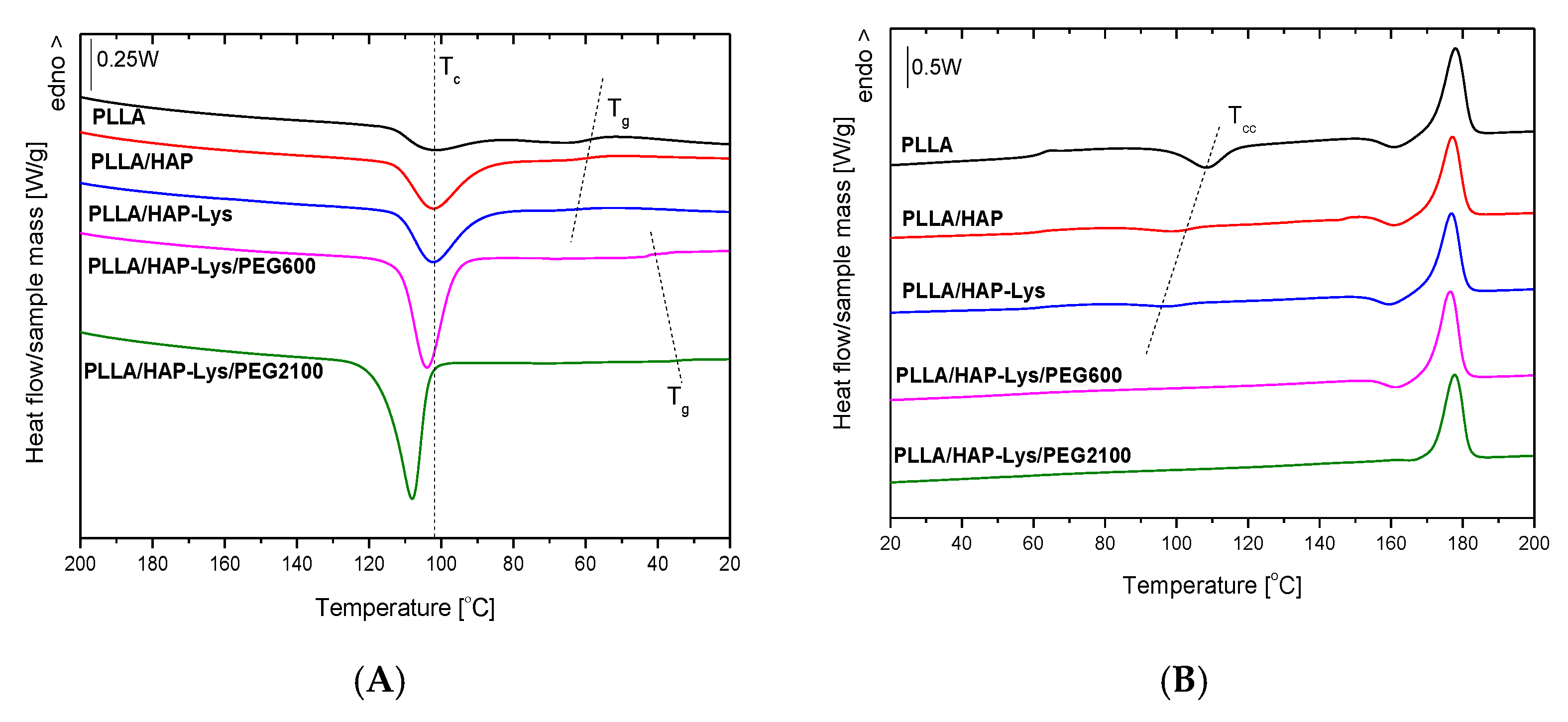
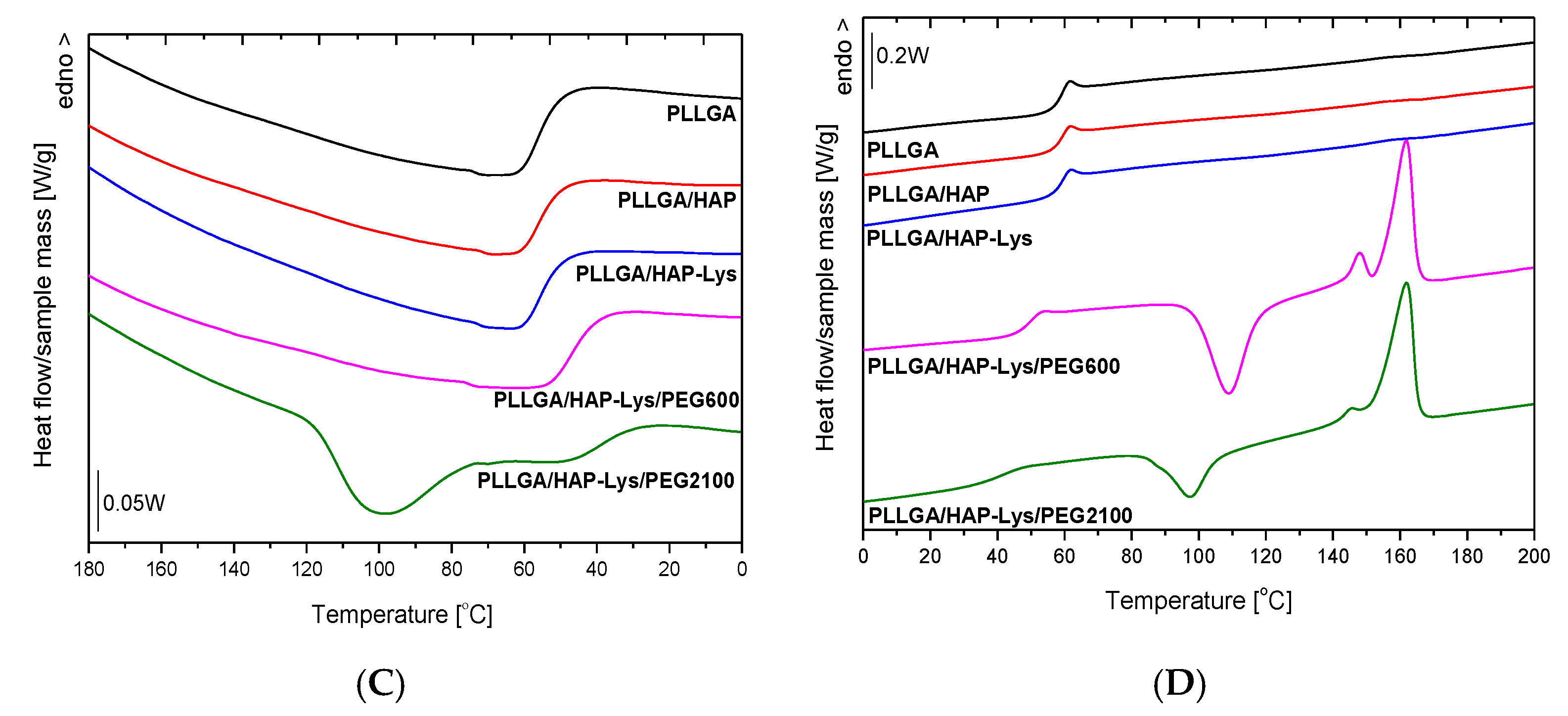
2.4. DSC Results of PLLA and PLLA-Based Composites
2.5. DSC Results of PLLGA and PLLGA-Based Composites
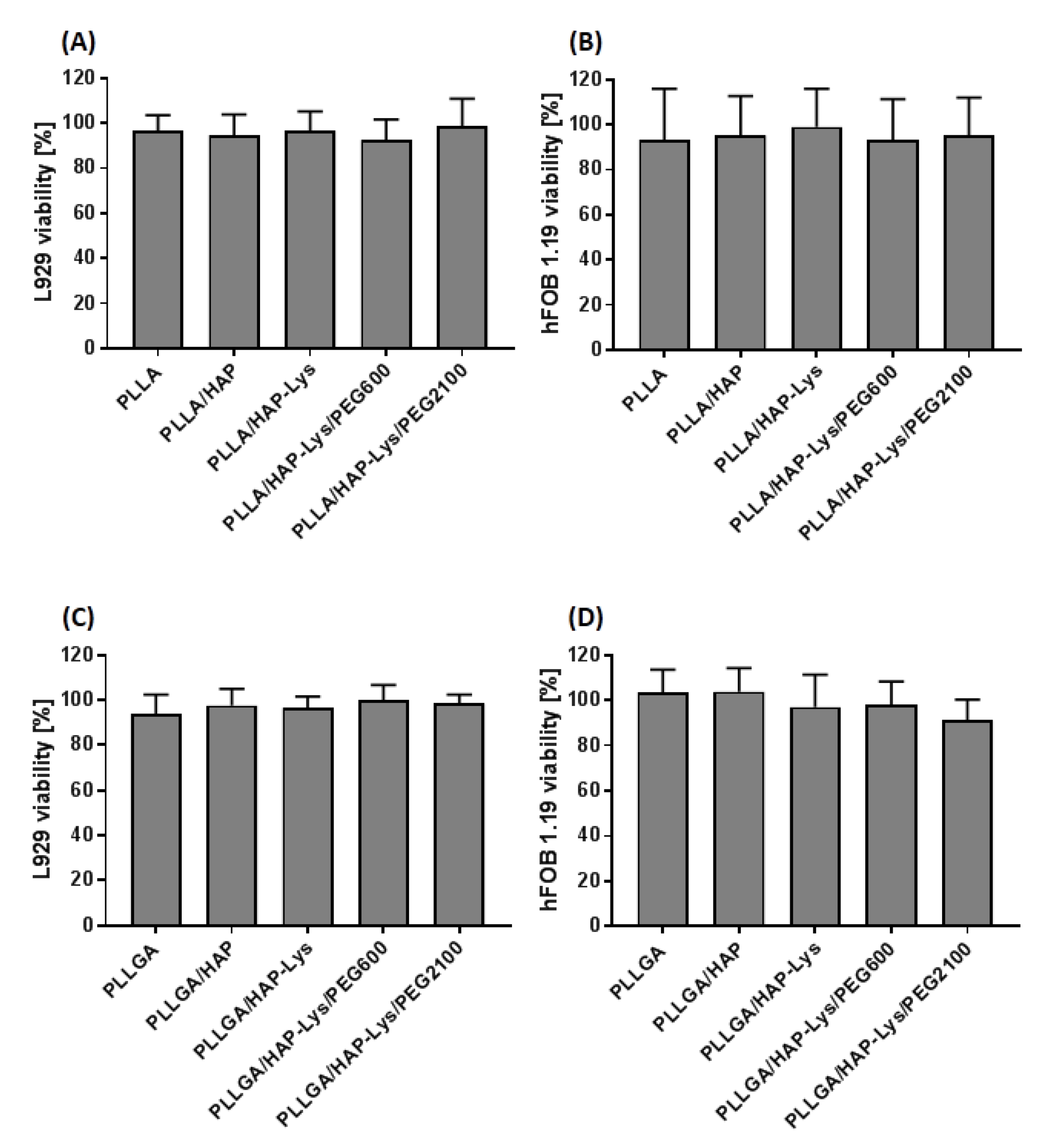
2.6. In Vitro Cytocompatibility of PLLGA and PLLGA-Based Composites
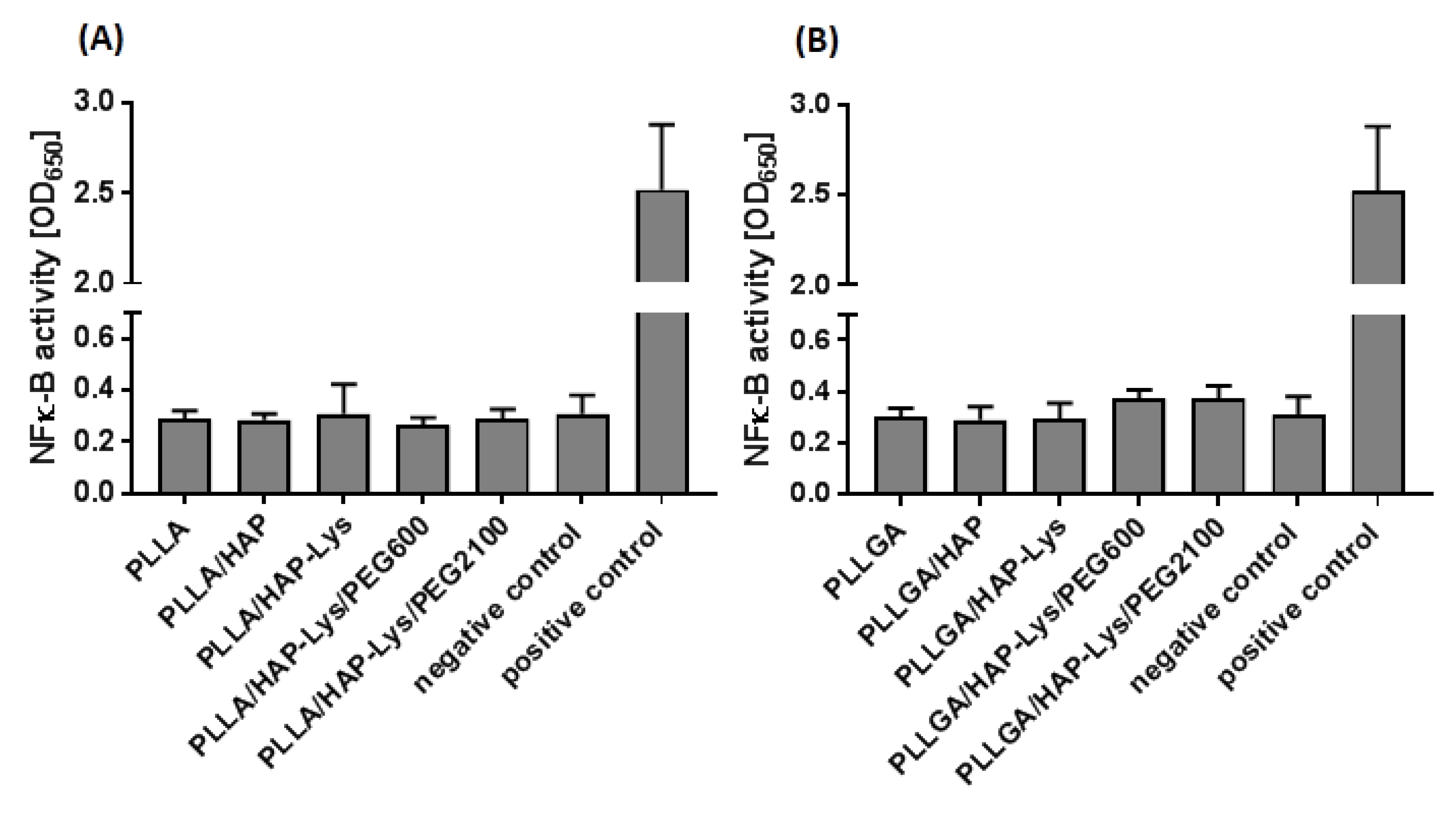
2.7. Immunocompatibility of THP1-Blue™ NF-κB Human Monocytes with PLLA and PLLGA Composites
3. Materials and Methods
3.1. Modification of Hydroxyapatite with l-Lysine
3.2. PEG Grafting to HAP Modified with l-Lysine
3.3. Preparation of Composites
3.4. PLLA/HAP-Lys/PEG2100 Composite Film Preparation
3.5. Fourier-Transform Infrared Spectroscopy
3.6. Thermogravimetry
3.7. Optical Microscopy
3.8. Scanning Microscopy
3.9. Water Contact Angle
3.10. Differential Scanning Calorimetry
3.11. In Vitro Cytocompatibility
3.11.1. Cell Culture Conditions
3.11.2. Direct Contact Cytotoxicity Assay
3.12. Proinflammatory Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LD | linear dichroism |
| ATR | attenuated total reflectance |
| CDI | 1,1′-carbonyldiimidazole |
| DCM | dichloromethane |
| DMSO | dimethyl sulfoxide |
| DSC | differential scanning calorimetry |
| EDS | energy-dispersive X-ray diffraction |
| EDTA | ethylenediaminetetraacetic acid tetrasodium salt dihydrate |
| FBS | fetal bovine serum |
| HAP | hydroxyapatite |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| LPS | lipopolysaccharide |
| Lys | l-lysine |
| MAPK | mitogen-activated protein kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OD | optical density |
| PEG2100 | O-methyl-O′-succinylpolyethylene glycol |
| PEG600 | O-(2-carboxyethyl)-O′-methyl-undecaethylene glycol |
| PLLA | poly(l-lactide) |
| PLLGA | poly(l-lactide-co-glycolide) |
| RANKL | Receptor Activator for Nuclear Factor-κB Ligand |
| RT | room temperature |
| SEAP | secreted embryonic alkaline phosphatase |
| SEM | scanning electron microscopy |
| TGA | thermogravimetry |
| TLR | toll-like receptor |
| Tc | peak temperature of melt crystallization exotherm |
| Tconset | temperature of onset of melt crystallization |
| Tcc | peak temperature of cold crystallization exotherm |
| Tcconset | temperature of onset of cold crystallization |
| Tg | glass transition temperature |
| Tm | peak temperature of melting endotherm |
| Tα’-α | temperature of α’-α phase transition |
| ΔHc | enthalpy of melt crystallization |
| ΔHcc | enthalpy of cold crystallization |
| ΔHα’-α | enthalpy of α’-α phase transition |
| ΔHm | enthalpy of melting |
References
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Zavgorodniy, A.V.; Rohanizadeh, R. High protein adsorptive capacity of amino acid-functionalized hydroxyapatite. J. Biomed. Mater. Res. Part A 2013, 101 A, 873–883. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Osteoblast-Osteoclast Interactions Xiao. Physiol. Behav. 2017, 176, 139–148. [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Qiu, X.; Han, Y.; Zhuang, X.; Chen, X.; Li, Y.; Jing, X. Preparation of nano-hydroxyapatite/poly(l-lactide) biocomposite microspheres. J. Nanoparticle Res. 2007, 9, 901–908. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly (L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Zin, W.; Yunus, W.; Hussein, M.Z. Plasticized Poly (lactic acid) with Low Molecular Weight Poly (ethylene glycol): Mechanical, Thermal, and Morphology Properties. J. Appl. Polym. Sci. 2013, 4576–4580. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kakigi, H.; Maeda, H.; Nishikawa, I.; Ikenaga, H. Bone Formation in a Scaffold Composed of Cylindrical Hydroxyapatite and Tryptophan- or Lysine-Coated Sponge in Vivo. J. Biomed. Sci. Eng. 2015, 8, 389–398. [Google Scholar] [CrossRef]
- Liuyun, J.; Lixin, J.; Chengdong, X.; Lijuan, X.; Ye, L. Effect of l-lysine-assisted surface grafting for nano-hydroxyapatite on mechanical properties and in vitro bioactivity of poly(lactic acid-co-glycolic acid). J. Biomater. Appl. 2016, 30, 750–758. [Google Scholar] [CrossRef]
- Yoshikawa, M. Effect of l-lysine in culture medium on nodule formation by bone marrow cells. J. Biomed. Sci. Eng. 2012, 5, 587–592. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated with Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence from Discordant Monozygotic Twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed]
- Ozhukil Kollath, V.; Van Den Broeck, F.; Fehér, K.; Martins, J.C.; Luyten, J.; Traina, K.; Mullens, S.; Cloots, R. A Modular Approach to Study Protein Adsorption on Surface Modified Hydroxyapatite. Chem. A Eur. J. 2015, 21, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M. Poly (Ethylene Glycol) Chemistry Biotechnical and Biomedical Applications; Springer Science + Buisness Media: New York, NY, USA, 1992; ISBN 9781489907059. [Google Scholar]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and Mechanical Properties of Plasticized Poly(L -lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, S.D.; Liang, J.Z.; Wang, J.Z. Effect of polyethylene glycol on the crystallization and impact properties of polylactide-based blends. Polym. Adv. Technol. 2015, 26, 465–475. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Ren, J.; Cao, E.; Fu, X.; Guo, W. Plasticizing effect of poly(ethylene glycol)s with different molecular weights in poly(lactic acid)/starch blends. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; De Wijn, J.R.; De Groot, K.; Van Blitterswijk, C.A. Surface modification of nano-apatite by grafting organic polymer. Biomaterials 1998, 19, 1067–1072. [Google Scholar] [CrossRef]
- Jegatheeswaran, S.; Sundrarajan, M. PEGylation of novel hydroxyapatite/PEG/Ag nanocomposite particles to improve its antibacterial efficacy. Mater. Sci. Eng. C 2015, 51, 174–181. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.W.; Franco, R.A.; Sarkar, S.K.; Lee, B.T. Fabrication of porous hydroxyapatite scaffolds as artificial bone preform and its biocompatibility evaluation. ASAIO J. 2014, 60, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Ergun, C.; Evis, Z.; Webster, T.J.; Sahin, F.C. Synthesis and microstructural characterization of nano-size calcium phosphates with different stoichiometry. Ceram. Int. 2011, 37, 971–977. [Google Scholar] [CrossRef]
- Aronov, D.; Rosen, R.; Ron, E.Z.; Rosenman, G. Tunable hydroxyapatite wettability: Effect on adhesion of biological molecules. Process Biochem. 2006, 41, 2367–2372. [Google Scholar] [CrossRef]
- Wang, Y.; Funari, S.S.; Mano, J.F. Influence of semicrystalline morphology on the glass transition of poly(l-lactic acid). Macromol. Chem. Phys. 2006, 207, 1262–1271. [Google Scholar] [CrossRef]
- Lai, W.C.; Liau, W.B.; Lin, T.T. The effect of end groups of PEG on the crystallization behaviors of binary crystalline polymer blends PEG/PLLA. Polymer 2004, 45, 3073–3080. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Hadasha, W.; Bezuidenhout, D. Poly(lactic acid) as Biomaterial for Cardiovascular Devices and Tissue Engineering Applications. In Industrial Applications of Poly(Lactic Acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 51–77. ISBN 978-3-319-75459-8. [Google Scholar]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yu, X.; Li, Y.; Sun, A.; Huang, C.; Xu, F.; Guo, J.; Sun, Y.; Zhang, X.; et al. Construction of vascularized pacemaker tissues by seeding cardiac progenitor cells and endothelial progenitor cells into Matrigel. Life Sci. 2017, 179, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.F.; Schuler, M.; Tosatti, S.; Textor, M.; Schwartz, Z.; Boyan, B.D. Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics. Clin. Oral Implants Res. 2011, 22, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Osada, S.; Narita, T.; Oishi, Y.; Kodama, H. Promotion of cell adhesion by low-molecular-weight hydrogel by Lys based amphiphile. Mater. Sci. Eng. C 2015, 47, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Cruz, O.; Avilés, F.; Vargas-Coronado, R.; Cauich-Rodríguez, J.V.; Chan-Chan, L.H.; Sessini, V.; Peponi, L. Mechanical properties of l-lysine based segmented polyurethane vascular grafts and their shape memory potential. Mater. Sci. Eng. C 2019, 102, 887–895. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, K.; Niu, L.; Jiao, Y.; Song, Q.; Shen, L.; Tay, F.R.; Chen, J. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 2017, 113, 203–216. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Lennerås, M.; Ekström, K.; Vazirisani, F.; Shah, F.A.; Junevik, K.; Thomsen, P.; Omar, O. Interactions between monocytes, mesenchymal stem cells, and implants evaluated using flow cytometry and gene expression. J. Tissue Eng. Regen. Med. 2018, 12, 1728–1741. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of crystalline forms (α′ and α) of poly(lactic acid) on its mechanical, thermo-mechanical, heat deflection temperature and creep properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Cocca, M.; Lorenzo, M.L.D.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Duan, J.; Yang, J.; Huang, T.; Qi, X.; Wang, Y. Comparison study of hydrolytic degradation behaviors between α′- and α-poly(l-lactide). Polym. Degrad. Stab. 2018, 148, 1–9. [Google Scholar] [CrossRef]
| HAP | HAP-Lys | HAP-Lys/PEG600 | HAP-Lys/PEG2100 | |||||
|---|---|---|---|---|---|---|---|---|
| Element | at% | wt.% | at% | wt.% | at% | wt.% | at% | wt.% |
| C | 39.89 ± 6.25 | 26.40 ± 5.52 | 45.15 ± 7.23 | 32.04 ± 4.92 | 28.59 ± 7.42 | 18.47 ± 4.16 | 34.63 ± 9.79 | 23.09 ± 8.15 |
| O | 44.32 ± 7.30 | 40.88 ± 7.05 | 33.10 ± 6.55 | 31.38 ± 6.21 | 44.24 ± 9.36 | 38.21 ± 7.63 | 38.31 ± 9.24 | 33.76 ± 10.80 |
| N | - | - | 6.49 ± 2.44 | 5.34 ± 0.51 | 8.47 ± 0.65 | 6.38 ± 0.43 | 8.56 ± 1.01 | 6.56 ± 0.73 |
| Ca | 9.84 ± 1.63 | 22.23 ± 3.22 | 8.99 ± 1.87 | 21.25 ± 4.20 | 12.03 ± 2.51 | 25.01 ± 5.19 | 12.18 ± 4.66 | 26.08 ± 7.96 |
| P | 5.86 ± 0.88 | 10.32 ± 1.32 | 4.93 ± 0.93 | 8.78 ± 1.57 | 6.51 ± 0.99 | 10.82 ± 1.42 | 6.41 ± 1.89 | 10.56 ± 2.38 |
| Ca/P molar ratio | 1.67 | 1.87 | 1.79 | 1.91 | ||||
| Ca/P atomic ratio | 1.68 | 1.89 | 1.85 | 1.90 | ||||
| Particle average size (µm2) | 11.36 ± 2.90 | 8.89 ± 2.12 | 5.48 ± 1.21 | 8.64 ± 3.92 | ||||
| Sample | Tconset (°C) | Tc (˚C) | ΔHc (J/g) | Tg (°C) |
|---|---|---|---|---|
| PLLA | 114.0 | 102.5 | 11.9 | 59.7 |
| PLLA/HAP | 112.8 | 102.2 | 23.8 | 58.6 |
| PLLA/HAP-Lys | 112.0 | 102.2 | 24.0 | 61.5 |
| PLLA/HAP-Lys/PEG600 | 111.2 | 104.0 | 32.1 | 43.7 |
| PLLA/HAP-Lys/PEG2100 | 116.5 | 108.2 | 37.9 | 35.2 |
| PLLGA | - | - | - | 56.3 |
| PLLGA/HAP | - | - | - | 56.2 |
| PLLGA/HAP-Lys | - | - | - | 55.5 |
| PLLGA/HAP-Lys/PEG600 | - | - | - | 47.0 |
| PLLGA/HAP-Lys/PEG2100 | 118.3 | 100.9 | 10.8 | 28.7 |
| Sample | Tg (°C) | Tcconset (°C) | Tcc (°C) | ΔHcc (J/g) | Tα’-α (°C) | ΔHα’-α (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|---|
| PLLA | 62.6 | 97.2 | 108.9 | 25.7 | 160.9 | 6.4 | 177.9 | 46.4 |
| PLLA/HAP | 61.4 | 84.9 | 99.1 | 8.7 | 6.1 | 170.8 | 42.7 | |
| PLLA/HAP-Lys | 63.1 | 84.3 | 97.8 | 6.7 | 159.5 | 5.7 | 170.6 | 41.8 |
| PLLA/HAP-Lys/PEG600 | 57.0 | 76.5 | 115.8 | 2.0 | 161.5 | 4.0 | 170.9 | 43.3 |
| PLLA/HAP-Lys/PEG2100 | 46.0 | - | - | - | - | - | 172.0 | 41.2 |
| PLLGA | 59.5 | - | - | - | - | - | - | - |
| PLLGA/HAP | 59.6 | - | - | - | - | - | - | - |
| PLLGA/HAP-Lys | 59.62 | - | - | - | - | - | - | - |
| PLLGA/HAP-Lys/PEG600 | 50.1 | 98.39 | 109.0 | 28.1 | - | - | 161.7 | 24.6 |
| PLLGA/HAP-Lys/PEG2100 | 41.6 | 86.68 | 97.5 | 14.8 | - | - | 161.8 | 28.7 |
| Material | Chemical Formula | Tg [°C] | Tm [°C] | Molecular Weight * |
|---|---|---|---|---|
| PEG600 |  | −64.4 | 20.2 | 588.86 g/mol |
| PEG2100 |  | −33.1 | 53.2 | ~2100 g/mol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazińska, M.; Krokos, A.; Kobielarz, M.; Włodarczyk, M.; Skibińska, P.; Stępak, B.; Antończak, A.; Morawiak, M.; Płociński, P.; Rudnicka, K. Influence of Hydroxyapatite Surface Functionalization on Thermal and Biological Properties of Poly(l-Lactide)- and Poly(l-Lactide-co-Glycolide)-Based Composites. Int. J. Mol. Sci. 2020, 21, 6711. https://doi.org/10.3390/ijms21186711
Gazińska M, Krokos A, Kobielarz M, Włodarczyk M, Skibińska P, Stępak B, Antończak A, Morawiak M, Płociński P, Rudnicka K. Influence of Hydroxyapatite Surface Functionalization on Thermal and Biological Properties of Poly(l-Lactide)- and Poly(l-Lactide-co-Glycolide)-Based Composites. International Journal of Molecular Sciences. 2020; 21(18):6711. https://doi.org/10.3390/ijms21186711
Chicago/Turabian StyleGazińska, Małgorzata, Anna Krokos, Magdalena Kobielarz, Marcin Włodarczyk, Paulina Skibińska, Bogusz Stępak, Arkadiusz Antończak, Milena Morawiak, Przemysław Płociński, and Karolina Rudnicka. 2020. "Influence of Hydroxyapatite Surface Functionalization on Thermal and Biological Properties of Poly(l-Lactide)- and Poly(l-Lactide-co-Glycolide)-Based Composites" International Journal of Molecular Sciences 21, no. 18: 6711. https://doi.org/10.3390/ijms21186711
APA StyleGazińska, M., Krokos, A., Kobielarz, M., Włodarczyk, M., Skibińska, P., Stępak, B., Antończak, A., Morawiak, M., Płociński, P., & Rudnicka, K. (2020). Influence of Hydroxyapatite Surface Functionalization on Thermal and Biological Properties of Poly(l-Lactide)- and Poly(l-Lactide-co-Glycolide)-Based Composites. International Journal of Molecular Sciences, 21(18), 6711. https://doi.org/10.3390/ijms21186711






